Research Article Open Access
Immunotoxicity of Zinc Oxide Nanoparticles and Municipal Effluents to Fathead Minnows
Gagne F1*, Fortier M1, Fournier M2, and Gagnon C21Aquatic Contaminants Research Division, Water Science and Technology, Environment Canada, 105 McGill, Montreal, QC, Canada
2INRS-Institut Armand-Frappier, 531 Boul. des Prairies, Laval, QC, Canada
- *Corresponding Author:
- Gagne F
Aquatic Contaminants Research Division
Water Science and Technology
Environment Canada, 105 McGill
Montreal, QC
Canada,
E-mail: francois.gagne@canada.ca
Received date: June 15, 2016; Accepted date: June 20, 2016; Published date: June 25, 2016
Citation: Gagne F, Fortier M, Fournier M, Gagnon C (2016) Immunotoxicity of Zinc Oxide Nanoparticles and Municipal Effluents to Fathead Minnows. Toxicol Open Access 2:113. doi:10.4172/2476-2067.1000113
Copyright: © 2016 Gagne F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
The presence of nanoparticles in sewage water has raised concerns about the cumulative toxicity of municipal effluent to aquatic organisms. The purpose of this study was to examine the effects of dietary zinc oxide nanoparticles (nano-ZnO) and ZnCl2 (at 100 ng total Zn/g food) in adult fathead minnows Pimephales promelas during continuous exposure to a physico-chemically treated effluent for 21 days. Immunocompetence was determined by assessing leucocyte viability, phagocytosis activity, oxidative stress (lipid peroxidation) and DNA strand breaks in gills. The results revealed that leucocyte viability decreased with increasing effluent concentration, while it increased somewhat in fish fed either form of Zn. The decrease in viability was not observed in fish exposed to the municipal effluent that were fed either form of Zn. Phagocytosis activity decreased after an initial increase at a low concentration of the effluent (5% v/v), while it readily decreased in fish fed either form of Zn. The decrease was also observed in fish fed either form of Zn that were exposed to the effluent. The data revealed that nano-ZnO toxicity differed from ZnCl2 effects, but when the fish were both exposed to the effluent and fed a nano-ZnOcontaminated diet, the overall effects closely resembled the effects in fish fed a ZnCl2 -supplemented diet. In conclusion, ingested nanoparticles in food could affect the immune system of fish exposed to municipal wastewaters differently than non-exposed fish, rendering the exposed fish more vulnerable to microorganisms.
Keywords
Leucocyte viability; Phagocytosis; Lipid peroxidation; DNA strand breaks; Fathead minnows
Introduction
Municipal effluents are a major source of contamination to aquatic biota. They comprise a variety of contaminants originating from industrial, domestic activities and urban runoffs. They are complex mixtures of contaminants in addition to microorganisms such as polycyclic aromatic and aliphatic hydrocarbons, heavy metals, pesticides, pharmaceutical and personal products [1]. The continuous discharge of municipal effluents could have therefore serious impacts on aquatic ecosystems. In some cases, these discharges contribute to bacterial contamination and eutrophication caused by inputs of nitrogen and phosphorus to aquatic habitats. As an emerging technology, nanotechnology is a major source of discharges of novel complex pollutants to aquatic ecosystems [2]. The determination of the behaviour, persistence and toxicity of nanoparticles in municipal effluent is a challenge given that nanoparticles can interact with the natural and municipal organic matter, form aggregates or degrade by oxidative processes, such as microbial aerobic digestion, chemical oxidation and sunlight.
Zinc oxide nanoparticles (nano-ZnO) are engineered materials having a size range of 1–100 nm in diameter and a high surface to volume ratio. Nano-ZnO is use in a wide variety of commercial applications, including personal care products such as transparent sunscreens, ointments and cosmetics [3], as well as pigments and lubricants. They also have biocidal properties, which have commercial value to consumers, and are associated with the slow release of Zn2+ in cream preparations [4]. On the one hand, nano-ZnO has low Zeta potential, which makes them susceptible to aggregation in surface water [5]. On the other hand, dissolved organic matter, which is present at high concentrations in some types of freshwater, especially those close to sources of urban pollution, could bind to the nanoparticles and prevent aggregation [6]. With the increasing use of sunscreens and cosmetics containing nano-ZnO, these pollutants will find their way into sewage wastewaters and will be released to the aquatic environment. The interaction of nano-ZnO and their aggregates with organic-rich municipal effluents is not well understood, but would likely modulate the toxicity of released Zn forms.
Like higher vertebrates, fish possess both innate and specific immunity [7]. Innate immunity involves macrophages, granulocytes and natural cytotoxic cells. Phagocytosis of macrophages and granulocytes is an important line of defence against invading microorganisms and foreign bodies [8]. Adaptive and specific immunity is mediated by B and T cells which are involved in the production of antibodies and cytotoxic agents (cytokines) to ensure destruction of pathogen-infected cells. Given that municipal effluents contain chemicals and suspended particles, they are known to disrupt immunocompetence in fish and other aquatic organisms which involves inflammation [9,10]. In addition, nanoparticles induce oxidative stress and inflammation, which could impair immunocompetence in fish [11]. Moreover, the cumulative effects on fish immunocompetence in fish exposed to both municipal effluents and nano-ZnO are not well understood at the present time.
The purpose of this study was therefore to examine the immunotoxicity of municipal effluent in the presence of nano-ZnO in adult fathead minnows. Given the instability of nano-ZnO in surface waters, the fish were exposed to nano-ZnO through food ingestion and was compared with feed spiked with ZnCl2. Immunocompetence was assessed by leucocyte viability, phagocytosis activity (proportion of cells that ingested at least one bead) and phagocytosis efficiency (proportion of cells that ingested at least three beads). Lipid peroxidation and DNA strand breaks were also measured in gills to determine the oxidative and inflammatory effects of 21-day continuous exposure to municipal effluents. An attempt was made to determine the interaction between municipal effluent in fish that were fed normal diet and in fish fed a diet spiked with either nano-ZnO or ZnCl2.
Materials and Methods
Fish were cultured and bred at a fathead minnow colony at the wet labs of the aquatic toxicology laboratory at the Montreal Wastewater Treatment Plant (Pointe-Claire, QC, Canada). The study employed a 21-day exposure regime with a 1:2 male to female ratio in 12 L tanks into which diluted municipal effluent was continuously pumped (1 L per hour). Briefly, two males and four females were held in a 12-L aquarium for a period of 7 days prior to exposure to the effluent, dietary Zncl2 and nano-ZnO. The aquariums contained spawning tiles made from two 8-cm lengths of polyvinyl pipes with a 10-cm diameter, cut in half longitudinally, which were monitored in the mornings for egg production. Successful fertilization of spawned eggs was confirmed by observation under a microscope, and groups exhibiting the highest egg fertilization rate were selected for the experiment. In fully active fish, exposure to the effluent was initiated using concentrations of 5, 10 and 20% v/v at 25°C. The effluent was constantly renewed (1 L/hr flow rate) and was pre-heated to 25°C before being pumped into the fish aquariums. The exposure experiments were repeated with two replicate tanks for each treatment group. In addition to real-time exposure to the municipal effluents, one group of fish was exposed to either nano- ZnO- or Zncl2-spiked feed at a nominal concentration of 100 ng of total Zn per g of feed. The fish were fed a commercial fish food once daily during the exposure experiments (10 g per aquarium). The food powder was placed in a Waring-type blender at low speed, and zinc (Zncl2 or nano-ZnO) was added at 10 × 100 µl volume increments to ensure homogenous distribution in the mixture. Nano-ZnO stock solution was purchased from Sigma-Aldrich (721077) at 50% w/v at pH 7.1. The nano-ZnO particles were capped with cationic 3- aminopropyltriethoxysilane to ensure stability in the stock solution. Analysis of the commercial sample in bidistilled water using dynamic light scattering analysis revealed a mean diameter of 60 ± 5 nm, which is technically close to the manufacturer's specifications (50-nm mean diameter) [12]. Controls were exposed to aquarium water and fed an unspiked diet. The fish were exposed to these conditions at 25°C for 21 days under constant aeration and a 16 h light/8 h dark photoperiod. Water pH, dissolved oxygen and temperature were monitored daily, and the spawning tiles were checked for egg production (the tiles were replaced with new ones when the egg density covered a large proportion of the tile). At the end of the exposure period, the fish were anesthetized in a 50 mg/l MS-222 solution (Sigma-Aldrich, Ontario, Canada) in accordance with the guidelines of the Animal Care Committee. Fork length and wet body weight, gonad weight and brain weight were recorded. The organs were then mixed with three different volumes of homogenization buffer before freezing at -85°C. The homogenization buffer consisted of 140 mM NaCl and 25 mM Hepes- NaOH, at pH 7.4, containing 1 mM dithiothreitol and 10 µg/ml aprotinin (protease inhibitor) and the tissues were homogenized using a Teflon pestle tissue grinder (5 passes) on ice. A portion of the homogenate was centrifuged at 3000X g for 15 min at 4°C, and the resulting supernatant (S3 fraction) was centrifuged at 15000X g for 30 min at 4°C. The supernatant (S15 fraction), S3 fraction and homogenate were then conserved at -85°C until analysis. The male and female secondary sexual characteristics were also evaluated: ovipositor, banding coloration in males, nuptial tubercles and head-sponge appearance.
Immunocompetence assessment
Leucocytes were isolated from the head kidneys under sterile conditions and crushed on a stainless steel mesh (50 µm mesh size) with a rubber spatula in RPMI 1640 cell culture medium (Sigma, ON, Canada) supplemented with heparin (10 U/ml), HEPES (10mM; pH 7.2) (Sigma, On, Canada), penicillin (100 U/ml)/ streptomycin (100 mg/ml) (Sigma, ON, Canada), and 10% heat inactivated foetal bovine serum (FBS) (Sigma, ON, Canada). The cell suspension was then layered over Lympholyte-M (density=1.085; Cedarlane, On, Canada) and centrifuged at 600X g for 20 min at room temperature. Cells (leucocytes) were collected at the Lympholyte-M interface, washed two times in phosphate-buffred saline (Sigma, ON, Canada) and resuspended in cell culture medium containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 10 mM HEPES-NaOH, pH 7.2.
Cell viability was determined using trypan blue stain (0.4%; Sigma- Aldrich Chemical Co., Canada). Counts of viable and dead (stained) cells were then performed on a hemocytometer at 200X magnification under a binocular microscope. Phagocytic activity was measured in macrophages (fish) based on a previously published protocol. Briefly, fluorescent latex beads (d=1.72 µm; Polysciences, PA, USA) were added to the cell suspensions at a ratio of 100 beads per cell and incubated at 15ºC for 18 h in the dark. After the incubation period, the cell suspensions were layered on top of 4 ml of sterile RPMIc supplemented with 3% bovine serum albumin (BSA) (Sigma, On, Canada). Elimination of free beads was performed by centrifugation at 150 g for 8 min at 4°C. Cell pellets were then resuspended in 0.5 ml of 0.5% formaldehyde (Sigma, ON, Canada) and 0.2% sodium azide (Sigma, ON, Canada) diluted in BDFacsflowTM (Becton Dickinson, CA, USA). Cells were analyzed by flow cytometry (FACSCalibur, Becton Dickinson, CA, USA) and at least 10000 events were recorded. Analyses were done using two endpoints operationally defined as phagocytosis activity: the percentage of phagocytes containing one bead or more (M1) and then as the phagocytosis efficiency or capacity, phagocytes containing three beads or more (M2).
Lipid peroxidation (LPO) was determined in the gill homogenates using the thiobarbituric acid method for malonaldehyde (and other aldehydes) detection [13]. A 100 µl volume of the homogenate was mixed with 300 µl of 10% trichloroacetic acid containing 1 mM FeSO4 and 150 µl of 0.7% thiobarbituric acid and heated at 70-80°C for 10 min. The mixture was cooled at room temperature and centrifuged at 10000X g for 5 min to remove any precipitates. A 200 µl volume was transferred to a 96-well dark microplate and fluorescence readings were taken at 520 nm excitation and 600 nm emission. Standard solutions of tetrametoxypropane (stabilized form of malonaldehyde) were prepared for calibration in the blank (homogenization buffer). Results were expressed as µmole thiobarbituric acid reactants (TBARS)/mg total proteins in the homogenate. DNA damage was determined using the alkaline precipitation assay [14], which is based on the potassium detergent precipitation of DNA-proteins. Proteinfree DNA strand breaks were measured in the supernatant using fluorescence spectroscopy. A 25 µl volume of the homogenate was mixed with 225 µl of detergent solution (2% SDS containing 10 mM EDTA, 10 mM Tris base and 40 mM NaOH) for one min, followed by the addition of 250 µl of 0.12 M KCl. The mixture was mixed by inversion, heated at 60°C for 10 min, cooled on ice for 15 min and centrifuged at 8000X g for 10 min. A 50 µl volume of the supernatant was mixed to 150 µl of 100 µg/ml Hoescht in 0.1 Tris-Acetate, pH 8.5, containing 4 mM sodium cholate and 0.4 M NaCl and fluorescence readings taken at 360 nm excitation and 450 nm emission. A standard solution of salmon sperm DNA was prepared for calibration. The data were expressed as µg DNA strand/mg total protein in homogenate.
Data analysis
The exposure experiment consisted of 2 males and 4 females per treatment aquarium and 3 tanks per treatment were used (6 males and 12 females in total per treatment). Tissue biomarkers were analyzed in N=6 males and N=6 females fish using two-way factorial analysis of variance (exposure groups and sex as the main factors) after verifying for homogeneity of variance and normality using Levene’s test and the Shapiro-Wilk test. Correlation analysis was also performed using the Pearson product moment correlation. Discriminant function analysis and factor analysis were used to determine the physiological changes induced by exposure to the municipal effluent and the two zinc formulations in the feed. All statistical tests were performed using Statistica software (version 8). Significance was set at a=0.05.
Results
The basic physico-chemical characteristics of the municipal effluent are reported in Table 1. Water chemistry in the aquariums was monitored every 2 days during the 21-day exposure period. The water chemistry parameters were kept relatively constant over the period. In the 20% dilution of municipal effluent: total nitrogen, ammonia, turbidity and conductivity were increased 4.5, 2.5, 2.1 and 1.2 fold respectively. Dissolved ammonia concentrations were below the toxic threshold (circa 17.6 mg/l total ammonia) at 0.029 mg/l and 0.85 mg/l for un-ionized and total ammonia, respectively. Total dissolved oxygen content decreased from 9.1 mg/l in the control tanks to 7.62 mg/l (94% saturation), but this level was not considered hypoxic for this fish species.
| Concentration | Temperature °C | Conductivity µs/cm | pH | Redox potential mv | NH4+ mg/l | NH3 mg/l | NO3-n mg/l | Turbidity NTU | Dissolved oxygen mg/l |
|---|---|---|---|---|---|---|---|---|---|
| 0-day 0 | 24.7 | 302 | 8.06 | 148.5 | 0.075 | 0.005 | 1.11 | 0 | 9.59 |
| 0-day 2 | 24.9 | 307 | 8.03 | 151.1 | 0.189 | 0.011 | 0.78 | 0.4 | 9.13 |
| 0-day 10 | 24.8 | 308 | 8.05 | 140.8 | 0.207 | 0.013 | 0.66 | 0 | 8.99 |
| 5-day 0 | 24.1 | 312 | 8.05 | 139.3 | 0.231 | 0.015 | 0.66 | 0 | 8.95 |
| 5-day 2 | 24.8 | 312 | 7.83 | 150.6 | 0.253 | 0.01 | 0.67 | 0 | 9.07 |
| 5-day10 | 24.4 | 312 | 7.94 | 145.1 | 0.242 | 0.0125 | 0.69 | 0 | 8.94 |
| 10-day 0 | 24.7 | 315 | 7.94 | 137.8 | 0.2795 | 0.014 | 0.69 | 0.4 | 8.93 |
| 10-day 2 | 24.7 | 316 | 7.97 | 140.2 | 0.289 | 0.015 | 0.71 | 0.8 | 8.88 |
| 10-day 10 | 24.7 | 314 | 7.92 | 135.5 | 0.27 | 0.013 | 0.67 | 0.1 | 8.94 |
| 20-day 0 | 24.7 | 374 | 7.77 | 128.3 | 0.851 | 0.028 | 0.76 | 1.1 | 7.82 |
| 20-day 2 | 24.8 | 366 | 7.84 | 122.4 | 0.736 | 0.029 | 0.78 | 1 | 7.62 |
| 20-day 10 | 24.6 | 383 | 7.69 | 134.3 | 0.967 | 0.027 | 0.74 | 1.2 | 7.61 |
Table 1: Physico-chemical characteristics of the municipal effluent.
The morphometric analysis of fathead minnows exposed to the municipal effluent, two forms of Zn and combined exposure is reported in Table 2. For fork length, factorial analysis of variance revealed that gender was significant with a significant interaction (gender*exposure treatments). Fork length in males was significantly higher (1.4 fold) than in females. Exposure to nano-ZnO significantly reduced fork length in males, while no effects were observed in females. Fork length was higher for male fish fed a nano-ZnO-spiked diet and exposed to 20% municipal effluent. In fish exposed to the effluent and each form of dietary Zn, the condition factor was not significantly affected by the municipal effluent and/or Zn treatments. There was a significant difference between the sexes, with males being about 3 times larger than females. However, there was a marginal interaction between these two factors (p=0.08). In females, no significant effects in fish exposed to either municipal effluent or dietary Zn were observed. In males, there was no significant change, although the data had more variance. However, the difference was not significant in fish exposed to dietary nano-ZnO. This effect was lost when municipal effluent was present. Correlation analysis revealed that condition factor was significantly correlated with fork length (r=0.61; p< 0.001). The gonado-somatic index (GSI) was significantly affected by both the effluent- dietary Zn treatments and sex (2-way factorial ANOVA: effluent/dietary Zn*sex interaction at p< 0.01). In control fish, the GSI in males was significantly higher than females. In males, the GSI was significantly higher at 20% municipal effluent, but decreased when the fish were fed a nano-ZnO- or ZnCl2-spiked diet, which suggests than effluent exposure increases GSI while dietary Zn exposure decreases GSI. In females, the GSI increased with municipal effluent concentrations of 5 and 10%, while exposure to both forms of dietary Zn had significant effects on the GSI; ZnCl2 increased the GSI compared to female controls and females exposed to nano-ZnO.
| Sex | Fork length (mm) | Fish weight(g) | CF | GSI | Gill-SI | |
|---|---|---|---|---|---|---|
| Controls | M | 68.3 ± 1.7 | 5.59 ± 0.68 | 0.082 ± 0.009 | 0.09 ± 0.01 | 0.11 ± 0.02 |
| F | 46.25 ± 2.1b | 1.25 ± 0.13b | 0.027 ± 0.002 | 0.022 ± 0.007b | 0.025 ± 0.01b | |
| 5% ME | M | 77 ± 1 | 8.39 ± 1.1 | 0.11 ± 0.02 | 0.014 ± 0.007a | 0.024 ± 0.003a |
| F | 44 ± 6b | 1.07 ± 0.41b | 0.02 ± 0.006b | 0.049 ± 0.005b | 0.023 ± 0.001 | |
| 10% ME | M | 73 ± 22 | 7.2 ± 0.41 | 0.09 ± 0.026 | 0.07 ± 0.001 | 0.023 ± 0.001a |
| F | 53 ± 15b | 3.09 ± 2b | 0.05 ± 0.029b | 0.048 ± 0.04 | 0.023 ± 0.001 | |
| 20 % ME | M | 75 ± 3 | 7.35 ± 0.27 | 0.098 ± 0.007 | 0.011 ± 0.001a | 0.03 ± 0.002a |
| F | 45 ± 3b | 1.48 ± 0.24b | 0.03 ± 0.003b | 0.013 ± 0.001 | 0.03 ± 0.002 | |
| nanoZnO | M | 58 ± 2 | 3.41 ± 0.05 | 0.06 ± 0.003 | 0.045 ± 0.005a | 0.03 ± 5e-4a |
| (100 ug/g) | F | 50 ± 1 | 1.54 ± 0.05 | 0.03 ± 0.01 | 0.045 ± 0.005a | 0.01 ± 2e-4 |
| ZnCl2 | M | 66.5 ± 1.5 | 5.16 ± 0.24 | 0.078 ± 0.005 | 0.07 ± 0.005 | 0.065 ± 0.01a |
| (100 ug/g) | F | 49.9 ± 0.5 | 1.5 ± 0.05b | 0.03 ± 0.001b | 0.09 ± 0.005a | 0.01 ± 2e-4b |
| 5% ME-ZnO | M | 69 ± 10 | 5.44 ± 2.2 | 0.077 ± 0.02 | 0.065 ± 0.005 | 0.035 ± 0.02a |
| F | 47.5 ± 1.5b | 1.43 ± 0.15b | 0.03 ± 0.002b | 0.14 ± 0.001a,b | 0.01 ± 2e-4 | |
| 5% ME-ZnCl2 | M | 69.5 ± 5.5 | 6.13 ± 1.6 | 0.087 ± 0.016 | 0.08 ± 0.02 | 0.11 ± 0.03a |
| F | 49.5 ± 4.5b | 1.45 ± 0.32b | 0.03 ± 0.004b | 0.11 ± 0.001a | 0.025 ± 0.01b | |
| 10% ME-ZnO | M | 80 ± 1a | 7.4 ± 0.93 | 0.092 ± 0.01 | 0.045 ± 0.01a | 0.14 ± 0.02a |
| F | 46 ± 4b | 1.28 ± 0.2b | 0.028 ± 0.002b | 0.07 ± 0.03a | 0.015 ± 0.005b | |
| 10% ME- ZnCl2 | M | 68.5 ± 6.5 | 5.0 ± 0.8 | 0.07 ± 0.005 | 0.086 ± 0.02 | 0.09 ± 0.02 |
| F | 50 ± 1 | 1.45 ± 0.14b | 0.03 ± 0.002b | 0.085 ± 0.02a | 0.02 ± 0.01b | |
| 20 % ME-ZnO | M | 76.5 ± 7.5a | 6.54 ± 0.85 | 0.085 ± 0.003 | 0.11 ± 0.02 | 0.12 ± --- |
| F | 48.5 ± 1.5 b | 1.43 ± 0.42b | 0.03 ± 0.008b | 0.22 ± 0.02a,b | 0.02 ± 0.01b | |
| 20 % ME- ZnCl2 | M | 67.5 ± 3.5 | 5.09 ± 0.72 | 0.075 ± 0.007 | 0.08 ± 0.03 | 0.11 ± 0.01 |
| F | 50.5 ± 4.5b | 1.87 ± 0.34 | 0.037 ± 0.003b | 0.28 ± 0.003a,b | 0.03 ± 0.01b |
Table 2: General health status of fathead minnows exposed to municipal effluent, nanoZnO and dissolved Zn.
A significant interaction between the two factors was also observed. In the presence of municipal effluent and either form of dietary Zn, the GSI in females tended to be higher than in males. Hence, males and females seem to respond differently to exposure to municipal effluent and Zn diets. In males, the GSI appeared to be more responsive to the reducing effects of dietary Zn than to stimulation by the municipal effluent, while in females, the effects of the municipal effluent were more apparent. The gill somatic index (Gill-SI) was significantly affected by exposure treatments to dietary Zn/municipal effluent and sex. In control fish, the Gill-SI values were significantly higher in males than in females. The Gill-SI was not significantly affected in females but was reduced in males exposed to the municipal effluent and by either form of dietary Zn. Combination of the municipal effluent with either form of dietary Zn resulted in a significant increase in Gill-SI in males. Females were impervious to the exposure treatment regime. Correlation analysis showed that Gill-SI was significantly correlated with fork length (r=0.76; p< 0.001) and condition factor (r=0.65; p< 0.001) (Table 3).
| Fork length | FC | GSI | Gill index | DNA | LPO | leucocyte | Phag | Phag | |
|---|---|---|---|---|---|---|---|---|---|
| strands | gills | viability | act. | eff | |||||
| Fork length | 1 | 0.62 | -0.05 | 0.76 | 0.22 | -0.06 | -0.18 | 0.29 | 0.32 |
| p<0.001 | p>0.1 | p<0.001 | p<0.05 | p>0.1 | p=0.07 | p<0.01 | p=0.001 | ||
| FC | 1 | -0.09 | 0.65 | -0.4 | -0.16 | -0.28 | -0.09 | 0.02 | |
| p>0.1 | p<0.001 | p<0.001 | p>0.1 | p<0.01 | p>0.1 | p>0.1 | |||
| GSI | 1 | 0.03 | -0.001 | -0.01 | -0.1 | 0.25 | 0.27 | ||
| p>0.1 | p>0.1 | p>0.1 | p>0.1 | p<0.05 | p<0.01 | ||||
| Gill | 1 | 0.16 | -0.17 | -0.58 | 0.43 | 0.52 | |||
| index | p>0.1 | p=0.1 | p<0.001 | p<0.001 | p<0.001 | ||||
| DNA | 1 | 0.43 | 0.01 | 0.52 | 0.51 | ||||
| strands | p<0.001 | p>0.1 | p<0.001 | p<0.001 | |||||
| LPO | 1 | -0.07 | 0.28 | 0.26 | |||||
| p>0.1 | p<0.01 | p<0.01 | |||||||
| Viability | 1 | -0.52 | -0.53 | ||||||
| (leucocyte) | p<0.001 | p<0.001 | |||||||
| Phag | 1 | 0.98 | |||||||
| act | p<0.001 |
Table 3: Correlation analysis.
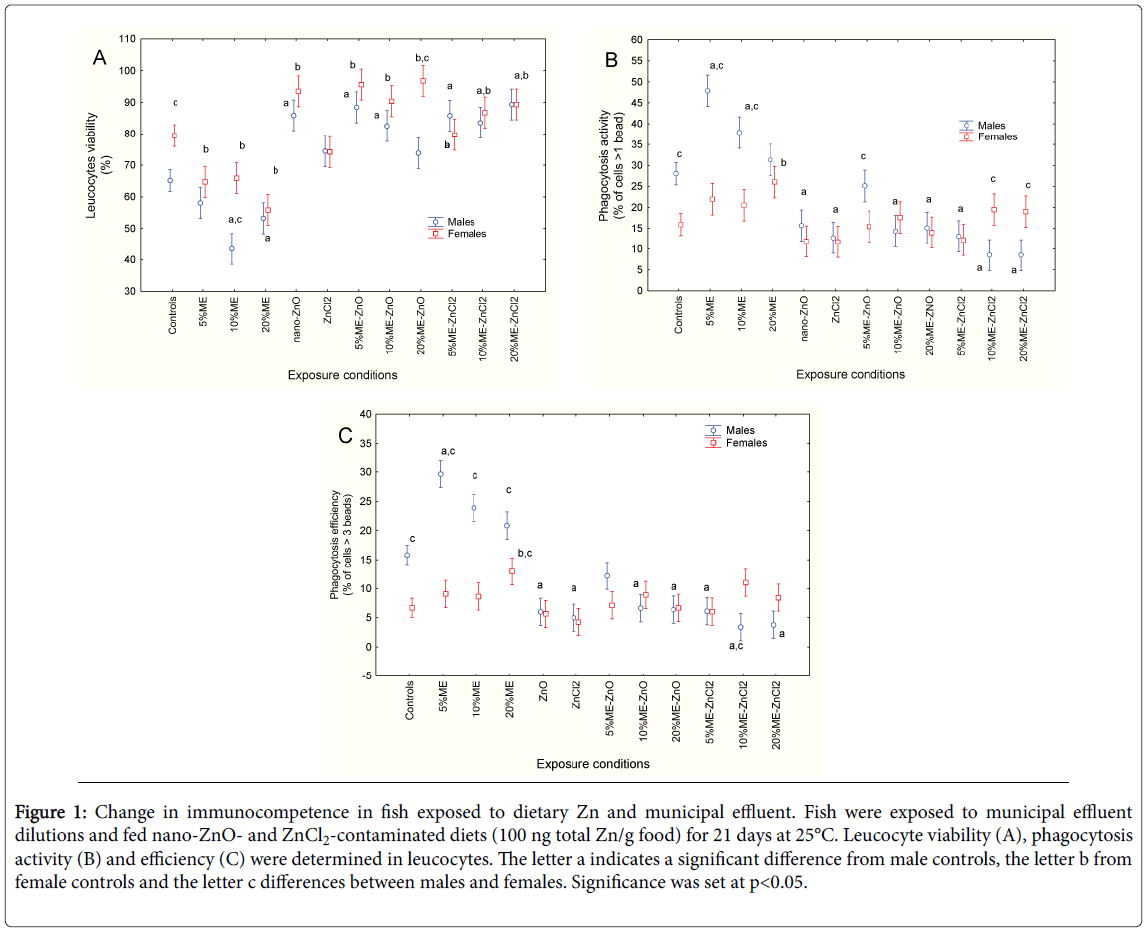
Immunocompetence in fish was assessed by measuring changes in leucocyte viability, phagocytosis activity and efficiency (Figures 1A, B and C). Both sex and exposure to dietary Zn and municipal effluent significantly influenced leucocyte viability, with no significant interaction between the two factors (Figure 1A). In control fish, leucocyte viability was higher in females than in males. Exposure to municipal effluent significantly reduced leucocyte viability in both males and females. In fish fed a diet contaminated with nano-ZnO, leucocyte viability was higher, while the Zncl2 diet had no effect. Coexposure to municipal effluent led to increased metabolic viability in fish leucocytes in both sexes. Correlation analysis revealed that leucocyte viability was significantly correlated with condition factor (r=-0.28; p< 0.01) and gill-SI (r=-0.58; p< 0.001). Phagocytosis activity (proportion of cells that ingested at least one bead) was also examined (Figure 1B). Factorial analysis of variance revealed that both sex and exposure treatment were significant factors. In control fish, phagocytosis activity was significantly higher in males. In males, phagocytosis activity was increased by the municipal effluent at low concentrations and then returned to control values at the highest concentration (20% v/v). In females, phagocytosis activity was only increased at 20% municipal effluent. Exposure to dietary nano-ZnO and Zncl2 significantly reduced phagocytosis activity in males but not in females. Exposure to municipal effluent did not remove the decrease. Phagocytosis activity returned to control levels in female fish exposed to the 10 and 20% municipal effluent and fed a Zncl2- contaminated diet but phagocytosis activity remained low in males. Correlation analysis showed that phagocytosis activity was significantly correlated with fork length (r=0.29; p< 0.01), GSI (r=0.25; p< 0.05), gill-SI (r=0.43; p< 0.001), and leucocyte viability (r=-0.52; p< 0.001). Phagocytosis efficiency was assessed by determining the proportion of cells that engulfed at least three beads (Figure 1C). Factorial analysis of variance revealed that both sex and dietary/ municipal effluent exposure treatments were significant factors, with a significant interaction between these two factors. As with phagocytosis activity, phagocytosis efficiency was significantly higher in males compared to females in the control group. Exposure to the municipal effluent led to an initial increase in phagocytosis efficiency in males, which returned to control values at the highest municipal effluent concentration (20% v/v). In females, a significant increase in efficiency was observed at 20% municipal effluent. The presence of dietary nano- ZnO or Zncl2 led to decreased activity in males only. Co-exposure with the municipal effluent did not remove this inhibiting effect in male fish but phagocytosis efficiency returned to control values in females. Correlation analysis revealed that phagocytosis efficiency was significantly correlated with GSI (r=0.27; p< 0.01), Gill-SI (r=0.52; p< 0.001), leucocyte viability (r=-0.53; p< 0.001) and phagocytosis activity (r=0.98; p< 0.0001).
Figure 1: Change in immunocompetence in fish exposed to dietary Zn and municipal effluent. Fish were exposed to municipal effluent dilutions and fed nano-ZnO- and ZnCl2-contaminated diets (100 ng total Zn/g food) for 21 days at 25°C. Leucocyte viability (A), phagocytosis activity (B) and efficiency (C) were determined in leucocytes. The letter a indicates a significant difference from male controls, the letter b from female controls and the letter c differences between males and females. Significance was set at p<0.05.
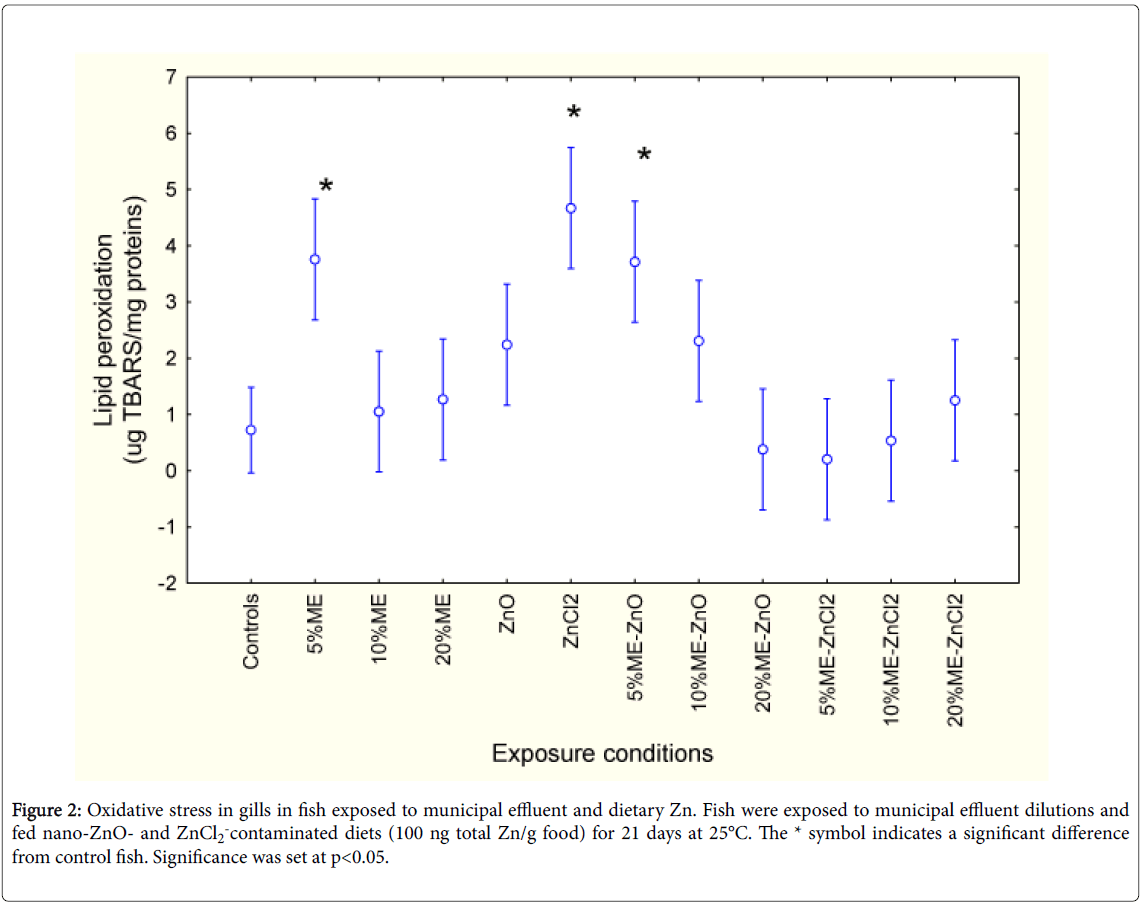
The extent of tissue damage was measured using gill LPO and DNA damage (Figures 2 and 3). Factorial analysis of variance revealed that only the exposure treatments were significant. LPO was higher in fish exposed to 5% municipal effluent and fed a ZnCl2-contaminated diet. The effects of ZnCl2 were lost with the presence of municipal effluent. In fish fed a nano-ZnO diet and exposed to 5% municipal effluent, a similar increase was observed. Increasing the concentration of municipal effluent led to a return of LPO to control levels. Correlation analysis revealed that gill LPO was significantly correlated with phagocytosis activity (r=0.28; p< 0.01) and efficiency (r=0.26; p<0.01).
Figure 2: Oxidative stress in gills in fish exposed to municipal effluent and dietary Zn. Fish were exposed to municipal effluent dilutions and fed nano-ZnO- and ZnCl2-contaminated diets (100 ng total Zn/g food) for 21 days at 25°C. The * symbol indicates a significant difference from control fish. Significance was set at p<0.05.
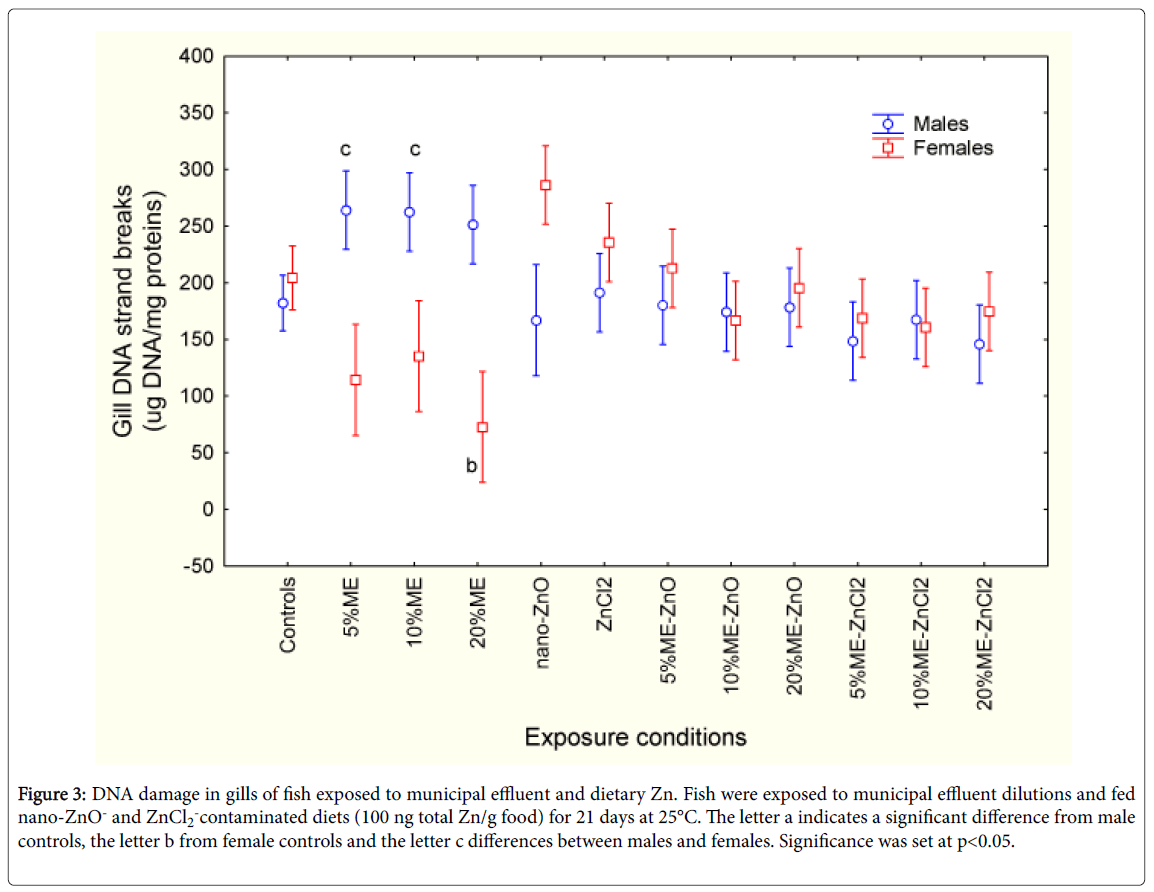
The levels of DNA strand breaks were assessed in fish exposed to municipal effluent, in fish fed diets contaminated with nano-ZnO and ZnCl2 in the absence/presence of the municipal effluent (Figure 3). Factorial analysis of variance revealed significant interaction between sex and municipal effluent/dietary Zn exposure treatments. In control fish, the levels of DNA strand breaks were similar. In males, DNA breaks were significantly increased at 5% effluent and returned to control values as the effluent concentration increased. In females, DNA strand breaks were reduced at 5 and 10% and increased at 20% municipal effluent dilutions. The presence of nano-ZnO and ZnCl2 in feed had no effects on DNA strand break levels. The combination of exposure to municipal effluent with either form of dietary Zn produced no significant change in DNA damage levels. Correlation analysis revealed that DNA strand breaks were correlated with fork length (r=0.22; p< 0.05), condition factor (r=-0.40; p< 0.001), LPO (r=0.43; p< 0.001), phagocytosis activity (r=0.52; p< 0.001) and efficiency (r=0.51; p< 0.001).
Figure 3: DNA damage in gills of fish exposed to municipal effluent and dietary Zn. Fish were exposed to municipal effluent dilutions and fed nano-ZnO- and ZnCl2-contaminated diets (100 ng total Zn/g food) for 21 days at 25°C. The letter a indicates a significant difference from male controls, the letter b from female controls and the letter c differences between males and females. Significance was set at p<0.05.
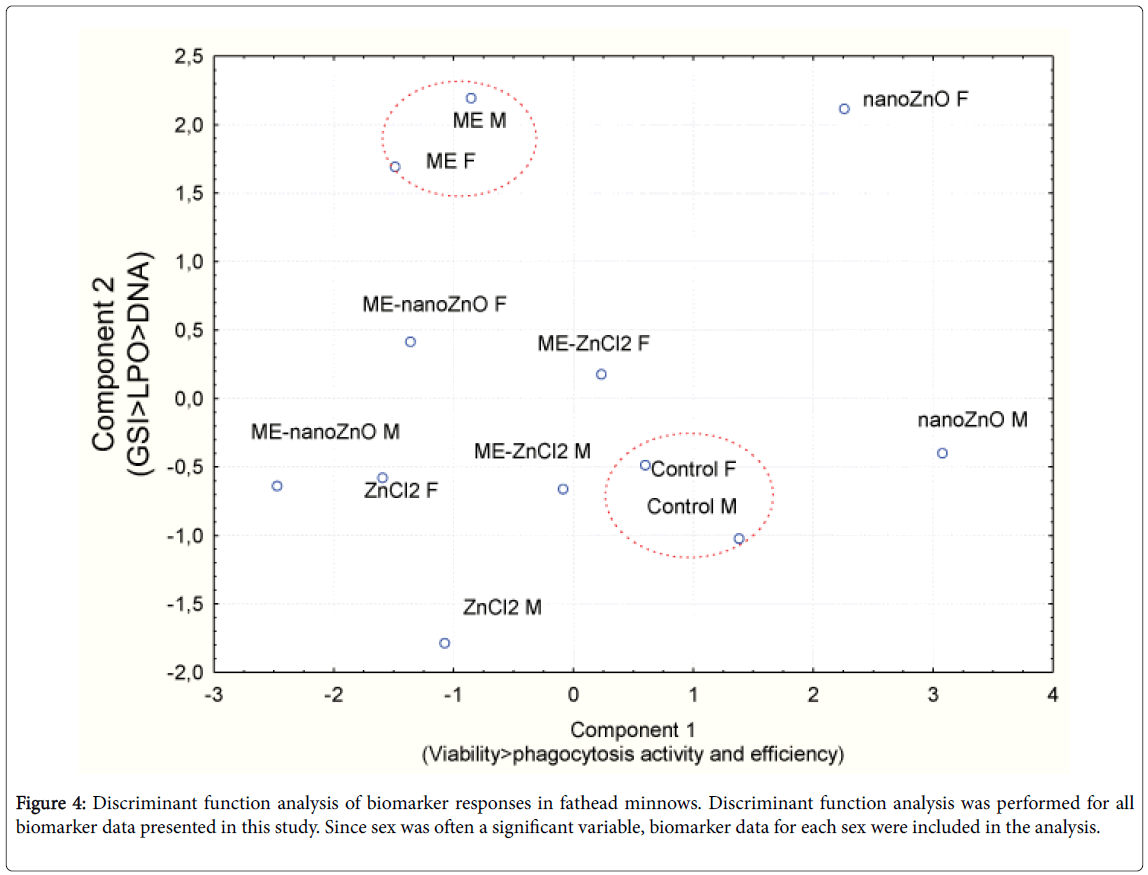
In an attempt to gain an overall understanding of fish responses to municipal effluent and either form of dietary Zn, a discriminant function analysis was performed (Figure 4). The analysis revealed that the responses of male and female fish from the controls and the municipal effluent exposure group formed a distinct and compact cluster. The response pattern of fish exposed to dietary nano-ZnO was also distinct but the males were separated from females, which suggests a strong sex-specific response to GSI, LPO and DNA damage in gills. The factorial weights of combined exposure to municipal effluent and either dietary nano-ZnO or ZnCl2 were close to the municipal effluent and ZnCl2 responses, suggesting the strong influence of the municipal effluent over exposure to dietary nano-ZnO.
Discussion
In this study, fathead minnows were fed dietary Zn (100 µg/kg) and continuously exposed to concentrations of municipal effluent for 21 days. There are few studies on the effects of nano-ZnO on the immune system in fish. In tilapia exposed to two sizes of nano-ZnO (20 and 100 nm) for 7 and 14 days, an increase in respiratory burst and cell killing was observed in leucocytes [15]. However, in fish exposed to large size nano-ZnO (100 nm), a decrease in leucocyte activity was observed. In addition, water exposure to nano-ZnO also caused osmoregulatory changes by inhibition of Na+, K+-ATPase activity fish. Leucocyte viability and phagocytosis activity were reduced in fathead minnows exposed to dietary nano-ZnO alone, and co-exposure to the municipal effluent did not change this outcome. In fish fed nano-ZnO, gill LPO was not observed, which suggests that exposure to dietary nano-ZnO does not produce oxidative stress in fish gills. Exposure of white sucker to nano-ZnO in the water column led to increased LPO and Na+,K+- ATPase activity in gills, which indicates that this nanoparticle can led to oxidative stress through water exposure but not through dietary exposure [16]. Interestingly, caspase 3 and 7 were increased, which suggests apoptosis following oxidative stress from mitochondrial dysfunction. In another study with white suckers exposed to nano- ZnO, increased oxidative stress was observed in the liver [17]. Aconitase activity, which contains a Fe-thiolate active centre, was inactivated by nano-ZnO and could be restored by Fe2+ supplementation, suggesting the release of Zn2+.
The release of Zn2 + was also associated with LPO, indicating that nano-ZnO-mediated release of Zn2 + involved oxidative processes in cells. It was further demonstrated that Zn2 + but not nano-ZnO decreased LPO and increased GSH reductase activity. Hence, the release of Zn2 + from nano-ZnO in leucocytes is possible given the significant relationship between oxidative stress and phagocytosis activity. Interestingly, phagocytosis activity was also negatively correlated with leucocyte viability, indicating immunotoxicity of dietary nano-ZnO and Zncl2.
Much more is known about the effects of municipal effluent on immunocompetence in aquatic organisms than those of nanoparticles. Phagocytosis activity showed a biphasic response, i.e., was induced at the low effluent concentration and decreased at higher effluent concentrations. This suggests that at high concentrations, phagocytosis is inhibited by the effluent, which contains significant amounts of microorganisms (1-3 × 106 coliform bacteria/100 ml) in addition to miscellaneous contaminants. Another explanation would be the capacity of fish to adapt to municipal effluent. The latter explanation was also proposed in a recent study in which fish exposed subchronically to a highly treated effluent had decreased phagocytosis initially, which returned to control levels when the fish were exposed for longer periods (>7 days) [18]. Reduction in phagocytosis activity in fish could be associated with the presence of estrogenic compounds in the municipal effluent and could explain why phagocytosis activity in females is lower than in males. Exposure to ethynylestradiol-17ß (0.1 and 1 ug/l EE2 for 1 and 7 days) down-regulated genes involved in phagocytosis (major histocompatibility complex II and TGF-1) in sexually immature male rainbow trout [19]. Injection of the common carp Cyprinus carpiowith ß-estradiol, progesterone, cortisol and 11- ketotestosterone significantly reduced phagocytosis activity, superoxide anion generation and nitric oxide production [20].
If this holds true, the presence of microorganisms, which would increase immunocompetence, could be hindered by the presence of estrogenic compounds, in part at least, in the effluent. A similar observation was reported in field-collected spottail shiners upstream and downstream of a major municipal effluent discharge [21]. Phagocytosis activity and efficiency was significantly correlated with ambient fecal coliform levels, but when the activities were corrected against microbial exposure, decreased phagocytosis activity was observed in fish collected at downstream sites. This is consistent with the estrogenic properties of this effluent reported earlier [22].
It was noteworthy that the inclusion of both dietary nano-ZnO and Zncl2 eliminated the initial increase in phagocytosis activity in fish exposed to municipal effluent. This suggests that both forms of dietary Zn contributed to reduced immunocompetence in fish exposed to municipal effluent [23]. This is consistent with discriminant analysis results where nano-ZnO effects in fish co-exposed to municipal effluent were closely related to the effects of fish exposed to municipal effluent and in those fed Zncl2 only. The suppressive effects of municipal effluents on phagocytosis has also been reported in juvenile female rainbow trout [9]. Phagocytosis activity was suppressed after a one-week exposure to a primary-treated effluent, but returned to control levels after four weeks, suggesting adaptation of the fish immune system to sustained exposure to municipal effluents [24-27]. The suppression of non-activated proliferation of lymphocytes was consistent with the estrogenic properties of the municipal effluent as well. In conclusion, exposure to both forms of dietary Zn and to primary-treated municipal effluents significantly reduced phagocytosis activity and contributed to gill LPO in fathead minnows. Males responded more to municipal effluent than females in respect to immunosuppressive responses to dietary Zn and exposure to municipal effluents [28]. Oxidative stress was more evident in fish fed a Zncl2–contaminated diet than those fed a nano-ZnO-contaminated diet, but this effect was eliminated in the presence of municipal effluent. Conversely, nano-ZnO-mediated oxidative stress was more evident in the presence of municipal effluent, which suggests the release of ionic Zn from nano-ZnO. Future research is required to confirm whether nano-ZnO liberates Zn2+ (oxidative degradation) or releases free Zn2+ from intracellular stores. More experiments would be required on the stability of nano-ZnO in the presence of municipal effluents.
Acknowledgements
The work was funded under the Chemical Management Plan and St. Lawrence Action Plan of Environment Canada. The authors thank Sophie Trépanier for the technical assistance for fish exposure and fish handling at the City of Montreal ecotoxicology laboratory.
References
- Holeton C, Chambers PA, Grace L (2011) Wastewater release and its impacts on Canadian waters. Can J Fish AquatSci 68: 1836-1859.
- Wang D, Chen Y (2016) Critical review of the influences of nanoparticles on biological wastewater treatment and sludge digestion. Crit Rev Biotechnol 36: 816-828.
- Kundu P, Anumol EA, Ravishankar N (2013) Pristine nanomaterials: synthesis, stability and applications. Nanoscale 5: 5215-5224.
- Shi LE, Li ZH, Zheng W, Zhao YF, Jin YF, et al. (2014) Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food AdditContam Part A Chem Anal Control Expo Risk Assess 31:173-186.
- Merdzan V, Domingos RF, Monteiro CE, Hadioui M, Wilkinson KJ (2014) The effects of different coatings on zinc oxide nanoparticles and their influence on dissolution and bioaccumulation by the green alga, C. reinhardtii. Sci Total Environ 488-489: 316-324.
- Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, et al. (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ SciTechnol 44: 1962-1967.
- Watts M, Munday BL, Burke CM (2001) Immune responses of teleost fish. Aust Vet J 79: 570-574.
- Secombes CJ (1996) The nonspecific immune system: cellular defenses. In: Iwama, G, Nakanishi T. (Eds), The Fish Immune System: Organism, Pathogen, and Environment. Academic Press, San Diego, Calif 63-103.
- Salo HM, Hebert N, Dautremepuits C, Cejka P, et al. (2007) Effects of Montreal municipal sewage effluents on immune responses of juvenile female rainbow trout (Oncorhynchusmykiss). AquatToxicol 84: 406-414.
- Arstikaitis J, Gagne F, Cyr DG (2014) Exposure of fathead minnows to municipal wastewater effluent affects intracellular signaling pathways in the liver. Comp BiochemPhysiol C ToxicolPharmacol 64: 1-10.
- Gagne F, Andre C, Cejka P, Hausler R, Fournier M (2011) Evidence of neuroendocrine disruption in freshwater mussels exposed to municipal wastewaters. Sci Total Environ 409: 3711-3718.
- Gagne F, Auclair J, Peyrot C, Wilkinson KJ (2015) The influence of zinc chloride and zinc oxide nanoparticles on air-time survival in freshwater mussels. Comp BiochemPhysiol C ToxicolPharmacol 172-173: 36-44.
- Wills ED 1987. Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell, K, Mullock B (Eds) Biochemical Toxicology: A Practical Approach. IRL Press, Washington, USA, 12.
- Olive PL (1988) DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen 11: 487-495.
- Kaya H, Aydin F, Gurkan M, Yilmaz S, Ates M, et al. (2015) A comparative toxicity study between small and large size zinc oxide nanoparticles in tilapia (Oreochromisniloticus): Organ pathologies, osmoregulatory responses and immunological parameters. Chemosphere 144: 571-582.
- Bessemer RA, Butler KM, Tunnah L, Callaghan NI, Rundle A, et al. (2015) Cardiorespiratory toxicity of environmentally relevant zinc oxide nanoparticles in the freshwater fish Catostomuscommersonii. Nanotoxicology 9: 861-870.
- Dieni CA, Callaghan NI, Gormley PT, Butler KM, Maccormack TJ (2014) Physiological hepatic response to zinc oxide nanoparticle exposure in the white sucker, Catostomuscommersonii. Comp BiochemPhysiol C ToxicolPharmacol 162: 51-61.
- Singh A, Havixbeck JJ, Smith MK, Shu Z, Tierney KB, et al. (2015) UV and hydrogen peroxide treatment restores changes in innate immunity caused by exposure of fish to reuse water. Water Res 71: 257-273.
- Massart S, Milla S, Redivo B, Flamion E, Mandiki SN, et al. (2014) Influence of short-term exposure to low levels of 17a-ethynylestradiol on expression of genes involved in immunity and on immune parameters in rainbow trout, Oncorhynchusmykiss. AquatToxicol 157: 57-69.
- Watanuki H, Yamaguchi T, Sakai M (2002) Suppression in function of phagocytic cells in common carp Cyprinuscarpio L. injected with estradiol, progesterone or 11-ketotestosterone. Comp BiochemPhysiol C ToxicolPharmacol 132: 407-413.
- Menard L, Escarne R, Marcogliese DJ, Cyr D, Fournier M, et al. (2010) The impacts of urban pollution on the immune system of spottail shiners (Notropishudsonius) in the St. Lawrence River. Fresenius Environ Bull 19: 1369-1374.
- Aravindakshan J, Paquet V, Gregory M, Dufresne J, Fournier M, et al. (2004) Consequences of xenoestrogen exposure on male reproductive function in spottail shiners (Notropishudsonius). ToxicolSci 78: 156-165.
- Bonacci S, Browne MA, Dissanayake A, Hagger JA, Corsi I, et al. (2004) Esterase activities in the bivalve molluscAdamussiumcolbecki as a biomarker for pollution monitoring in the Antarctic marine environment. Mar Pollut Bull 49: 445-455.
- Bradford MB (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Gagne F, Andre C, Skirrow R, Gelinas M, Auclair J, et al. (2012) Toxicity of silver nanoparticles to rainbow trout: a toxicogenomic approach. Chemosphere 89: 615-622.
- Gagne F (2014) Oxidative stress. In: Biochemical Ecotoxicology-Principles and Methods. First Edition, Chapter 6. Elsevier Inc, USA 103-115.
- Haase H, Maret W (2004) A differential assay for the reduced and oxidized states of metallothionein and thionein. Anal Biochem 333: 19-26.
- Reinhold KA, Percitelli SM (1990) Effects of Cold Temperature on Toxicity of Ammonia to Rainbow Trout, Bluegills, and Fathead Minnows. Aquatic Ecology Technical Report, US Environmental Protection Agency, USA, Contract 68-01-5832/B.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 11397
- [From(publication date):
October-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10517
- PDF downloads : 880