Immunostimulation Pathways of Extracellular Nucleosomes
Received: 17-Dec-2020 / Accepted Date: 04-Jan-2021 / Published Date: 11-Jan-2021 DOI: 10.4172/2332-0877.1000448
Abstract
Circulating nucleosomes released from dying or damaged cells have been observed to be associated with in-flammation and autoimmune diseases, indicating that extracellular nucleosomes have immunostimulation. Early studies have demonstrated that extracellular nucleosomes can induce immune responses in a TLR9-dependent mechanism. A very recent study reported that extracellular nucleosomes can be taken up by mammalian cells. After cellular uptake, nucleosomes bind and activate cGAS in the cytoplasm, inducing the expression and secre-tion of type I interferons and proinflammatory cytokines. Thus, the cGAS-dependent mechanism represents a new pathway for the immunostimulation of extracellular nucleosomes.
Keywords: Nucleosome; Immunostimulation; cGAS; TLR9
Description
Nucleosomes composed of DNA fragments and histone octamers (which contain H2A, H2B, H3, and H4), are the basic structural repeating units of chromatin [1]. Under certain physiological or pathological conditions, apoptotic fragmentation of chromatin occurs, leading to the release of nucleosomes into the blood circulation system [2]. Increased nucleosomes in circulation are observed in both lupus patients and lupus mice, which had spurred scientists to reveal the potential relationship between extracellular nucleosomes with immunostimulation.
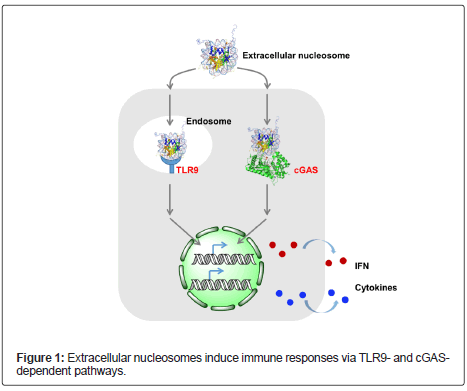
DNA fragments are pathogen-associated molecular patterns. Tolllike Receptor 9 (TLR9) is a well-studied Pat-Tern Recognition Receptor (PRR) that senses DNA fragments in the endolysosomal compartment and triggers im-mune responses [3]. Gowda et al. demonstrated that extracellular nucleosomes induced bone marrow cells to secret proinflammatory cytokines in a TLR9-specific manner. This suggests that TLR9 is a receptor of nucleoso-mal DNA (Figure 1) [4]. Decker et al. also showed that purified extracellular nucleosomes activate both dendritic cells and neutrophils to secrete cytokines. However, the activation was found not dependent on TLRs [5,6]. These results corroborated that extracellular nucleosomes have immunostimulation and their activities may depend on distinct receptors in different cell lines. The pathway of TLR-independent immunostimulation of extracellular nucleosomes remained to be uncovered.
cGMP-AMP synthase (cGAS) is a PRR of dsDNA and is recently discovered in both the cytoplasm and the nu-cleus of mammalian cells [7]. Upon binding to pathogenic dsDNA, cGAS is activated to synthesize the second messenger cyclic [G (2’,5’)pA(3’,5’)p] (cGAMP) using ATP and GTP as substrates. cGAMP binds and activates STING, which finally leads to antiviral immune responses. Recently, we revealed that nucleosomes, both reconstituted in vitro and isolated from HeLa cells, can be efficiently taken up by different types of mammalian cell lines. The uptake of nucleosomes is energy-dependent and the positively charged histone tails play important roles for the efficient uptake [8]. After cell entry, nucleosomes bind and activate cGAS in the cytoplasm, triggering the production of type I interferons IFN-β and proinflammatory cytokines IL-6. Though nucleosomes exhibit considerably lower potency in cGAS activation than dsDNA, the cGAS-dependent mechanism represents a TLR-independent pathway for the immunostimulation of extracellular nucleosomes. These results provide additional evidence for the relationship between circulating nucleosomes with inflammation and immune responses.
There are two key issues to be addressed regarding the cGASdependent immunostimulation of extracellular nucleosomes. First, how extracellular nucleosomes are taken up by mammalian cells and how they are released into the cytoplasm. Second, how nucleosomes bind and activate cGAS. For dsDNA, a cGAS dimer interacts with two parallel-aligned dsDNA molecules, forming a cGAS2-DNA2 complex [9]. This complex formation changes the conformation of cGAS, activating cGAS to synthesize cGAMP. In the nucleus, the activity of cGAS seems to be inhibited by the chromatin. Several recent structural studies showed that cGAS interacts with nucleosome by binding to the acid patch of the histones H2A and H2B dimer, which provide a reasonable explanation for the inhibition of cGAS by chromatin [10]. However, the cGAS-dependent immunostimulation of extracellular nucleosomes suggests that another interaction model between nucleosome and cGAS may exist. Further research in these directions will on one hand deepen our understanding of how cGAS distinguishes between extracellular nucleosomes and self chromatin, and on the other hand facilitate the design and development of therapies targeting the cGAS pathway to treat a wide range of human diseases, including cancer, autoimmune and inflammatory diseases.
Acknowledgement
This work was supported by the Natural Science Foundation of China [91953115, 21877064] and Natural Science Foundation of Tianjin City [20JCZDJC00830].
References
- McGinty RK, Tan S (2015) Nucleosome structure and function. Chem Rev 115: 2255-2273.
- Marsman G, Zeerleder S, Luken BM (2016) Extracellular histones, cell-free DNA, or nucleosomes: Differences in immunostimulation. Cell Death Dis 7: e2518.
- Schlee M, Hartmann G (2016) Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol 16: 566-580.
- Gowda NM, Wu X, Gowda DC (2011) The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of plasmodium falciparum that activates dcs. PLOS ONE 6: e20398.
- Decker P, Singh-Jasuja H, Haager S, Kötter I, Rammensee H-G (2005) Nucleosome, the main autoantigen in systemic lupus erythematosus, induces direct dendritic cell activation via a myd88-independent pathway: Consequences on inflammation. J Immun 174: 3326-3334.
- Ronnefarth VM, Erbacher AIM, Lamkemeyer T (2006) TLR2/TLR4-independent neutrophil activation and recruitment upon endocytosis of nucleosomes reveals a new pathway of innate immunity in systemic lupus erythematosus. J Immun 177: 7740-7749.
- Ablasser A, Chen ZJ (2019) cGAS in action: Expanding roles in immunity and inflammation. Science 363: aat8657.
- Wang H, Zang C, Ren M (2020) Cellular uptake of extracellular nucleosomes induces innate immune responses by binding and activating cGMP-AMP synthase (cGAS). Sci Rep 10: 15385.
- . Li X, Shu C, Yi G (2013) Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 39: 1019-1031.
- Pathare GR, Decout A, Glück S (2020) Structural mechanism of cGAS inhibition by the nucleosome. Nature 587: 668-672.
Citation: Wang H, Zhou C (2021) Immunostimulation Pathways of Extracellular Nucleosomes. J Infect Dis Ther 9:448. DOI: 10.4172/2332-0877.1000448
Copyright: © 2021 Wang H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2677
- [From(publication date): 0-2021 - Jan 18, 2026]
- Breakdown by view type
- HTML page views: 1835
- PDF downloads: 842