Research Article Open Access
Immunological Properties of Breast Milk: A Prospective Study in 589 Children
Cantani A*Department of Pediatrics, Allergy and Clinical Immunology Division, University of Rome “La Sapienza”, Rome, Italy
- *Corresponding Author:
- Cantani A
Division of Pediatric Allergy and Immunology
Roma University “La Sapienza”, Italy
Tel: 0644230256
E-mail: acantani13@gmail.com
Received date: November 17, 2014; Accepted date: December 07, 2014; Published date: December 09, 2014
Citation: Cantani A (2014) Immunological Properties of Breast Milk: A Prospective Study in 589 Children. Interdiscip J Microinflammation 1:127. doi: 10.4172/2381-8727.1000127
Copyright: © 2014 Cantani A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Background: Normal neonates are equipped with a limited immunocompetence, therefore they need breast milk (BM), which represent an excellent immune protection for the neonate during the critical period of intestinal vulnerability, due to a great variety of functionally interactive immunological, antibacterial, antiviral, anti-inflammatory and immunomodulating factors. Evidence suggests that the protection afforded by human milk to the recipient infant is greatest when breast-feeding is exclusive and of substantial duration.
Material and methods: In this update of an old topic, we shall review its role in atopy prevention as an introduction to the immunological and non-immunological components of BM and colostrum, and the spectrum and mechanisms of the protection of host defenses. Accordingly, we analyzed the propensity for breastfeeding in 289 children with respiratory disease and in 300 control children.
Results: The net result is that a high proportion of atopic children (273/289) were breastfed from their mothers and for a longer period of time.
Conclusion: This is the best demonstration that breastfeeding is the most effective single nutritional strategy that has been identified for the prevention of the atopic march in vulnerable infants. Therefore we stress that breast-feeding can prevent or ameliorate allergies, although some authors have emphasized the increased hazard of sensitization in breast-fed infants.
Keywords
Atopy prevention; Breast milk; Colostrum; Neonate; Breastfeeding; Essential fatty acids; Nucleotides; Atopic march
Breast Milk in the Allergy Prevention
There is a continuous flow of studies stressing the effectiveness of exclusive breastfeeding (associated or not with soy protein formulas (SPF) and/or hydrolysate formulas (HFs), along with food and inhalant allergens avoidance) in decreasing the prevalence of allergic diseases in genetically at risk neonates [1]. The protective effects of breastfeeding are indeed a positive natural selection process [2]. The allergy-preventive importance of BM has been evaluated by both prospective and retrospective studies [3-5]. We have prepared a partial list of both types of studies (Tables 1 and 2) [3-5], which show that the protective value of BM is confirmed by 37/41 (90.3%) of prospective, and by 4/9 (44.4%) retrospective studies (p=0.001, Fisher=0.005). Therefore the studies showing either no differences or an increase of allergic disease in breast-fed in comparison with the bottle-fed children total to 8/40. As was pointed out [4-9] in some cases the infants were breastfed for less than 3 months (47% of children in one study) [10], sometimes less than 6 weeks [11-16]. Solid foods were introduced very early in the infants’ diet (75% of cases at the 4th month in another study) [13] with foreseeable effects [17-19], e.g. the start of the atopic march.
| Authors Diseases |
Ref | Year | No. of Cases | Alimentation of significance | Data in bracket=OR | Duration of BM | F-U (y) | Effect on atopic | Results in the study group (1st No.) Compared to controls (2nd No.) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chandra [6] | 6 | 1979 | 37 | 37 | BM | >5 m | 2 | ⇓AD | 10.8/56.7 p <0.001, Asthma 2.7/21.6 p <0.01 | ||
| Saarinen et al.[7] | 7 | 1979 | 54 | 105 | BM | 6 m | 3 | ⇓AD | 0/18p <0.05, FA 4/24 p <0.05 | ||
| Ziering et al.[8] | 8 | 1979 | 25 | 25 | BM | 6 m | 2 | ⇓AD | 32/64 p = 0.0235 | ||
| Kaufman andFrick [9] | 9 | 1981 | 38 | 56 | BM | 6 w | 2 | ⇓Asthma | 5.3/17.9 p <0.05 | ||
| Hide andGuyer [10] | 10 | 1981 | 204 | 62 | BM | 6 m | 1 | = AD | 7.9/8.9, Asthma 11.8/11.3 NS ^ | ||
| Gruskay [11] | 11 | 1982 | 48 | 201 | BM | 3 m | 15 | ⇓AD | 8.3/12 Asthma 8.3/15,4 AR 8/10.4 NS | ||
| Juto et al.[12] | 12 | 1982 | 54 | 11 | BM | >1 m | 1 | ⇓Asthma Atopy | 0.06 ± 0.3/1.o2.1 p <0.001 1.4 ± 1.8/3.2 ± 4.5, p <0.05 |
||
| Businco et al.[34] | 4 | 1983 | 49 | 41 | BM +SM | 6 m | 2 | ⇓Atopy | 18/37 Fisher=0.0381 | ||
| Fergusson et al.[56] | 13 | 1983 | 199 | 911 | BM | 4 m | 4 | ⇓= Asthma | 8.5/6.6 NS | ||
| Kajosaari and Saarinen [14] | 14 | 1983 | 70 | 65 | BM | 6 m | 1 | ⇓FA | 7/37, AD 14/35 p <0.001 – 0.01 | ||
| Pratt 15] | 15 | 1984 | 19 | 58 | BM | >3 m | 5 | ⇓AD | 15.8/37.9 p <0.05 | ||
| Hide andGuyer [16] | 16 | 1985 | 115 | 52 | BM | 6 m | 4 | = AD | 14.8/17.3, Asthma 10.4/11.5 NS | ||
| Moore et al.[17] | 17 | 1985 | 224 | 35 | BM | 3 m | 1 | ⇓AD | 13/20 p <0.05 | ||
| Chandra et al. [18] | 18 | 1986 | 35 | 20 | BM | 3 m | 1 | ⇓AD | 14/60 Fisher =0.0046 | ||
| Vandenplas andSacre [19] | 19 | 1986 | 47 | 228 | BM | 3 m | 0,4 | ⇓Atopy | 8.5/39.9 p=0.0001 | ||
| Miskelly et al.[20] | 20 | 1988 | 189 | 293 | BM +SM | ± 3 m | 1 | ⇓ Asthma | 21.7/42.7, p <0.001 | ||
| Hattevig et al.[21] | 21 | 1989 | 65 | 50 | BM +CH | ± 3 m | 1, 5 | ⇓AD | 10.8/28 p=0.033 | ||
| Chandra et al. [22] | 22 | 1989 | 97 | 40 | BM | ± 6 m | 1, 5 | ⇓AD | 22/70 p <0.001 | ||
| Chandra et al.[23] | 23 | 1989 | 72 | 72 | BM | ± 4 m | 1, 5 | ⇓AD | 18/30 Asthma 0/4.5 AR 1.5/7.5 p<0.05 | ||
| Lucas et al. [24] | 24 | 1990 | 38 | 37 | BM | 5w | 2 | ⇓AD | 6/15 (3.6), Asthma 8/11 (1,6), Atopy 13/24 (3.6) |
||
| Chandra andHamed[25] | 25 | 1991 | 60 | 68 | BM | 4 m | 1, 5 | ⇓AD | 20/35.8 p=0.0484 | ||
| Arshad andHide [37] | 5 | 1992 | 420 | 747 | BM | 3 m | 1 | ⇓ Asthma | 6.7/12 p < 0.01 | ||
| Sigurs et al. [26] | 26 | 1992 | 65 | 60 | BM +CH | ± 3 m | 4 | ⇓AD | 29.2/50 p=0.0038 | ||
| Burr et al. [27] | 27 | 1993 | 179 | 274 | BM | ± 3 m | 7 | ⇓Asthma | 59/74 p <0.001 AD, AR NS | ||
| Halken et al. [28] | 28 | 1993 | 20 | 75 | BM | 6 m | 1,5 | ⇓CMA | 0/20 Fisher 0.0207 | ||
| Kajosaari [29] | 29 | 1994 | 51 | 62 | BM | 6 m | 5 | ⇓AR | 20/37 p=0.04,Asthma 8/15 NS | ||
| Høst et al. [30] | 30 | 1995 | 88 | 75 | BM +CH | 6 m | 5 | ⇓CMA/CMI | 5.7/20 p=0.0055 | ||
| Saarinen andKajosaari [31] | 31 | 1995 | 48 | 102 | BM | <1->6 m | 17 | ⇓Atopy | 42/65 p=0.02, grave 8/54 p <0.0001 AD p=0.03, FA p=0.02 , Asthma p = 0.01 |
||
| Chandra [32] | 32 | 1997 | 60 | 68 | BM | 4 m | 5 | ⇓AD, Asthma | AD=10/29.9 p=0.0057, Asthma 6.6/23.9 p=0.0079 |
||
| With environmental controls | |||||||||||
| Matthew et al. [33] | 33 | 1977 | 23 | 19 | BM+SM | 3 m | 1 | ⇓AD | 13/47.4 Fisher 0.0171 | ||
| Businco et al.[34] | 34 | 1987 | 179 | 65 | BM +SM | 6 m | 3,6 | ⇓AD ⇓ Atopy | 4.5/15.4 p=0.0039, Asthma 7.2/20 p=0.0073 14.5/38.5 p=0.0001 |
||
| Savilahti et al. [35] | 35 | 1987 | 142 | 31 | BM | 6-9 m | 1 | ⇓Atopy | 38/13 NS | ||
| Zeiger et al.[36] | 36 | 1989 | 103 | 185 | BM +CH | ± 6 m | 2 | ⇓AD,FA | 7.2/20.1 p=0.005 | ||
| Arshad et al.[37] | 37 | 1992 | 58 | 62 | BM +IS | 9 m | 1 | ⇓AD | 4/12 (>3), FA 3/7 (>3), Asthma 7/19(>4) |
||
| Halken et al.[38] | 38 | 1992 | 105 | 85 | BM +CH/SPH | ≥ 3 m | 1,5 | ⇓Atopy Asthma | 32/74 p <0.01, AD 14/31 p <0.01, 13/37 p <0.01, FA 6/17 p <0.05 |
||
| Zeiger et al.[39] | 39 | 1992 | 103 | 185 | BM +CH | ± 6 m | 4 | ⇓Atopy, FA, AR | at 12 months p ≤ 0.05 – 0.01 | ||
| Bardare et al.[40] | 40 | 1993 | 145 | 196 | BM +SM | 6 m? | 1 | ⇓Atopy | 13.3/28.9 p=0.0044 | ||
| Bruno et al.[41] | 41 | 1993 | 114 | 14 | BM +SM | 6 m | 4,3 | ⇓Atopy | 11/21 p=0.001 | ||
| Hide et al. [42] | 42 | 1994 | 58 | 62 | BM+IS | 9 m | 2 | ⇓Atopy Asthma | 29.3/58.1 p<0.05,AR 3.4/11.3 p < 0.1, (SPT+) 6.9/22.6 p=0.0162 |
||
| Machado et al.[43] | 43 | 1994 | 333 | 87 | BM+SM | 6 m | 4 | ⇓Atopy | 17/32 p=0.0028 | ||
| Zeiger et al.[44] | 44 | 1995 | 53 | 106 | BM+CH | ± 6 m | 7 | ⇓ FA | 10/22 p=0.06 *** | ||
| Hide et al. [45] | 45 | 1996 | 58 | 62 | BM+SH | 9 m | 4 | ⇓Atopy | 32.7/54.8 (2.73) AD 13.8/24.2 (3.4), | ||
Table 1: Prevention of Atopy based on BM: Results of Prospective Studies according to the publication year.
| Authors | No. of Cases | Alimentation of S group | Duration of BM (s, m) |
F-U (y) | Effect on atopic diseases (as above) | |
|---|---|---|---|---|---|---|
| S | C | |||||
| Grulee and Sanford [3] | 200 | 61 | BM | 9 m | 0,75 | ⇓AD0.2/3.5 p ≤ 0.0000 |
| Halpern et al.[46] | 193 | 349 | BM, SM, CM | 2 w | 7 | = Atopy 11.1/19.9 NS |
| Koivikko [47] | 73 | 486 | BM | ≥6 m | 1 | ⇓ Asthma p <0.0005 = AD |
| Blair [48] | 80 | 59 | BM | ≥2 m | 20 | ⇓ Asthma 25/64 p <0.05 |
| Kramer and Moroz [49] | 59 | 102 | BM | ≥2 m | 0.8 | = AD 41.5/31.1 NS |
| Gordon et al.[50] | 112 | 85 | BM | ≥ 3 m | 2 | = AD, Asthma 22/15 NS |
| Golding [51] | 221 | 1567 | BM | ≥ 3 m | 5 | ⇓AD 1.6/6.6 p <0.001 = Asthma 0.2/1.4 NS |
| Magnusson [52] | 48 | 142 | BM +SM | ≥ 3 m | 1.5 | = Atopy 16.7/21.1 NS |
| Wjst et al. [53] | 484 | 2352 | BM | 2 m | 1 | = Asthma, AR NS |
Table 2: Prevention of Atopy based on BM: Results of Retrospective Studies according to the publication year.
As regards the importance of environmental controls, as we proposed and applied together with dietary manipulations (Table 3 and 4) since 1983 [4], Arshad et al. [5] confirm these measures, demonstrating that they are highly effective in reducing the prevalence of allergic disease: 14% vs 40% according to the employment or not of environmental controls in babies, who were all fed either with BM or a soy HF.
| - Exclusive breast feeding for the first 6 months of life |
| - No more than 200 ml of milk/day and no more than 2 eggs/week to the nursing mothers |
| - Soy milk (Isomil) supplement when breast milk is not sufficient |
| - Selected weaning after the 6th month of life |
| - Egg and fish after the 1st year of age |
| - Cow's milk and dairy products after the 6th month and gluten shortly afterwards |
Table 3: DIetary manipulations followed by the study group
| - Absolutely no smoking in the house |
| - Strict environmental controls for the elimination of house dust |
| - No pets in the house |
| - Day-care centers attendance delayed to after 3 years of age |
Table 4: Environmental measures given to the study group
In our studies the figures are quite similar according to the dietary manipulations (breast or SPF in the study group, cow’s milk-CM-in the controls) associated with the environmental controls. The results showed that 51/244 children developed atopic symptoms during the follow-up: when we consider together the study group, only 14.5% (26/179) compared to 38.5 (25/65) of the control group developed atopic manifestations during the follow-up [4] (p ≤0.0000), thus confirming that dietary and environmental measures are able to significantly prevent atopy onset in high-risk babies, at least until the age of 3 years and 8 months. Similar results we obtained in a multicenter study comprising 2270 infants. At the last follow-up [20-23], 732 children are 3 years old, 98 (13%) have two or more relatives, 634 (87%) have one relative affected by atopic disease. 242/732 (33%) were exclusively breast feed until the 6th month of life, 139/732 (19%) received exclusively SPF, 212/732 (29%) received BM and SPF supplement, and 139/732 (19%) were CM formula feed. The prevalence of atopic disease was 13% (77/593 of the study group vs. 48/139=34.5% of the controls, p=0.0000).
In this prospective study in high-risk babies we have verified how many of them were breastfed, in comparison with the children of the control group.
Patients and Methods
In order to explore how long a baby genetically at risk of atopy is breastfed, we have enrolled into this prospective study 289 children, 169 males and 120 females, aged 3.5 to 7.5 years. These children attended the Allergy and Clinical Immunology Division of the Pediatric Department of Rome University, because they were affected by respiratory allergy, previously diagnosed with family history, SPTs and IgE antibodies. As controls there were 300 children comparable for age and sex with no respiratory illness recruited from our outpatient clinic. In particular we asked the accompanying parent(s) of the 589 children whether they were breastfed, and in case of positive response, which was the duration of breastfeeding. The parents of all children gave their informed consent. Data were analyzed using the X2 method.
Results
Analysis males versus females p=0.0001. The mothers of the study group have breastfed 273/289 off springs (94.5%) of cases (p=0.0225) for 136 days (mean 125, range 33-211) p=0.0001. The control children were breastfed in 89.6% of cases, for a mean of 98 days (mean 80 days, range 10-110). In both groups breastfeeding was not always exclusive.
Discussion
The mothers of children at genetic risk were much more motivated, and have breastfed their children for a significantly longer period of time than the mothers of the control babies. A basic knowledge of controversial results in studies on atopy prevention, examining the prospective versus the retrospective ones has been outlined in Table 5 [24-34]. On the contrary, the prospective preventive studies have largely confirmed the high value of breastfeeding alone or associated with dietary manipulations, along with environmental controls in the prevention of allergic disease in at risk babies. It was already stressed elsewhere that postponing the atopy development leads to a lessening of the severity of the clinical manifestations, and even to atopy avoidance forever [34-46].
| 1. Selection criteria (atopic or non-atopic parents) |
| 2. Methods used to diagnose the atopic disease (parental diagnosis, general practitioners, pediatricians, allergists, dermatologists, questionnaires) |
| 3. Lack of supportive immunologic data |
| 4. Lack of statistical analysis of data |
| 5. Social demographic characteristics |
| 6. Sex of babies |
| 7. Drop-out rates |
| 8. Small number of subjects (possible type 2 error) |
| 9. Exclusive nature and duration of breast-feeding |
| 10. Dietary restriction in mothers (cow's milk, egg) |
| 11. Age of solid food introduction |
| 12. Type of solid foods |
| 13. Maternal and child compliance |
| 14. Attendance at day care facilities |
| 15. Environmental measures (smoking, dust, pets) |
| 16. Duration of the follow-up |
| 17. Prospective versus retrospective studies |
| Modified from reference 57 |
Table 5: Possible causes of controversial results in studies on atopy prevention.
However, there are very sophisticated measures to cast doubt on the “completeness” of BM, such as indirectly throwing discredit on breastfeeding. There are some controversies and the reader can conclude that the above studies have yielded conflicting results, and that the ability of breastfeeding to delay the onset or to reduce the severity of allergic disease is only equivocal. For example Vandenplas [47-58] affirms that although the majority of studies suggest that exclusive breastfeeding exerts a protective effect, a number of other studies fails to show this assumption and points out that breastfeeding is associated with a decrease in the subsequent risk of atopic disease. In addition, breastfeeding has been associated even with a rise in prevalence of atopic diseases, and a possible interference with normal growth [59]. There are further, direct measures to comply this effect, such as advertising infant formulas in hospitals [60] or the use of HFs in the Maternity Hospitals and even for atopyprevention in at risk infants [61].
In addition we refer to the necessity stressed by several authors of supplementing the diet of breastfeeding mothers with essential fatty acids (EFAs) and/or administering EFAs to children with atopic dermatitis (AD). Since 1929 we have learned that a deficit of EFAs may be the cause of skin lesions in animals [62] and in atopic children compared with healthy controls [63]. EFAs are polyunsaturated fatty acids that cannot be synthesized by vertebrates and must therefore be obtained from the diet. Dietary EFAs and their derived compounds are important structural components of cell membranes. They are the principle determinants of membrane fluidity and affect the activity of a number of membrane-associated enzymes [64].
These eicosanoids play an important role in immunological and inflammatory processes both as effector signals and as immunoregulatory mediators [65-69]. Increased proportions of LA and decreased proportions of its derived fatty acids have been found in the plasma of AD patients [70-73]. Supplementation of the diet in these patients with g-linolenic acid (GLA) in the form of evening primrose oil produces a tendency towards normal of the EFA (essential fatty acids) profile and an improvement in symptoms [74-77].
Another possible drawback of BM resides in the so-called BM allergy, starting from the supplements of CM formulas occasionally given to full term healthy neonates in nurseries, even in breast-fed babies. The amount of CM proteins in such supplements is enormous compared with the extremely low amount provided by BM [78]. We know that 40 ml of BM contains an amount of ß-lactoglobulin (ßLG) of 0.012ng/l [78], whereas 40 ml of CM contains 1610ng/l of ßLG! As a consequence of these occasional supplements, sensitization may occur in a predisposed baby, and the minute amount of CM proteins of BM may subsequently act as a booster dose, triggering allergic reactions. The results of several studies support this hypothesis: CM allergy (CMA) was significantly more common in babies who received supplements of CM formulas early in life in comparison to fully breastfed babies [30,79,80]. All the fully breastfed babies who developed CMA received feedings of CM formula in the nurseries and none of the fully breast fed babies without supplements of CM formula developed CMA [30].
Lindfors et al. [81] have documented that children with skin prick tests (SPT) and specific IgE antibodies against egg all were fed CM during the first days of life. Total IgE levels at the 5th day of life were significantly correlated with the amount of early post natal CM supplementation (p=0.013) [82], maintaining the significativity until the age of 12 months mainly in at risk babies [83]. This data confirms the studies cited so far and the classic Jarrett one (the repeated little doses of allergens are more sensitizing than larger ones for the predisposed individual) [84]. In addition, 93% [85], 68% [86], or 64% [80] of breast-fed infants were exposed to less or more inadvertent supplements in the neonatal nursery. The babies presented CMA proved by challenge on an average after 7 weeks of life [80], or immediate symptoms at the first introduction of CM, at the age of 1-8 months (median 4.0) [85]. We emphasize that when the “pirate bottle” has administered HFs: the neonates presented with anaphylactic reactions when they were fed such formulas on weaning from BM [86,87]. The infants kept an immunologic memory of the type of supplement received at that time. Since several years in the Maternities of Northern countries [59,88] and in a London hospital [33], these CM feedings are no more permitted.
A typical case is reported by Lifschitz et al. [89], an anaphylactic shock due to CM protein hypersensitivity in a newborn who was mistakenly fed BM that had been expressed before CM products were eliminated from his mother’s diet is correctly shown in the title, however in the abbreviated title and in the discussion is referred to as “anaphylactic shock in a breast-fed infant” [89].
The anaphylactic reactions triggered in young infants by the first CM administration show that apparently it is not easy to protect neonates at risk of atopy. Such reactions could be explained by transfer of maternal antigens directed versus the antigen-binding site of anti-idiotypic antibodies: if anti-idiotype antibodies against poliovirus antigens can be transferred from the mother to the offspring, similarly anti-idiotype antibodies to food antigens (e.g. lactoprotein), having the capacity of recognizing an idiotope within the paratope, could replace the antigen, mimicking its functional properties, and be transferred from the mother via the placenta to the fetus, acting like antigens during the neonatal period or subsequently [90].Therefore IgE-mediated sensitization through BM is rather rare: 0.042% [91] or 0.28% [30].
Immunology of breast milk: Nutrition and defense
It is increasingly manifest that BM contains a wealth of immune factors, which are designed to nourish and protect the vulnerable newborns during the critical postpartum period. Thanks to the mammary gland, a true immune organ [16,66], BM represents an excellent protection against the dangers of a deficient intestinal defence system, based on unique immunological, anti- infective, anti-inflammatory and immunomodulating factors functionally interacting among themselves (Table 6) [92-105]
| Components | Properties |
|---|---|
| sIgA 240 μg/mg/day until 3rd day, ~20 μg/mg/day from 15 days to 6 months | Does not activate complement, suppresses PMN chemotaxis, blocks adhesion of microbial pathogens, prevents infections, limits the allergenic penetration |
| IgM about 100 mg/day | Activates complement, forms antibodies against bacteria and virus, retains opsonic activity after traversing the intestinal canal |
| IgG about 70 mg/day | Activates complement, has heat-stable opsonic activity, blocks toxins and virus |
| IgG subclasses | Anti-infective activity |
| IgD | Forms antibodies against bacteria |
| α-2-glycoprotein associated with pregnancy | Inhibits the lymphocytes, lymphocyte blastogenesis, ADCC, and immunoglobulin productions |
| Antioxidants: ascorbic acid, cysteine, β-carotene,atocopherol | Contrast superoxide production |
| Arylsulphatase | Degrades leukotrienes |
| Catalase | Degrades H2O2 |
| Cytokines [95,97,103,105]: IFN-γ (0,5 UI/ml) | Increases chemotaxis and opsonization, enhances Th1 and antagonizes Th2 cells |
| IFN-γ (0,5 UI/ml) | Increases chemotaxis and opsonization, enhances Th1 and antagonizes Th2 cells |
| IL1 (1 ng/ml) | Activates T lymphocytes |
| IL6 (50 pg/ml) | Enhances IgA production and favors oral tolerance |
| IL10 (3.000 pg/ml) | Carries on anti-inflammatory activity in the infants' gut, has effects opposed to those of IFN-g on Th1 e Th2 cells |
| M-CSF, GM-CSF | Induces macrophage differentiation |
| TGF-β (1.000 ng/ml) | Enhances isotype switching to BIgA+ cells and favors oral tolerance |
| TNF-α (500 pg/ml) | Induces proliferation of epithelial cells from G-CSF; activates macrophage motility and SC production |
| Glycoproteins, glycolipids | Antiviral activity, offer protection against bacterial colonization |
| fibronectins | membrane protein, mediates cell interactions and adhesion plasma protein with opsonic functions |
| Histaminase | Catabolize histamine |
| Inhibitors of proteases | Reduce inflammation |
| α-1-antichimotripsina | Degrades the enzymes active in the inflammatory reactions |
| α-1-antitripsina | |
| Inhibitors of viruses | |
| Lactoferrin | Inhibits complement and inflammation, is bacteriostatic and iron-binding factor |
| Lipids | Inhibits superoxide production, disrupt enveloped virus |
| Lysozyme | Inhibits PMN chemotaxis and free radicals formation, antibacterial activity |
| Macrophagesc | Have sIgA, produce lysozyme and PGE2which reduces intestinal permeability |
| Modulators of growth | |
| EGF | Enhances maturation of epithelial cells: levels in "mature" BM 20-111 ng/ml, versus pasteurized CM 155 ng/ml (101) |
| NGF | neuropeptides: GIP, bombesin, cholecystokinin, gastrin, etc |
| taurine | |
| Oligosaccharides | Receptors for certain microbes, block their attachment to mucosal sites |
| Prostaglandins | Inhibit degranulation of neutrophils, activation of lymphocytes, are cytoprotec¬tive, promote intestinal and cellular integrity, release brush border enzymes |
| Protein binding B12 | Antibacterial activity, is bacteriostatic |
| Receptor analogues | Anti-infective activity, protection of mucosal barrier |
| Evaluation of BM immune activity | |
| Poor or absent cellular reactivity | |
| Absence of basophils, mast-cells, eosinophils, platelets | |
| T lymphocytes respond weakly to allogenic cells | |
| Poor activity of NK cells and of ADCC | |
| Slow motility of neutrophils | |
| Active cellular reactivity | |
| Macrophages: contain IgA, produce IFN and lysozyme, modulate phagocytosis, epithelial growth, immunoregulation | |
| Neutrophils: contain IgA, regulate chemotaxis, phagocytosis | |
| B lymphocytes: Ig synthesis | |
| T lymphocytes: cellular immunity, produce IFN, modulate cytotoxicity, immunoregulation | |
| * Features denoting that BM lymphocytes are activated [99] | |
| T cells: IFN-γ, CD45RO, CD25, HLA-DR↑ | |
| macrophages: motility↑CD11b↑CD62L↓ | |
| neutrophils: chemotactic response↓CD11b↑CD62L↓ | |
| * Number of lymphocytes and other cells/mm3(mean ± SD) (94,98,100) | |
| Total lymphocytes=1.196.7± 358.7 | |
| B lymphocytes=376.2 ± 138.1 | |
| T lymphocytes=222.3 ± 251, CD4 = 504.2 ± 155.3, CD8 = 318.4 ± 98.6; CD4/CD8 = 1.6 ± 0.15 | |
| Macrophages=2.325.5 ± 660.5 | |
| Neutrophils=948.9 ± 368.3 | |
| * Determinants (% of positive cells ± SD) (93, 96) | |
| CD3 (T lymphocytes) | 25.6 ± 14.9 |
| TcRαβ | 24.5 ± 15.6 |
| TcRγδ | 4.0 ± 3.6 |
| CD4 | 13.6 ± 8.7 |
| CD8 | 12.2 ± 7.0 |
| CD14 (macrophages) | 64.0 ± 18.2 |
| CD19 (B lymphocytes) | 10.2 ± 5.3 |
| CD103 | 4.7 ± 1.6 |
| IL2R | 10.5 ± 4.6 |
Table 6: Breast milk components with immunological, anti-infective, anti-inflammatory and immunomodulating activities
• Provides, together with colostrum, in addition to T and B lymphocytes and Ig, IFN, complement components and other bioactive molecules protecting against bacterial and viral infections [106], is rich of vital cells (up to 106/ml), thus insuring a continuing supply of factors with a high immune role in the defense of the vulnerable infant. IgA and sIgA antibodies inhibit or prevent the penetration of harmful luminal antigens [107]. IgA and sIgA have the potential for lessening or abrogating possible hypersensitivity reactions [92,93,102], with levels equal also to 0.5-1 g/die or 0.2-0.3g/kg of sIgA [107], in addition to anti- infective factors to protectinfants against pathogens during the critical period [108], such as anti-VRS sIgA [109]. BM stimulates IgA and sIgA secretion, thus actively facilitating the maturation of the neonatal immune system, unlike bottle-fed neonates [110]. It appears that sIgA are significantly more elevated in the saliva of breastfed babies [111], whereas the production is developmentally delayed in the recipient baby: for example 4 weeks-12 months are needed for sIgA maturation, 1-2 years for lysozyme and about 2 years for memory (CD45RO) cells [2,112].
• Idiotype/anti-idiotype antibodies may have lasting effects on the offspring immune system, activating both B- and T-cell clones, and thus priming protective immune responses [113] also inducing tolerance versus environmental antigens, such as food antigens [113,114]. Such antibodies generate in 4-month old breastfed infants serum and secretory responses to vaccines (tetanus and diphtheria toxoids) statistically more significant than in formula- fed babies [115], and can also explain the occasional cord blood (CB) findings of IgE antibodies to several antigens [116,117]. This finding would also agree with the enhanced antibody responses to oral poliovirus vaccine in babies breastfed for 6 months compared to babied fed a conventional formula supplemented with nucleotides; moreover the titresanti-Haemophilusinfluenzae type b are higher if breastfeeding is longer than 6 months [118].
• EFA, polyunsaturated (PUFA), long chain (LCP) C20 and C22: arachidonic (AA, 20:4w6), docosahexaenoic (22:6w3), dihomo-g-linolenic (DGLA 20:3w6) necessary also for intellectual development [119], notably the ratio 18:2w6 /18:3w3 allowing the incorporation of 22:6w3 into the neonatal neural tissue and the retina [120]. CB studies suggest a preferential and selectivematerno- fetal transfer of LCP [121], therefore the breastfed premature infants receive a higher LCP supply [122] and in general a threefold higher dietary LCP phospholipide concentration than LCP-enriched formulas, apparently because the infants transform dietary LCP into structural lipids sparing them from oxidation [121]. On the other hand, the EFA abnormality found in umbilical CB in newborns ‘at risk’ of atopic disease was shown to correlate with IgE levels, suggesting that the EFA abnormality may be of fundamental importance in the development of the atopic state [123]. Several studies yielded controversial results about the presence of an EFA deficit in BM of atopic mothers, and suggested that an EFA supplementation could be useful for preventing atopy in genetically at risk infants [124-129]. But the small cohort size [125,126] can introduce a possible type II error in the data. But atopic mothers provide with BM normal amounts of w-6-fatty acids; such data were supported by extensive statistical analyses [129]. In addition, w-6-fatty acids were of no value in children with atopic bronchial asthma [130,131], nor in AD being no differences between treated and control children [132]. The EFA levels (Table 7) [133,134], higher in both control babies and in those fed supplemented formulas [135], regularly absorbed [136], are to be found in BM for at least 8 weeks with a ratio w6:w3=5:1 [137]: the w6 level is twofold higher than that of w3 in brain and 1.3-fold higher in retina [120]. Although the EFA levels are variable, taking into consideration the marked differences in methods and dietary composition of the population studied [133], most EFA levels are comparatively similar in several European studies [133,138] (Table 7).
| EFA | Mean of 14 studies done in Europe (N. 329) | EFA | Mean of 10 studies done in Africa (No. 259) | |
| ω-6 PUFA | ω-6 PUFA | |||
| C18:2ω-6 | 11.0 (6.9-16.4) | C18:2ω-6 | 12.7 (5.7-17.2) | |
| C18:3ω-6 | 0.54 (0.16-0.9) | C18:3ω-6 | 0.2 (0.1-0.3) | |
| C20:2ω-6 | 0.35 (0.2-0.5) | C20:2ω-6 | 0.41 (0.3-0.83) | |
| C20:3ω-6 | 0.32 (0.2-0.7) | C20:3ω-6 | 0,4 (0.2-0.5) | |
| C20:4ω-6 | 0.55 (0.2-1.2) | C20:4ω-6 | 0.66 (0.3-1.0) | |
| C22:4ω-6 | 0.08 (0.0-0.1) | C22:4ω-6 | 0.08 (0.0-0.1) | |
| C22:5ω-6 | 0.15 (0.1-0.3) | C22:5ω-6 | 0.15 (0.1-0.3) | |
| ω-3 PUFA | ω-3 PUFA | |||
| C18:3ω-3 | 0.87 (0.7-1.4) | C18:3ω-3 | 0.86 (0.1-<2.0) | |
| C20:3ω-3 | 0.06 | C20:3ω-3 | 0.14 | |
| C20:5ω-3 | 0.23 (0.04-0.6) | C20:5ω-3 | 0.22 (0.1-0.48) | |
| C22:5ω-3 | 0.2 (0.1-0.52) | C22:5ω-3 | 0.19 (0.1-0.39) | |
| C22:6ω-3 | 0.29 (0.1-0.59) | C22:6ω-3 | 0.38 (0.1-0.9) | |
| Modified from reference 120 | Modified from reference 290 | |||
Table 7: BM values of essential fatty acids (EFA) polyunsaturated (PUFA) in 14 European and 10 African studies; mean and highest and lowest values.
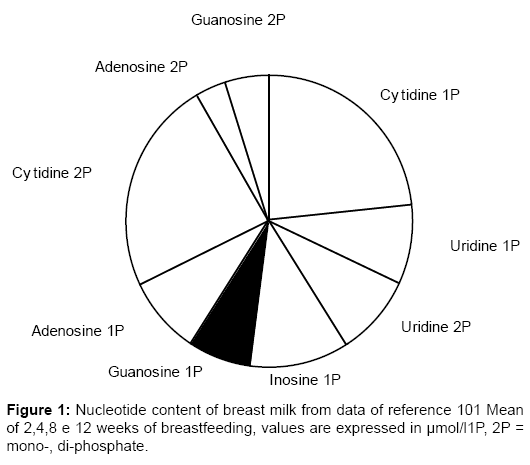
• Nucleotides, monomeric units of polymeric DNA and RNA, present in BM (% of nitric products) five times more than in CM [139], are essential in energy metabolism, enzymatic reactions, and during rapid growth, have been reported to be especially important for the growth and maturation of the developing gut, in addition to enhancing immune system potentialities in neonates [140]. The human body synthesizes nucleotides to cover its metabolic needs, and is also dependent on external supplies, such as dietary nucleotides; BM levels are shown in Figure 1 [141], in Table 8 [140] and in comparison with supplemented formulas (Table 9) [142]. BM is provided with the complete enzyme sequence to convert purine nucleotides to uric acid [142]. Nucleotides influence several indices of neonatal immune function [143]. They are essential nutrients for normal development, maturation and repair of the gastrointestinal tract, since rapidly growing tissues such as the intestinal epithelium and lymphoid cells have limited de novo synthetic capacity and require exogenous sources of purine and pyrimidine bases [144]. Dietary purines are not significantly incorporated into hepatic nucleic acids, but pyrimidines are: both are taken up by gut cells, which convert to uric acid the excess purines [144]. Carver [143] has documented that breastfed infants have higher NK cell and IL2 production compared with a group of healthy babies fed a CM formula supplemented with nucleotides (41.7 ± 4.7 versus 32.2 ± 3.4), and levels significantly higher than infants fed a standard nonnucleotide- supplemented formula; at 4 months only breastfed babies maintained the statistical differences versus the standard CM-fed group [143]. High levels of NK cells and IL2 may represent a defense against pathogenic invasion [143]. Principally in preterm infants [145], dietary nucleotides may influence the conversion into LCPs of linoleic (w-6 series) and a-linolenic (w-3 series) acids.
| Type of BM | uridine | cytidine | guanosine | adenosine | TPAN |
| Colostrum Mean + (range) | 26 (21-30) | 71 (33-84) | 21 (15-26) | 21 (13-26) | 139 (82-164) |
| Transitional BM Mean + (range) | 32 (23-37) | 86 (76-100) | 30 (19-43) | 29 (17-42) | 177 (144-210) |
| Early mature BM Mean + (range) | 48 (30-67) | 102 (79-146) | 45 (23-91) | 46 (21-97) | 240 (172-402) |
| Mature BM Mean + (range) | 47 (36-58) | 96 (73-124) | 28 (22-40) | 31 (24-49) | 202 (156-259) |
| General mean ± SD | 38 ± 13 | 88 ± 24 | 31 ± 18 | 32 ± 20 | 189 ± 70 |
| Range | 21-67 | 33-146 | 19-92 | 13-97 | 82-402 |
Table 8: Nucleotides as such and total potentially available nucleoside (TPAN) (μmol/l) in a pool of 100 samples of breast milk (BM) distinct by stage of lactation
| Compound | Breast milk | Not supplemented formula | Supplemented formula |
| Nucleic acid | 23 ± 19 (8,6-71) | <2 | <2 |
| Nucleic acid* | 68 ± 55 (25-209) | <6 | <6 |
| 5'-CMP | 66 ± 19 (41-106) | - | 60 |
| 5'-UMP | 11 ± 5.3 (4.8-21) | - | 22 |
| 5'-GMP | 1.5 ± 1.6 (0-5.9) | - | 8.4 |
| 5'-IMP | - | - | 9.8 |
| 5'-AMP | 5.7 ± 4.9 (1.7-19) | - | 14 |
| Cytidine | 5.4 ± 1.6 (3.6-9.8) | 6.2 | 8.5 |
| Uridine | 4.9 ± 1.3 (2.8-7.8) | 10 | 9.7 |
| Guanosine | - | 1.4 | 1.2 |
| Inosine | - | 1.5 | 1.5 |
| Adenosine | - | - | - |
| Guanine | 0.76 ± 1.3 (0-3.3) | - | - |
| Hypoxanthine | - | - | - |
| Xanthine | - | - | - |
| uric acid | 69 ± 12 (47-86) | 35 | 33 |
| orotic acid | - | 240 | 188 |
Table 9: Levels (mean ± standard error of the mean and limits) of nucleic acids and their metabolites found in breast milk at 3-24 weeks of breastfeeding, and in formulas supplemented or not of ribonucleotides.
• Several factors insure a nonspecific protection against potential pathogens, including the enteric colonization with nonpathogenic bacteria, lectins, additional carbohydrates different from lactose providing a protective effect for the developing mucosa, lipids with antibacterial, antiviral and antiprotozoan activity, and growth factors stimulating gut closure etc [146].
• Leukocytes (Table 6) consist of (%): neutrophils 55-60, macrophages 35-40, lymphocytes 5.80% of which are T lymphocytes [147], including memory T cells [148]; the total lymphocytes, B and T cells, CD4 and CD8 lymphocytes, macrophages and neutrophils are present in preterm babies in a significantly greater number [149]. Moreover are present more T cells than those found in peripheral blood, such as TcRgd, CD8 and CD103 and an increased proportion of activated cells, which impart to BM cells a “mucosal” phenotype [150]. What is more, healthy breastfed infants have significantly fewer CD4 T cells, and a greater number of CD8 T cells, so that the CD4/CD8 ratio is lower than in age-matched bottle-fed babies, whereas in peripheral blood the ratio is 2:1, in addition to a greater number of NK cells [151]. It was suggested that maternal T cells carry on a prominent activity, promoting the secretion of maternal IgA [152].
• Macrophages are functionally active by means of M-CSF (macrophage colony- stimulating factor) present with levels 10-100 times above the levels found in human blood [153], mean 30 times [150], ready to bridge the early neonatal “deficits” since they are provided with receptors for sIgA and may be activated via these receptors [154]. BM macrophages are able to increase sIgA levels up to 5-10% of BM content [104], yet intracellular sIgA, a valid means to progressively transport the antibodies directly into critical areas [154], in addition to phagocyting the immune complexes formed by sIgA to exclude potential pathogens invading BM. Macrophage concentration is greatest at the 6th day but can persist and act up to the 6th month [93], they can also be primed to release large amounts of O2 metabolites and could thus contribute to the protection of newborns against invading microorganisms [155]. In keeping with these findings the ability of immunocompetent cells to survive sticking to the intestinal mucosal sites [149]and to secrete their soluble products, is also a means to potentiate the local immune responses of neonatal gut as well as their systemic ones;
• In addition to the ILs listed in Table 6, almost all generated by macrophages, there are several others including IL1a, IL2-5, IL8 [156] and IL2 produced by T cells [148]. Such ILs are therefore able to meet all requirements of breastfed neonates, moreover the leukocytes if properly stimulated produce TNF-a [157] and mononucleates GMCSF likewise [156]. In particular IL10, present in placental lysates of 2nd and 3rd trimesters and in amniotic fluid [158], could represent a bridge among the anti-inflammatory and immunomodulating factors forming the defense system of BM [159], also because it is necessary for the synthesis of IgA antibodies [160]. The undetectable expression of IL10 in preterm babies [161], confirmed by the decreased secretion by neonatal monocytes and T cells [162], is in relationship with severe respiratory manifestations and can predispose to chronic lung inflammation in preterm neonates [161].
• Regarding the chemokines IL8 and RANTES, it is intriguing the hypothesis that they facilitate the transfer of maternal cells into BM, their adhesion to intestinal walls and their migration into infantile immune tissues, leading in prospective to the modulation of neonatal immunity [105].
• Some oligosaccharides (Table 7) contribute to augment the defense potential of babies against infectious agents, acting as receptors for E. coli and Vibrio cholerae, preventing their adhesion to the intestinal mucosa, so decreasing the inflammatory reactions at the mucosal level.
Immunology of colostrum
Centuries were necessary in order that the alimentary value was acknowledged, the immunologic one was still denied in 1939 [163]. Contains:
• IgA, IgM, IgG; as a compensation of poor quantity of IgG (3% of maternal IgGs), colostrum and BM contain significant concentration of subclasses, » 50% of maternal titres [157]; Table 10 shows also the salivary values [164].
| IgG1 | IgG2 | IgG3 | IgG4 | IgGtotali | |
| Colostro | 0.0372 (46.6) | 0.0349 (43.9) | <0.0034 (<4.0) | 0.0049 (6.2) | 0.0804 |
| Siero | 6.209 (64.0) | 2.585(28.5) | 0.577(6.0) | 0.194(2.1) | 9.986 |
| RapportoC/S (%) | 0.6 | 1.1 | <0.4 | 1.4 | 0.8 |
| LM | 0.0251 (46.9) | 0.0196 (43.1) | <0.0016 (<3.7) | 0.0042 (5.7) | 0.0469 |
| Siero | 7.546(63.0) | 3.204 (29.0) | 0.786(7.0) | 0.201 (2.0) | 12.480 |
| RapportoLM/S (%) | 0.3 | 0.6 | <0.2 | 0.9 | 0.4 |
| Saliva | 0.0097 (27.9) | 0.0187 (53.7) | <0.0016 (<4.6) | 0.0013 (3.7) | - |
| Siero | 8.246 (60.7) | 4.504(33.2) | 0.473(3.5) | 0.161 (0.8) | - |
| Rapporto Saliva/S (%) | 0.1 | 0.4 | <0.3 | 0.8 | - |
Table 10: Levels (geometric mean, g / l) of the subclasses IgG in colostrum, milk maternoe saliva (average levels, g/l) (with relative percentage of each IgG) in comparison with the percentage values with the serum (S) corresponding.
• sIgA, macrophages and EGF with titres higher than the BM ones (Table 11) [93,101,149,164-167]. IgA antibodies (5-10 g/l) are transferred to newborns during the first 3 days after delivery: IgA can represent up to 80% of total content of proteins [104,105], while CD154 could increase their levels influencing the B-cell isotype switching [168].
| *Lymphocytes (about 10-15% of total cells) x ml (x 104) | |||
| CD3 | 74.7 ± 2.5 | ||
| CD4 | 50.6 ± 2.3 | ||
| CD8 | 24.0 ± 1.7 | ||
| (mean ± ESM) T4/T8 cell ratio higher than that of circulation | |||
| (mean and range) | colostrum | autologous blood | |
| CD3+ | 69 (55-81) | 75 (65-84) | |
| CD3+/CD45RA+ | 12 (4-31) | 49 (28-69) | |
| CD3+/CD45RO+ | 78 (56-98) | 54 (40-85) | |
| * Immunoglobulins (Igs) | |||
| IgA (mg/dl) days 1-2 | 619.0 ± 110.6 | days 3-4 | 239.3 ± 55.8 |
| IgG (mg/dl) days 1-2 | 31.4 ± 12.3 | days 3-4 | 14.1 ± 5.0 |
| IgM (mg/dl) days 1-2 | 38.3 ± 7.8 | days 3-4 | 5.3 ± 1.6 |
| IgG subclasses (about 50% of maternal levels, see Table 6) | |||
| sIgA with levels higher than BM levels | |||
| * IFN-γ(U/ml) | colostrum | autologous blood | |
| anti-CD3 | 39.5 ± 9.6 | 33.8 ± 10.7 | |
| anti-CD2 | 20.6 ± 6.5 | 22.3 ± 6.2 | |
| * macrophages 30-47% of total cells (levels higher than BM levels) | |||
| * antioxidants (may serve to block neutrophil-generated toxic oxygen metabolites) * EGF |
|||
| pre-colostrum | 130-180 ng/ml | ||
| colostrum | 35-438 ng/ml | ||
| * enzymes and proteins (levels higher than BM levels) | |||
| * lactate-dehydrogenase | |||
| * trace elements (levels higher than BM levels) | |||
| Human colostrum activities: | |||
| * hastens the development of an intact mucosal barrier | |||
| * enhances brush-border enzyme production (lactase, sucrase, and alkaline phosphatase) | |||
| * cytotoxic activity | |||
| ADCC | |||
| lectine-dependent | |||
| NK cells | |||
| * decrease of antigen penetration in neonates | |||
| * presence of IgE suppressor factors | |||
| No. of lymphocytes and other cells/mm3 (mean ± SD) | |||
| Total lymphocytes | 1.532 ± 520.2 | ||
| B lymphocytes | 414.2 ± 218.2, T=1.095 ± 347.6, T4=662.2 ± 218.4, T8=425.5 ± 143,9; T4/T8 = 1,6 ± 0.35 | ||
| Macrophages | 2.860 ± 860.3 | ||
| Neutrophils | 1.2019 ± 479 | ||
Table 11: Immunologic factors in human colostrum.
• Nucleotide levels not much lower than those of transitional BM (Table 12)
| Xenobiotic | Milk/plasma rate |
| Caffeine | 0.8 |
| Chloramphenicol | 0.5-0.6 |
| Ethanol | 0.9-0.95 |
| Iodum131 | 65 |
| Nicotine | 0.17 |
| Streptomycin | 0.5-1.0 |
| Theobromine | 0.7 |
| Theophylline | 0.7 |
| Tetracycline | 0.62-0.81 |
Table 12: Maternal milk/plasma rate of certain xenobiotics.
• Factors binding IgE and specifically suppressing IgE synthesis by B cells of atopicindividuals [169].
• Factors eliciting a non-specific protective function at the level of mucosal barrier because the existent antibodies, not being absorbed, stick to the intestinal wall, carrying on a function of passive defense [170].
• In addition an amount of EFA equal to the recommended ones [171]
• Titres of CD45RO+ and T-CD3+ cells mostly expressing CD103 and of IFN-g which assume a highly positive significance in the context of the above alluded to inadequacy of such neonatal cells [172] moreover there is a double volume of TcRgd cells compared with that in the peripheral blood [173].
• Substances able to accelerate the development of an intact mucosal barrier, enhance the production of brush-border enzymes (lactase, sucrase, alkaline phosphatase), and decrease food antigen penetration through an anti-inflammatory activity within the intestinal mucosa [174].
• Antioxidant substances which could be feasible to inactivate O2 toxic metabolites deriving from the neutrophil excessive secretion [175].
• Colostral B cells respond with Ig secretion to the antigen stimulation [176]; similar growth has been observed in culture, therefore such results have given credit to the theory that B cells partially maintain their functional activities after having colonized the colostrum/BM [176]. This hypothesis was not confirmed since the IgE concentrations, similar in atopic and not atopic mothers, are so low that they have no significant effect on IgE regulation in the neonatal age [177].
As far as we have as yet discussed it seems antiscientific and antimedical depriving the neonate of colostrum and early milk in the very first days of extrauterine life adds to the risk that potential pathogens from other foods and fluids may cause infections [178].
All evidence as yet gathered tends to prove that maternal breast is an immune organ belonging to MALT [102]: BM not only has components protecting the vulnerable infant against the first infective and inflammatory episodes, but is also the vehicle for the transfer of immune regulation from the mother to her offspring, thus contributing to the maturation of the immune system of the newborn infant [179]. Several immunomodulating factors present in BM that may actively modulate the immunologic growth of the baby, many of which are produced and are common to other mucosal sites, often share synergical features, provide a protective activity without inducing inflammation, moreover their production decreases with reference to the duration of breastfeeding and synchronously with the increased secretion of those factors from the neonate [179]. In addition EGF has been shown to play a role in reducing macromolecular absorption and to promote the functional maturation of the epithelial cells of the gut barrier (Tables 6 and 11): indeed the proliferation of the intestinal epithelium is more rapid in breastfed animals compared to the artificially fed ones [101]. However pasteurized CM contains EGF values almost similar, which likely do not resist to subsequent manipulations [180].
sIgA is the major antibody of BM [92,93,102]: primed IgA-B cells home to the mammary gland through the enteromammary axis and are transferred to the suckling newborn, where they act against noxious intraluminal antigens and also respiratory microorganisms [92,93,102]. In the neonatal gut were detected specific sites where maternal sIgA antibodies bind the glycocalyx of epithelial cells more firmly than the endogenous ones [170]. Furthermore, maternal IgA antibodies have been shown to block effectively the antigen entrance into BM [181]. Clearly, maternal MALT is in turn “educated” and, again through the enteromammary pathway, contributes to the de novo synthesis of sIgA; next as previously alluded to, the studies on anti-idiotypic antibodies show that they are favored in infants by maternal antibodies [90,182]. Even human milk macrophages play a role in the local protection of the infantile gut [154]. Beginning from the first week of breastfeeding, the baby receives spermine and spermidine, with a virtual protective effect on food allergy. Consequently it appears to be pivotal the immune defense insured to the offspring firstly by colostrum and then by BM, in a particularly critical period concerning a possible sensitization [179], in which the maturation of the gastrointestinal barrier and the antibody secretion still are inadequate [158]. In conclusion, breastfeeding can prevent atopy development in genetically at risk newborns/infants and promote la maturation of the gastrointestinal tract with several mechanisms [2], however there are conditions potentially associated with immature mucosal barrier, for instance the elimination of gut flora following an antibiotic treatment. It is not out of place to state that the most common immunodeficiency may be that of the young infant deprived of BM with the ensuing paucity of sIgA and other immune and defense factors [178]. This is demonstrated by the study of 24 variables, as presumed causes of neonatal septicemia: only the protection insured by BM versus CM or formula reached the statistical significativity (p<0.001) [178].
As regards possible effects of malnutrition on breastfeeding, neither the nutritional status, nor the ethnic origin influence the immunological components; instead Rotavirus infections may cause a significant rise in intestinal permeability to antigenic macromolecules (ßLG), in particular if associated with malnutrition, so that CM introduction can trigger an inflammatory reaction if the local microflora is reduced or absent [89].
Conclusion
The development of sensitization to food antigens depends on genetic factors, however the phenotypic expression of the disease is modulated by the age of the baby or child and by the different diets administered early in life. Early exposure to food allergens in infancy is associated with an increased risk of sensitization, favoring the start of the atopic march. Exclusive, prolonged breast feeding and delayed weaning should be encouraged in babies of atopic families. Elimination of the most common offending foods from the maternal diet should be considered during the period of lactation (Tables 1 and 2) [34,92]. There is no agreement on the most suitable formula if the mother cannot breastfeed. SPFs have been employed for many years and their safety and nutritional adequacy has been definitely documented. In the last few years HFs have been suggested by several studies. However, at present, there are no data on the safety and nutritional adequacy on vulnerable babies exclusively fed these products since birth and for many months. Finally, due to the significant amount of immune reactive epitopes and intact CM proteins, HFs may be immunogenic in a predisposed host and should therefore not be given as a BM substitute [146]. Further studies are necessary in order to rule out this likelihood before widely using these products in atopic prone babies. Only BM can prevent the atopic march, as confirmed by recent studies. This is best demonstrated by the high proportion of atopic children breastfed from their mothers in this study=89.6%.
References
- Björkstén B,Kjellman NI (1990) Perinatal environmental factors influencing the development of allergy. ClinExp Allergy 20 Suppl 3: 3-8.
- Goldman AS (1993)The immune system of human milk: anti¬microbial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis 12: 664-672
- Grulee CG, Sanford HN (1936) The influence of breast and artificial feeding on infantile eczema. J Pediatr 9: 223-225
- Businco L, Marchetti F, Pellegrini G, Cantani A, Perlini R (1983) Prevention of atopic disease in "at-risk newborns" by prolonged breast-feeding. Ann Allergy 51: 296-299.
- Arshad SH, Hide DW (1992) Effect of environmental factors on the development of allergic disorders in infancy. J Allergy ClinImmunol 90: 235-241.
- Chandra RK (1979) Prospective studies of the effect of breast feeding on incidence of infection and allergy. ActaPaediatrScand 68: 691-694.
- Saarinen UM, Kajosaari M, Backman A, Siimes MA (1979) Prolonged breast-feeding as prophylaxis for atopic disease. Lancet 2: 163-166.
- Ziering RW, O'Connor R, Mellon M (1979) University of California in San Diego prophylaxis of allergy in infancy study: an interim report. J Allergy ClinImmunol 63: 199A
- Kaufman HS, Frick OL (1981) Prevention of asthma. Clin Allergy 11: 549-553.
- Hide DW, Guyer BM (1981) Clinical manifestations of allergy related to breast and cows' milk feeding. Arch Dis Child 56: 172-175.
- Gruskay FL (1982) Comparison of breast, cow, and soy feedings in the prevention of onset of allergic disease: a 15-year prospective study. ClinPediatr (Phila) 21: 486-491.
- Juto P, Möller C, Engberg S, Björkstén B (1982) Influence of type of feeding on lymphocyte function and development of infantile allergy. Clin Allergy 12: 409-416.
- Fergusson DM, Horwood LJ, Shannon FT (1983) Asthma and infant diet. Arch Dis Child 58: 48-51.
- Kajosaari M, Saarinen UM (1983) Prophylaxis of atopic disease by six months' total solid food elimination. Evaluation of 135 exclusively breast-fed infants of atopic families. ActaPaediatrScand 72: 411-414.
- Pratt HF (1984) Breastfeeding and eczema. Early Hum Dev 9: 283-290.
- Hide DW, Guyer BM (1985) Clinical manifestations of allergy related to breast- and cow's milk-feeding. Pediatrics 76: 973-975.
- Moore WJ, Midwinter RE, Morris AF, Colley JR, Soothill JF (1985) Infant feeding and subsequent risk of atopic eczema. Arch Dis Child 60: 722-726.
- Chandra RK, Puri S, Suraiya C, Cheema PS (1986) Influence of maternal food antigen avoidance during pregnancy and lactation on incidence of atopic eczema in infants. Clin Allergy 16: 563-569.
- Vandenplas Y, Sacre L (1986) Influences of neonatal serum IgE concentration, family history and diet on the incidence of cow's milk allergy. Eur J Pediatr 145: 493-495.
- Miskelly FG, Burr ML, Vaughan-Williams E, Fehily AM, Butland BK, et al. (1988) Infant feeding and allergy. Arch Dis Child 63: 388-393.
- Hattevig G,Kjellman B, Sigurs N, Björkstén B, Kjellman NI (1989) Effect of maternal avoidance of eggs, cow's milk and fish during lactation upon allergic manifestations in infants. ClinExp Allergy 19: 27-32.
- Chandra RK, Puri S, Hamed A (1989) Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants. BMJ 299: 228-230.
- Chandra RK, Singh G, Shridhara B (1989) Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Ann Allergy 63: 102-106.
- Lucas A, Brooke OG, Morley R, Cole TJ, Bamford MF (1990) Early diet of preterm infants and development of allergic or atopic disease: randomised prospective study. BMJ 300: 837-840.
- Chandra RK,HamedA (1991) Cumulative incidence of atopic disorders in high risk infants fed whey hydrolysate, soy, and conventional cow milk formulas. Ann Allergy 67: 129-132.
- Sigurs N,Hattevig G, Kjellman B (1992) Maternal avoidance of eggs, cow's milk, and fish during lactation: effect on allergic manifestations, skin-prick tests, and specific IgE antibodies in children at age 4 years. Pediatrics 89: 735-739.
- Burr ML, Limb ES, Maguire MJ, Amarah L, Eldridge BA, et al. (1993) Infant feeding, wheezing, and allergy: a prospective study. Arch Dis Child 68: 724-728.
- Halken S,Høst A, Hansen LG, Osterballe O (1993) Preventive effect of feeding high-risk infants a casein hydrolysate formula or an ultrafiltrated whey hydrolysate formula. A prospective, randomized, comparative clinical study. Pediatr Allergy Immunol 4: 173-181.
- Kajosaari M1 (1994) Atopy prevention in childhood: the role of diet. Prospective 5-year follow-up of high-risk infants with six months exclusive breastfeeding and solid food elimination. Pediatr Allergy Immunol 5: 26-28.
- Høst A,Husby S, Osterballe O (1988) A prospective study of cow's milk allergy in exclusively breast-fed infants. Incidence, pathogenetic role of early inadvertent exposure to cow's milk formula, and characterization of bovine milk protein in human milk. ActaPaediatrScand 77: 663-670.
- Saarinen UM,Kajosaari M (1995) Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet 346: 1065-1069.
- Chandra RK (1997)Five-year follow-up of high-risk infants with family history of allergy who were exclusively breast- fed or fed partial whey hydrolysate, soy and conventional cow milk formulas. J PediatrGastroenterolNutr 24: 380-388
- Matthew DJ, Taylor B, Norman AP, Turner MW (1977) Prevention of eczema. Lancet 1: 321-324.
- Businco L,Cantani A, Meglio P, Bruno G (1987) Prevention of atopy: results of a long-term (7 months to 8 years) follow-up. Ann Allergy 59: 183-186.
- Savilahti E, Tainio VM, Salmenperä L, Siimes MA, Perheentupa J (1987) Prolonged exclusive breast feeding and heredity as determinants in infantile atopy. Arch Dis Child 62: 269-273.
- Zeiger RS, Heller S, Mellon MH, Forsythe AB, O'Connor RD, et al. (1989) Effect of combined maternal and infant food-allergen avoidance on development of atopy in early infancy: a randomized study. J Allergy ClinImmunol 84: 72-89.
- Arshad SH, Matthews S, Gant C, Hide DW (1992) Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 339: 1493-1497.
- Halken S, Høst A, Hansen LG, sterballe O (1992) Effect of an allergy prevention program on incidence of atopic symptoms in infancy. A prospective study of 159 "high-risk" infants. Allergy 47: 545-553
- Zeiger RS, Heller S, Mellon MH, Halsey JF, Hamburger RN (1992) Genetic and environmental factors affecting the development of atopy through age 4 in children of atopic parents: a prospective randomized study of food allergen avoidance. Pediatr Allergy Immunol 3: 110-127
- Bardare M,Vaccari A, Allievi E, Brunelli L, Coco F, et al. (1993) Influence of dietary manipulation on incidence of atopic disease in infants at risk. Ann Allergy 71: 366-371.
- Bruno G,Milita O, Ferrara M, Nisini R, Cantani A, et al. (1993) Prevention of atopic diseases in high risk babies (long-term follow-up). Allergy Proc 14: 181-186.
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, et al. (1994) Effect of allergen avoidance in infancy on allergic manifestations at age two years. J Allergy ClinImmunol 93: 842-846.
- Machado E, Bartellini M, Testaferrata A (1994) Results of a multicentric study for allergy prevention: A 48-month follow-up. Immunità&Allergia, nuovipercorsi per ilpediatra. Brescia 26-29.
- Zeiger RS, Heller S (1995) The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy ClinImmunol 95: 1179-1190.
- Hide DW, Matthews S, Tariq S, Arshad SH (1996) Allergen avoidance in infancy and allergy at 4 years of age. Allergy 51: 89-93.
- Halpern SR, Sellars WA, Johnson RB, Anderson DW, Saperstein S, et al. (1973) Development of childhood allergy in infants fed breast, soy, or cow milk. J Allergy ClinImmunol 51: 139-151.
- Koivikko A (1974) Childhood asthma in Finland. A survey of 559 patients. ActaAllergol 29: 30-72.
- Blair H (1977) Natural history of childhood asthma. 20-year follow-up. Arch Dis Child 52: 613-619.
- Kramer MS, Moroz B (1981) Do breast-feeding and delayed introduction of solid foods protect against subsequent atopic eczema? J Pediatr 98: 546-550.
- Gordon RR, Noble DA, Ward AM, Allen R (1982) Immunoglobulin E and the eczema-asthma syndrome in early childhood. Lancet 1: 72-74.
- Golding J, Butler NR, Taylor B (1982) Breastfeeding and eczema/asthma. Lancet 1: 623.
- Magnusson CG1 (1988) Cord serum IgE in relation to family history and as predictor of atopic disease in early infancy. Allergy 43: 241-251.
- Wjst M,Dold S, Reitmeier P, Wulff A, Nicolai T, et al. (1992) [Does breast feeding prevent asthma and allergies? Results of the Munich asthma and allergy study]. MonatsschrKinderheilkd 140: 769-774.
- Burr ML (1983) Does infant feeding affect the risk of allergy? Arch Dis Child 58: 561-565.
- Zeiger RS1 (1994) Dietary manipulations in infants and their mothers and the natural course of atopic disease. Pediatr Allergy Immunol 5: 33-43.
- Fergusson DM,Horwood LJ, Shannon FT (1990) Early solid feeding and recurrent childhood eczema: a 10-year longitudinal study. Pediatrics 86: 541-546.
- Businco L, Cantani A (1991)The prevention of asthma. Interasma News Bull: 3-7.
- Vandenplas Y1 (1997) Myths and facts about breastfeeding: does it prevent later atopic disease? ActaPaediatr 86: 1283-1287.
- Isolauri E,Tahvanainen A, Peltola T, Arvola T (1999) Breast-feeding of allergic infants. J Pediatr 134: 27-32.
- Price C, Jackson K (1991) Advertising infant formulas in hospitals. BMJ 303: 1058.
- Businco L,Lucenti P, Arcese G, Ziruolo G, Cantani A (1994) Immunogenicity of a so-called hypoallergenic formula in at-risk babies: two case reports. ClinExp Allergy 24: 42-45.
- Burr GO, Burr MM (1973) Nutrition classics from The Journal of Biological Chemistry 82:345-67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr Rev 31: 248-249.
- Hansen AE, Wiese HF, Boelsche AN, Adam DJD (1936) Role of linoleic acid in infant nutrition. Clinical and chemical study of 428 infants fed on milk mixtures varying in kind and amount of fat. Pediatrics 31: 171-192
- Murphy MG1 (1990) Dietary fatty acids and membrane protein function. J NutrBiochem 1: 68-79.
- Henderson WR Jr (1987) Eicosanoids and lung inflammation. Am Rev Respir Dis 135: 1176-1185.
- Rola-Pleszczynski M1 (1985) Immunoregulation by leukotrienes and other lipoxygenase metabolites. Immunol Today 6: 302-307.
- Aussel C, Mary D, Fehlmann M (1987) Prostaglandin synthesis in human T cells: its partial inhibition by lectins and anti-CD3 antibodies as a possible step in T cell activation. J Immunol 138: 3094-3099.
- Goodman MG, Brunton LL, Weigle WO (1981) Modulation of lymphocyte activation. II. Alteration of intracellular cyclic nucleotide concentrations by an oxidation product of arachidonic acid. Cell Immunol 58: 85-96.
- Horrobin DF (1980)The regulation of prostaglandin biosynthesis: negative feed-back mechanisms and the selective control of formation of 1 and 2 series prostaglandins: relevance to inflammation and immunity. Med Hypotheses 6: 687-709
- Hansen AE (1937) Serum lipids in eczema and other pathological conditions. Am J Dis Child 53: 933-946
- Manku MS, Horrobin DF, Morse NL, Wright S, Burton JL (1984) Essential fatty acids in the plasma phospholipids of patients with atopic eczema. Br J Dermatol 110: 643-648.
- Muhlemann MF, Manku MS, Leonard TJ, Cream JJ (1987) Essential fatty acids in the plasma phospholipids of patients with atopic cataracts. Br J Dermatol 116: 179-182.
- Wright S (1985) Atopic dermatitis and essential fatty acids: a biochemical basis for atopy? ActaDermVenereolSuppl (Stockh) 114: 143-145.
- Cornbleet T (1935) Use of maize oil (unsaturated fatty acids) in the treatment of eczema. Arch DermatolSyph 31: 224-234
- HANSEN AE, KNOTT EM, et al (1947) Eczema and essential fatty acids. Am J Dis Child 73: 1-18.
- Lovell CR, Burton JL, Horrobin DF (1981) Treatment of atopic eczema with evening primrose oil. Lancet 1: 278.
- Wright S, Burton JL (1982) Oral evening-primrose-seed oil improves atopic eczema. Lancet 2: 1120-1122.
- Sorva R,Mäkinen-Kiljunen S, Juntunen-Backman K (1994) Beta-lactoglobulin secretion in human milk varies widely after cow's milk ingestion in mothers of infants with cow's milk allergy. J Allergy ClinImmunol 93: 787-792.
- Bergmann KE, Lau-Schadendorf S, Wahn U (1993)The atopic career in childhood - The German multicenter study (MAS-90). ACI News 5: 49-51
- Stintzing G, Zetterström R (1979) Cow's milk allergy, incidence and pathogenetic role of early exposure to cow's milk formula. ActaPaediatrScand 68: 383-387.
- Lindfors A,Enocksson E (1988) Development of atopic disease after early administration of cow milk formula. Allergy 43: 11-16.
- Schmitz J,Digeon B, Chastang C, Dupouy D, Leroux B, et al. (1992) Effects of brief early exposure to partially hydrolyzed and whole cow milk proteins. J Pediatr 121: S85-89.
- Strobel S (1996)Einfluß des Stillens und der Flaschenernährung auf der Entwicklung der ImmunitätimKindesalter. MonatsschrKinderheilkd 144: S161-S168
- Jarrett EE (1984) Perinatal influences on IgE responses. Lancet 2: 797-799.
- Cantani A,Gagliesi D (1996) Severe reactions to cow's milk in very young infants at risk of atopy. Allergy Asthma Proc 17: 205-208.
- Schwartz RH (1991)IgE-mediated allergic reactions to cow's milk. Immunol Allergy Clin North Am 11: 717-741
- Björkstén B (1994) Risk factors in early childhood for the development of atopic diseases. Allergy 49: 400-407.
- Lifschitz CH, Hawkins HK, Guerra C, Byrd N (1988) Anaphylactic shock due to cow's milk protein hypersensitivity in a breast-fed infant. J PediatrGastroenterolNutr 7: 141-144.
- Hahn-Zoric M,Carlsson B, Jeansson S, Ekre HP, Osterhaus AD, et al. (1993) Anti-idiotypic antibodies to poliovirus antibodies in commercial immunoglobulin preparations, human serum, and milk. Pediatr Res 33: 475-480.
- Cantani A, Ragno V, Businco L (1991) Natural history of IgE-mediated food allergy in fully breast-fed babies. Report of twenty-one cases (Follow-up to 19 years). Pediatr Allergy Immunol 2: 131-134
- Hanson LA (1998) Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol 81: 523-533.
- Bertotto A, Fabietti GM, Castellucci G, Vagliasindi C (1995)Proprietàimmunologiche del latte umano. Perugia: Istituto di Pediatriadell'Università di Perugia.
- Bertotto A,Castellucci G, Fabietti G, Scalise F, Vaccaro R (1990) Lymphocytes bearing the T cell receptor gamma delta in human breast milk. Arch Dis Child 65: 1274-1275.
- Garofalo R,Chheda S, Mei F, Palkowetz KH, Rudloff HE, et al. (1995) Interleukin-10 in human milk. Pediatr Res 37: 444-449.
- Gibson CE,Eglinton BA, Penttila IA, Cummins AG (1991) Phenotype and activation of milk-derived and peripheral blood lymphocytes from normal and coeliac subjects. Immunol Cell Biol69 : 387-393.
- Hara T,Irie K, Saito S, Ichijo M, Yamada M, et al. (1995) Identification of macrophage colony-stimulating factor in human milk and mammary gland epithelial cells. Pediatr Res 37: 437-443.
- Jain N,Mathur NB, Sharma VK, Dwarkadas AM (1991) Cellular composition including lymphocyte subsets in preterm and full term human colostrum and milk. ActaPaediatrScand 80: 395-399.
- Keeney SE,Schmalstieg FC, Palkowetz KH, Rudloff HE, Le BM, et al. (1993) Activated neutrophils and neutrophil activators in human milk: increased expression of CD11b and decreased expression of L-selectin. J LeukocBiol 54: 97-104.
- Keller MA, Faust J, Rolewic LJ, Stewart DD (1986) T cell subsets in human colostrum. J PediatrGastroenterolNutr 5: 439-443.
- Ménard D, Arsenault P (1988) Epidermal and neural growth factors in milk: Effects of epidermal growth factor on the development of the gastrointestinal tract. Nestlé Nutr Workshop Ser 15: 105-120
- Ogra PL, Ogra SS (1988) Cellular aspects of immunologic reactivity in human milk. NestlèNutr Work Ser 15: 171-183
- Rudloff HE,Schmalstieg FC Jr, Mushtaha AA, Palkowetz KH, Liu SK, et al. (1992) Tumor necrosis factor-alpha in human milk. Pediatr Res 31: 29-33.
- Wold AE, Hanson LÅ (1994) Defense factors in human milk. CurrOpinGastroenterol 10: 652-658
- Quie PG1 (1990) Antimicrobial defenses in the neonate. SeminPerinatol 14: 2-9.
- Xanthou M1 (1997) Human milk cells. ActaPaediatr 86: 1288-1290.
- Machtinger S, Moss R (1986) Cow's milk allergy in breast-fed infants: the role of allergen and maternal secretory IgA antibody. J Allergy ClinImmunol 77: 341-347.
- Okamoto Y,Ogra PL (1989) Antiviral factors in human milk: implications in respiratory syncytial virus infection. ActaPaediatrScandSuppl 351: 137-143.
- Banzhoff A,Dulleck A, Petzoldt S, Rieger CH (1994) Salivary anti-RSV IgA antibodies and respiratory infections during the first year of life in atopic and non-atopic infants. Pediatr Allergy Immunol 5: 46-52.
- Avanzini MA,Plebani A, Monafo V, Pasinetti G, Teani M, et al. (1992) A comparison of secretory antibodies in breast-fed and formula-fed infants over the first six months of life. ActaPaediatr 81: 296-301.
- Fitzsimmons SP, Evans MK, Pearce CL, Sheridan MJ, Wientzen R, et al. (1994) Immunoglobulin A subclasses in infants' saliva and in saliva and milk from their mothers. J Pediatr 124: 566-573.
- Chheda S,Palkowetz KH, Rassin DK, Goldman AS (1996) Deficient quantitative expression of CD45 isoforms on CD4+ and CD8+ T cell subpopulations and subsets of CD45RA(low)CD45RO(low) T cells in newborn blood. Biol Neonate 69: 128-132.
- Hanson LÅ, Dahlman-Höglund A, Lundin S (1996)The maturation of the immune system. Monogr Allergy 32: 10-15.
- Hanson LA,Telemo E, Wiedermann U, Dahlman A, Saalman R, et al. (1993) Sensitization and development of tolerance via the gut. Pediatr Allergy Immunol 4: 16-20.
- Hahn-Zoric M,Fulconis F, Minoli I, Moro G, Carlsson B, et al. (1990) Antibody responses to parenteral and oral vaccines are impaired by conventional and low protein formulas as compared to breast-feeding. ActaPaediatrScand 79: 1137-1142.
- Hahn-Zoric M,Carlsson B, Björkander J, Osterhaus AD, Mellander L, et al. (1992) Presence of non-maternal antibodies in newborns of mothers with antibody deficiencies. Pediatr Res 32: 150-154.
- Mellander L, Carlsson B, Hanson LA (1984) Appearance of secretory IgM and IgA antibodies to Escherichia coli in saliva during early infancy and childhood. J Pediatr 104: 564-568.
- Pickering LK,Granoff DM, Erickson JR, Masor ML, Cordle CT, et al. (1998) Modulation of the immune system by human milk and infant formula containing nucleotides. Pediatrics 101: 242-249.
- Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA (1994) Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J ClinNutr 60: 189-194.
- GuesnetPh, Couet C, Alessandri JM, Antoine JM, Durand G, et al. (1995)Effets de la teneur en acidelinoléiqueet du rapport 18:2n-6/18:3n-3 des lipides du laitmaternelsur la teneur en acidedocosahexaénoïque des structures nerveuses du jeune rat allaité. Ann Pédiatr 42: 289-284
- Koletzko B (1992) Long chain polyunsaturated fatty acids in the diets of premature infants. Nestlé Nutr Workshop Ser 28: 135-146
- Luukkainen P,Salo MK, Nikkari T (1994) Changes in the fatty acid composition of preterm and term human milk from 1 week to 6 months of lactation. J PediatrGastroenterolNutr 18: 355-360.
- StrannegÅrd IL, Svennerholm L, StrannegÅrd O (1987) Essential fatty acids in serum lecithin of children with atopic dermatitis and in umbilical cord serum of infants with high or low IgE levels. Int Arch Allergy ApplImmunol 82: 422-423.
- Wright S, Bolton C (1989) Breast milk fatty acids in mothers of children with atopic eczema. Br J Nutr 62: 693-697.
- Businco L,Ioppi M, Morse NL, Nisini R, Wright S (1993) Breast milk from mothers of children with newly developed atopic eczema has low levels of long chain polyunsaturated fatty acids. J Allergy ClinImmunol 91: 1134-1139.
- Duchén K, Yu G, Björkstén B (1998) Atopic sensitization during the first year of life in relation to long chain polyunsaturated fatty acid levels in human milk. Pediatr Res 44: 478-484.
- Morse PF,Horrobin DF, Manku MS, Stewart JC, Allen R, et al. (1989) Meta-analysis of placebo-controlled studies of the efficacy of Epogam in the treatment of atopic eczema. Relationship between plasma essential fatty acid changes and clinical response. Br J Dermatol 121: 75-90.
- Bamford JT, Gibson RW, Renier CM (1985) Atopic eczema unresponsive to evening primrose oil (linoleic and gamma-linolenic acids). J Am AcadDermatol 13: 959-965.
- Schroten H, Schöls K, Melnik B, von Kries R, Wahn V, et al. (1992) Breast milk of atopic mothers provide their infants of normal amounts of w-6-fatty acids. PediatrAl¬lergyImmunol 3: 140-143
- Berth-Jones J, Graham-Brown RA (1993) Placebo-controlled trial of essential fatty acid supplementation in atopic dermatitis. Lancet 341: 1557-1560.
- Paul K, Leichsenring M, Bremer HJ (1993) Omega-6-fatty acids in children with atopic bronchial asthma (abstract). MonatsschrKinderheilkd 141: 364
- Hederos CA, Berg A (1996) Epogam evening primrose oil treatment in atopic dermatitis and asthma. Arch Dis Child 75: 494-497.
- Koletzko B, Thiel I, Abiodun PO (1992) The fatty acid composition of human milk in Europe and Africa. J Pediatr 120: S62-70.
- Auestad N,Montalto MB, Hall RT, Fitzgerald KM, Wheeler RE, et al. (1997) Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas with long chain polyunsaturated fatty acids for one year. Ross Pediatric Lipid Study. Pediatr Res 41: 1-10.
- Innis SM, Dyer R, Quinlan P, Diersen-Schade D (1995) Palmitic acid is absorbed as sn-2 monopalmitin from milk and formula with rearrangedtriacylglycerols and results in increased plasma triglyceride sn-2 and cholesteryl ester palmitate in piglets. J Nutr 125:73-81
- Decsi T,Koletzko B (1994) Polyunsaturated fatty acids in infant nutrition. ActaPaediatrSuppl 83: 31-37.
- GuesnetPh, Couet C, Alessandri JM, Antoine JM, Durand G, (1995)Variabilité de la teneur en acidelinoléique et du rapport 18:2n-6/18:3n-3 des lipidesdans le lait de femme en France. Ann Pédiatr 42: 282-288
- Gil A (1995)From theory to development. Symposium on nucleotides. 6th Annual Meeting of the European Society of Pediatric Allergy and Immunology 15-18.
- Leach JL, Baxter JH, Molitor BE, Ramstack MB, Masor ML (1995) Total potentially available nucleosides of human milk by stage of lactation. Am J ClinNutr 61: 1224-1230.
- Barness LA, Carver JD (1996) Nucleotides and the neonatal immune response. In Gil A, Uauy R, ed. Nutritional and biological significance of dietary nucleotides an nucleic acids. sl: Abbott Laboratories 195-205
- Thorell L,Sjöberg LB, Hernell O (1996) Nucleotides in human milk: sources and metabolism by the newborn infant. Pediatr Res 40: 845-852.
- Carver JD, Pimentel B, Cox WI, Barness LA (1991) Dietary nucleotide effects upon immune function in infants. Pediatrics 88: 359-363.
- Uauy R,Quan R, Gil A (1994) Role of nucleotides in intestinal development and repair: implications for infant nutrition. J Nutr 124: 1436S-1441S.
- Pita ML,Fernández MR, De-Lucchi C, Medina A, Martínez-Valverde A, et al. (1988) Changes in the fatty acids pattern of red blood cell phospholipids induced by type of milk, dietary nucleotide supplementation, and postnatal age in preterm infants. J PediatrGastroenterolNutr 7: 740-747.
- Björkstén B (1996) Immunological interactions between the mother and her infant in relation to the development of food allergy. Monogr Allergy 32: 16-24
- Wirt DP, Adkins LT, Palkowetz KH, Schmalstieg FC, Goldman AS (1992) Activated and memory T lymphocytes in human milk. Cytometry 13: 282-290.
- Hawkes JS, Neumann MA, Gibson RA (1999) The effect of breast feeding on lymphocyte subpopulations in healthy term infants at 6 months of age. Pediatr Res 45: 648-651.
- Ridge JP, Fuchs EJ, Matzinger P (1996) Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271: 1723-1726.
- Miles EA, Warner JA, Jones AC, Colwell BM, Bryant TN, et al. (1996) Peripheral blood mononuclear cell proliferative responses in the first year of life in babies born to allergic parents. ClinExp Allergy 26: 780-788.
- Robinson G,Volovitz B, Passwell JH (1991) Identification of a secretory IgA receptor on breast-milk macrophages: evidence for specific activation via these receptors. Pediatr Res 29: 429-434.
- Speer CP, Gahr M, Pabst MJ (1986) Phagocytosis-associated oxidative metabolism in human milk macrophages. ActaPaediatrScand 75: 444-451.
- Skansén-Saphir U,Lindfors A, Andersson U (1993) Cytokine production in mononuclear cells of human milk studied at the single-cell level. Pediatr Res 34: 213-216.
- English BK, Burchett SK, English JD, Ammann AJ, Wara DW, et al. (1988) Production of lymphotoxin and tumor necrosis factor by human neonatal mononuclear cells. Pediatr Res 24: 717-722.
- Warner JA, Jones AC, Miles EA, Colwell BM, Warner JO (1996) Maternofetal interaction and allergy. Allergy 51: 447-451.
- Lawton AR, Cooper MD (1996) Ontogeny of immunity. In Stiehm ER, ed. Immunologic disorders in infants and children, 4th ed. Philadelphia: WB Saunders Co 1-13.
- Jones CA, Cayabyab RG, Kwong KYC (1996) Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm neonates. Pediatr Res 39: 966-975
- Chheda S, Palkowitz KH, Garofalo R, Rassin DK, Goldman AS (1996) Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-a and its receptors. Pediatr Res 40: 475-486.
- Czerny A (1939) Die PädiatriemeinerZeit. Berlin: Springer.
- Jatsyk GV, Kuvaeva IB, Gribakin SG (1985) Immunological protection of the neonatal gastrointestinal tract: the importance of breast feeding. ActaPaediatrScand 74: 246-249.
- Kim K, Keller MA, Heiner DC (1992) Immunoglobulin G subclasses in human colostrum, milk and saliva. ActaPaediatr 81: 113-118.
- Sarfati M, Vanderbeeken Y, Rubio-Trujillo M, Duncan D, Delespesse G (1986) Presence of IgE suppressor factors in human colostrum. Eur J Immunol 16: 1005-1008.
- Bertotto A,Castellucci G, Radicioni M, Bartolucci M, Vaccaro R (1996) CD40 ligand expression on the surface of colostral T cells. Arch Dis Child Fetal Neonatal Ed 74: F135-136.
- Schreiber RA, Walker WA (1988) The gastrointestinal barrier: antigen uptake and perinatal immunity. Ann Allergy 61: 3-12.
- Rønneberg R,SkÅra B (1992) Essential fatty acids in human colostrum. ActaPaediatr 81: 779-783.
- Bertotto A,Castellucci G, Scalise F, Vaccaro R (1991) [Recent findings on the phenotype and function of T-lymphocytes in the human colostrum]. Pediatr Med Chir 13: 325-329.
- Murphey DK,Buescher ES (1993) Human colostrum has anti-inflammatory activity in a rat subcutaneous air pouch model of inflammation. Pediatr Res 34: 208-212.
- Buescher ES,McIlheran SM (1992) Colostral antioxidants: separation and characterization of two activities in human colostrum. J PediatrGastroenterolNutr 14: 47-56.
- Slade HB, Schwartz SA (1989) Antigen-driven immunoglobulin production by human colostral lymphocytes. Pediatr Res 25: 295-299.
- Duchén K,Björkstén B (1996) Total IgE levels in human colostrum. Pediatr Allergy Immunol 7: 44-47.
- Hanson LÅ, Ashraf RN, Hahn-Zoric M (1993) Breast milk: role in neonatal host defense. Bristol-Meyers Squibb/Mead Johnson SympNutr Res 11: 247-268
- Goldman AS,Chheda S, Garofalo R (1998) Evolution of immunologic functions of the mammary gland and the postnatal development of immunity. Pediatr Res 43: 155-162.
- Yagi H, Suzuki S, Noji T, Nagashima K, Kuroume T (1986) Epidermal growth factor in cow's milk and milk formulas. ActaPaediatrScand 75: 233-235.
- Slade HB, Schwartz SA (1987) Mucosal immunity: the immunology of breast milk. J Allergy ClinImmunol 80: 348-358.
- Revillard JP, Lafont S, Kaiserlian D (1998) Regulation of mucosal immunity: an overview with special emphasis on secretory IgA production and tolerance. Nestlé Nutr Workshop Ser 17: 35-50
- Romain N,Dandrifosse G, Jeusette F, Forget P (1992) Polyamine concentration in rat milk and food, human milk, and infant formulas. Pediatr Res 32: 58-63.
- Bines JE, Walker WA (1991) Growth factors and the development of neonatal host defense. AdvExp Med Biol 310: 31-39.
- Walker WA (1987)The role of immunologic immaturity in neonatal food allergy. In Chandra RK (ed) Food Allergy. St John's: Nutrition Research Education Foundation 45-66.
- Jalonen T, Isolauri E, Heyman M, Crain-Denoyelle A-M, Sillanaukee P, et al. (1991) Increased ß-lactoglobulin absorption during Rotavirus enteritis in infants: relationship to sugar permeability. Pediatr Res 30: 290-293.
- Uhnoo IS,Freihorst J, Riepenhoff-Talty M, Fisher JE, Ogra PL (1990) Effect of rotavirus infection and malnutrition on uptake of a dietary antigen in the intestine. Pediatr Res 27: 153-160.
- Isolauri E, Kaila M, Arvola T, Majamaa H, Rantala I, et al. (1993) Diet during rotavirus enteritis affects jejunal permeability to macromolecules in suckling rats. Pediatr Res 33: 548-553.
- Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, et al. (1999) Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ 319: 815-819.
- Cantani A,Lucenti P (1997) Natural history of soy allergy and/or intolerance in children, and clinical use of soy-protein formulas. Pediatr Allergy Immunol 8: 59-74.Cantani A,Micera M (2000) Immunogenicity of hydrolysate formulas in children (part 1). Analysis of 202 reactions. J InvestigAllergolClinImmunol 10: 261-276.
- Hasselbalch H, Engelmann MD, Ersboll AK, Jeppesen DL, Fleischer-Michaelsen K (1999) Breast-feeding influences thymic size in late infancy. Eur J Pediatr 158: 964-967.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 16173
- [From(publication date):
December-2014 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 11566
- PDF downloads : 4607