Research Article Open Access
Immunological Analysis of the Alternate Rubber Crop Taraxacum koksaghyz Indicates Multiple Proteins Cross-Reactive with Hevea brasiliensis Latex Allergens
Katrina Cornish1,2*, Wenshuang Xie1, David Kostyal3, David Shintani4 and Robert G Hamilton51Department of Horticulture and Crop Science, The Ohio State University, Wooster, USA
2Department of Food, Agricultural and Biological Engineering, Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, USA
3Akron Rubber Development Laboratory, Akron, OH 44305, USA
4College of Agriculture, Biotechnology AND# Natural Resources, University of Nevada, Reno, NV 89557, USA
5Johns Hopkins University School of Medicine, Baltimore, MD 21224, USA
- Corresponding Author:
- Katrina Cornish
Department of Horticulture and Crop Science
The Ohio State University
HCS and FABE, 1680 Madison Avenue, Wooster
Ohio 44691, USA
Tel: 330 263 3982
E-mail: cornish.19@osu.edu
Received date: October 08, 2015; Accepted date: November 17, 2015; Published date: November 24, 2015
Citation: Cornish K, Xie W, Kostyal D, Shintani D, Hamilton RG (2015) Immunological Analysis of the Alternate Rubber Crop Taraxacum kok-saghyz Indicates Multiple Proteins Cross-Reactive with Hevea brasiliensis Latex Allergens. J Biotechnol Biomater 5:207. doi:10.4172/2155-952X.1000207
Copyright: © 2015 Cornish K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The public health risk of Type I natural rubber latex allergy, caused by residual proteins in Hevea brasiliensis rubber latex (HNRL) products, has led to some medical examination and surgeon’s gloves and other health-related products being made from synthetic polymers. However, they are generally not preferred by healthcare providers due to their physical limitations. Guayule latex (GNRL), from an alternate rubber crop Parthenium argentatum, has been proven to contain none of the protein antigens present in H. brasiliensis natural rubber latex and its products. Guayule latex has also exhibited excellent film properties. Another alternate rubber crop, Taraxacum kok-saghyz, is now under commercial development, and internet reports assume that, like guayule, its latex derived rubber is free of proteins that are cross-reactive with HNRL specific IgE antibodies. Thus, the assumption is that it will not trigger allergic reactions in Type I HNR latex allergic individuals. Using ELISA and immunoblot methods, we have tested the reactivity of HNRL protein specific murine monoclonal and polyclonal antibodies, rabbit polyclonal IgG antibodies and human IgE antibodies from clinically HNRL allergic individuals against T. kok-saghyz latex and its purified rubber particles. We demonstrate that T. kok-saghyz latex contains multiple HNRL cross-reactive proteins, which importantly react with HNRL latex specific human IgE antibodies from Type I latex allergic individuals. Exposure of HNRL allergic individuals to T. kok-saghyz latex may thus place them at risk for allergic reactions.
Keywords
Type I latex allergy; Dandelion; Guayule; Taraxacum koksaghyz; Epitopes; Alternate rubber
Introduction
A quantity of at least 1.2 million MT/yr of irreplaceable natural rubber (defined as a strategic raw material) is currently required by the United States for its military, industrial, transportation, medical and consumer manufacturing sectors. There are over 40,000 different products made with natural rubber and over 400 medical devices [1].
Globally, almost all commercial natural rubber (>11 million MT/ yr) is collected from a single source, Hevea brasiliensis, the Brazilian or para rubber tree. About 89% is converted to solid rubber of various grades. The remainder is concentrated into a stabilized form of latex with approximately 60% rubber content. However, the use of a single species of natural rubber plant to generate the global supply of this strategic commodity is not necessary, because many different plants [1] make natural rubber, as do some Lactarius sp. fungi [2-4]. Of the many plants capable of natural rubber production, two temperate species stand out as commercial candidates, Parthenium argentatum Gray (guayule) and Taraxacum kok-sahgyz Rodin (Buckeye Gold, also known as Kazak dandelion, Russian dandelion and TKS). These alternate rubber species are under development for commercial use at a number of universities and companies on several continents. Both species make high quality rubber. T. kok-saghyz rubber is similar to H. brasiliensis rubber in composition and performance [5]. In contrast, guayule rubber is naturally softer and more elastic than the other two [6,7].
The highest value market for natural rubber latex is the medical arena, with medical examination and surgeon’s gloves being the largest consumer product type made of latex [8]. Unfortunately, a processing change in the 1980’s left high levels of soluble protein in the Hevea latex glove matrix which led to a large number of people becoming exposed and sensitized (IgE antibody positive) with subsequent manifestations of Type I hypersensitivity referred to as natural rubber latex allergy [9-13]. HNRL proteins in products elicited high levels of IgE anti-HNR latex protein that when inhaled or adsorbed caused the release of vasoactive mediators from mast cells and basophils following HNRL allergen exposure. The clinical evidence that an individual had become “sensitized” was upper airway rhinitis and lower airway related asthma. Some individuals who were highly sensitized to HNRL proteins experienced life-threatening allergic symptoms involving anaphylaxis following an airborne or contact exposure to HNR latex allergens. This proved fatal in a number of high profile cases [13-17]. Most manufacturers now ensure that their latex gloves and other medical products are thoroughly leached and a lower number of new cases of Hevea latex allergy are now reported. The incidence of new cases in most developed countries has decreased dramatically in recent years as a result of effective avoidance tactics and lower allergenic content in rubber products [12,18]. Natural rubber latex, however, remains the best and most suitable protective material for high performance medical gloves during surgery [18].
Guayule (Parthenium argentatum) latex (GNRL) is much lower in protein than HNRL, and it has no protein epitopes which cross-react with anti-HNRL protein specific antibodies [8]. A three year study of occupationally exposed workers found no evidence of sensitization [19]. HNRL allergic people can use dipped medical and consumer products made from GNRL, without exhibiting any allergy symptoms. This guayule-specific property has led to a widely disseminated assumption that other alternate rubber-producing species also will not contain proteins with epitopes which cross-react with anti-HNR latex protein antibodies. In this paper, we scientifically address the question of whether or not latex from T. kok-saghyz is free of allergenic proteins implicated in Type I HNRL allergy. This supposition is claimed in numerous internet listings, promoting use of T. kok-saghyz latex and rubber as a safer alternative to HNR latex, like GNRL, rather than as a supplement or addition to the H. brasiliensis rubber and latex supply. However, no scientific data support these assumptions.
Methods
Latex was harvested from the roots of T. kok-saghyzand either mixed with sample buffer, or collected in sufficient quantities to prepare 3x washed rubber particles (WRP). For WRP, T. kok-saghyz latex was collected from its roots into a collection buffer of 100 mM Tris-HCI, pH 7.5, 5 mM Mg2SO4 buffer. Washed rubber particles (WRP) were prepared from T. kok-saghyz latex as described previously [20]. -H. brasiliensis latex (from clone RIMM600) was obtained from a plantation in Malaysia and WRP also were prepared [20]. Latex and WRP of each species were mixed with 1x NuPAGE SDS sample buffer, with 50 mM dithiothreitol (DTT), vortexed, denatured for 5 min at 100ºC. The samples were vortexed, then spun for 3 min to remove rubber, and these two steps were repeated twice more. Proteins were quantified using the modified Lowry method. The denatured proteins were loaded onto NuPAGE 4-12% Bis-Tris gels and run in a buffer of 50 mM MES, 50 mM Tris base, 0.1% SDS, 1 mM EDTA, pH 7.3 for 35 min at 200V. Latex and WRP proteins latex and WRPs proteins were loaded onto the gels in the amount of 0.5 μg and 14 μg for silver stain and Coomassie Blue, respectively [20]. Similar gels were transferred onto PVDF membranes and reacted with various antibodies (Table 1).
| Protein | Animal | Anitibody | ASTM or lot # | # Samples |
| Total Hevea latex protein | Rabbit | Polyclonal IgG | D6499 | multiple |
| ditto | Mouse | Polyclonal IgG | none | few |
| Hev b1 | Mouse | Monoclonal (58/14.6 kD) | D7427 | n/a |
| Hev b3 | Mouse | Monoclonal (24-27 kD) | D7427 | n/a |
| Hev b5 | Mouse | Monoclonal (16 kDa) | D7427 | n/a |
| Hev b6.02 | Mouse | Monoclonal (4.7 kDa) | D7427 | n/a |
| Glove | Adults,HCW, spina bifida | Polyclonal IgG | F8135 | 101 |
| Glove | Children with spina bifida | Polyclonal IgG | F8137 | 53 |
Table 1: Summary of the different immunochemical antibodies used to test against T. kok-saghyz latex and WRP proteins.
Each gel was blotted to Immobilon-PVDF transfer membrane in a transfer buffer of 25 mM bicine, 25 mM Bis-Tris, 1 mM EDTA, 0.05 mM chlorobutanol (pH 7.2), 10% methanol for 60 min at 30V. The blots were then blocked for 30 min in 5% non-fat milk in 1xPBS/0.05% Tween-20. The blots were incubated with purified animal antibodies (mouse monoclonal antibodies, or rabbit IgG ASTM D6499, IRM# 914), or two distinct human serum pools: IgE anti--H. brasiliensis latex serum pool (n=53 children only with spina bifida, 1-16 yrs old, lot F8137); and serum from a mixed surgical pediatric and adult healthcare worker population containing IgE anti--H. brasiliensis latex (n=101, lot F8135). The antibodies used for the immunoblots blots were diluted at 1:20,000 (anti-AL); 1:500 (Hev b1, b3, b5, b6.02); 1:333 (F8137 and F8135); 1:15,000 (anti-rabbit IgG) and 1:15,000 (anti-Human IgE). All samples were incubated for 1 hr with shaking in 1xPBS/0.05% Tween-20, subsequently washed for three times for 10 min each in 1xPBS/0.05% Tween-20. The blots were then incubated with an anti- Human IgE peroxidase conjugate (Sigma-Aldrich) (1:15,000) and Bio-Rad Precision protein Streptactin-HRP conjugate (1:15,000) for 1 hr with shaking in 1xPBS/0.05% Tween-20 and then washed. The blots were then detected by Luminata Forte western HRP substrate (Millipore) and visualized by chemiluminescence imaging with Bio- Rad chemiDoc XRS.
For ELISA analysis, proteins were extracted from the T. koksaghyz latex following the procedure outlined in the ASTM D1076 Standard. A 500 μL sample of the T. kok-saghyz latex was mixed with 450 μL extraction buffer (50 mM Na2PO4 pH 7.4) and 50 μL 20% SDS with agitation for 2 h at room temperature. The samples were then centrifuged for 15 minutes at 21,000 x g and the clarified aqueous phase removed. Total protein content was determined according to the ASTM D5712 procedure both with and without background subtraction.
The ASTM D6499-12 ELISA Inhibition Assay was used to determine the amount of cross-reactive protein. The standard and test samples were serially diluted in a 96 well plate, after which an equal volume of diluted rabbit anti-HNRL polyclonal antibody was added. After a 2 h incubation, the sample from each well was transferred to the corresponding well in a HNRL-protein-coated plate which had been blocked with non-fat dry milk. After another 2h incubation, the plates were then washed with buffer and a 100 μl solution of Goat anti-Rabbit IgG-HRP was added. After a final incubation, plates were washed and the substrate O-phenylenediamine was added to each well. Color was allowed to develop until the reaction was stopped by the addition of H2SO4. The plates were then read at 490 nm and protein values were determined by interpolation from a reference calibration curve.
Results and Discussion
Protein concentration of T. kok-saghyz fractions
The total protein content of the T. kok-saghyzlatex and WRP fractions (Table 2) were determined on the clarified aqueous fraction. The more refined WRP clarified aqueous fraction was colorless while the crude latex fraction was brown in color. This pigmented material may be the reason for the higher background values seen in the crude latex fraction (Table 2). This pigmented crude T. kok-saghyz-latex material was assayed using the method in the ASTM D6499 ELISA which was developed to specifically detect HNRL proteins. The protein concentration of the extract varied between 32 and 55 μg/mL. This variation is likely due to the large dilution factors used as well as possible interference from those materials observed to interfere with the D5712 protein quantification test. Further purification of this material may be necessary to obtain more consistent results.
| Sample | 5712 with bs(µg/mL) | 5712 w/o bs(µg/mL) |
|---|---|---|
| Latex | 293 | 1,891 |
| WRP | 785 | 1,398 |
Table 2: ASTM D5712 protein concentrations of T. kok-saghyz fractions with and without background subtraction (bs).
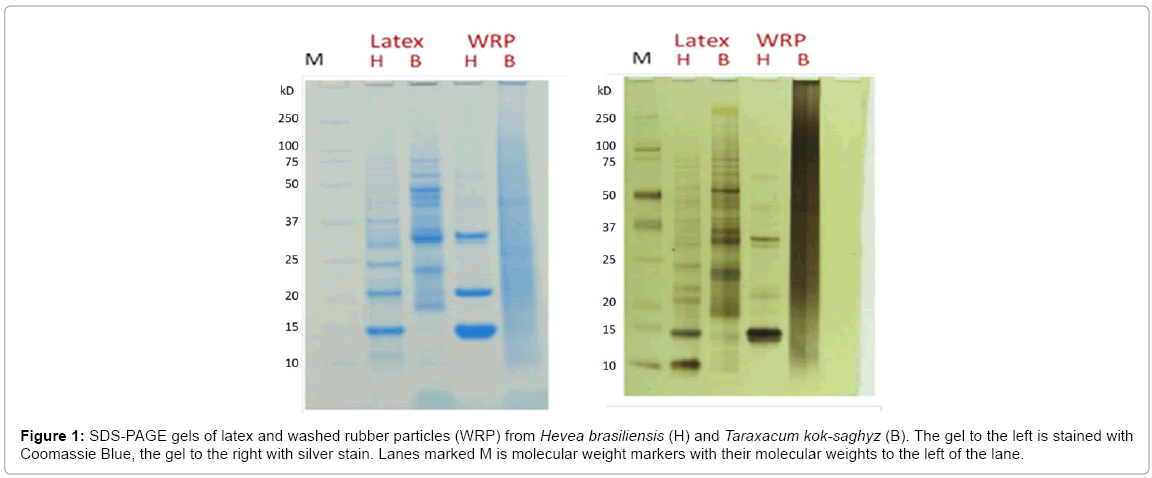
T. kok-saghyz latex and WRP contained similar amounts of protein to H. brasiliensis, and many proteins of different molecular weights are apparent in both their latex and rubber particles (Figure 1). In both gels, the staining was halted when any single lane verged on becoming overstained, and so not all proteins present were visualized. The proteins in the T. kok-saghyz WRP lanes are smeared, which suggests that some hydrolysis has occurred in this particular protein preparation. The HNRL antigenic protein ELISA (ASTM D6499) was used to evaluate protein preparations similar to those visualized in Figure 1.
Figure 1: SDS-PAGE gels of latex and washed rubber particles (WRP) from Hevea brasiliensis (H) and Taraxacum kok-saghyz (B). The gel to the left is stained with Coomassie Blue, the gel to the right with silver stain. Lanes marked M is molecular weight markers with their molecular weights to the left of the lane.
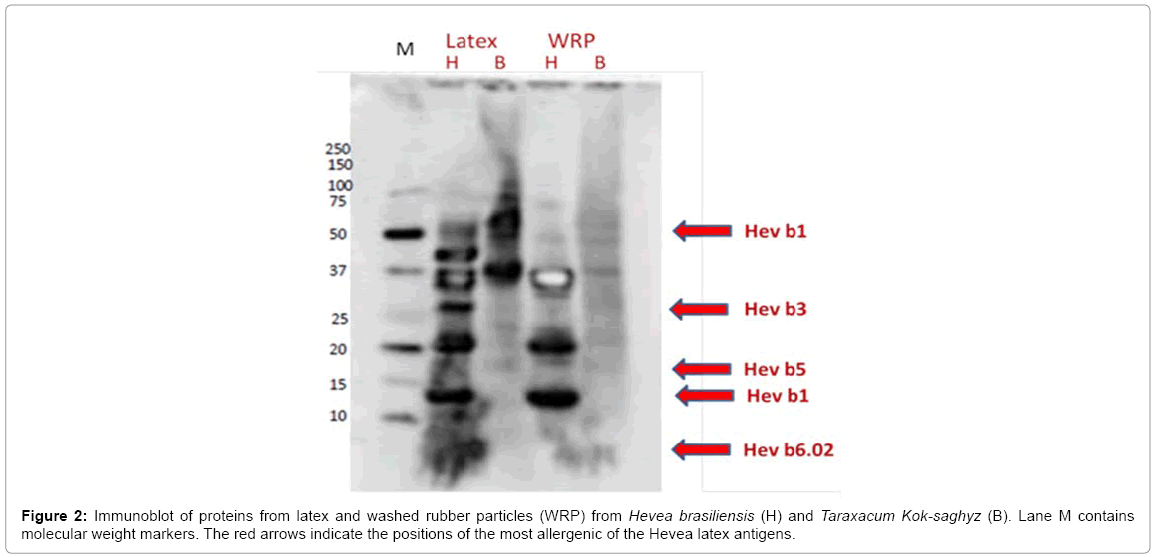
The rabbit anti-HNRL total protein IgG polyclonal antibodies (ASTM D6499, IRM# 914) reacted with H. brasiliensis and T. koksaghyz proteins blotted onto nitrocellulose membranes from the gels (Figure 2).
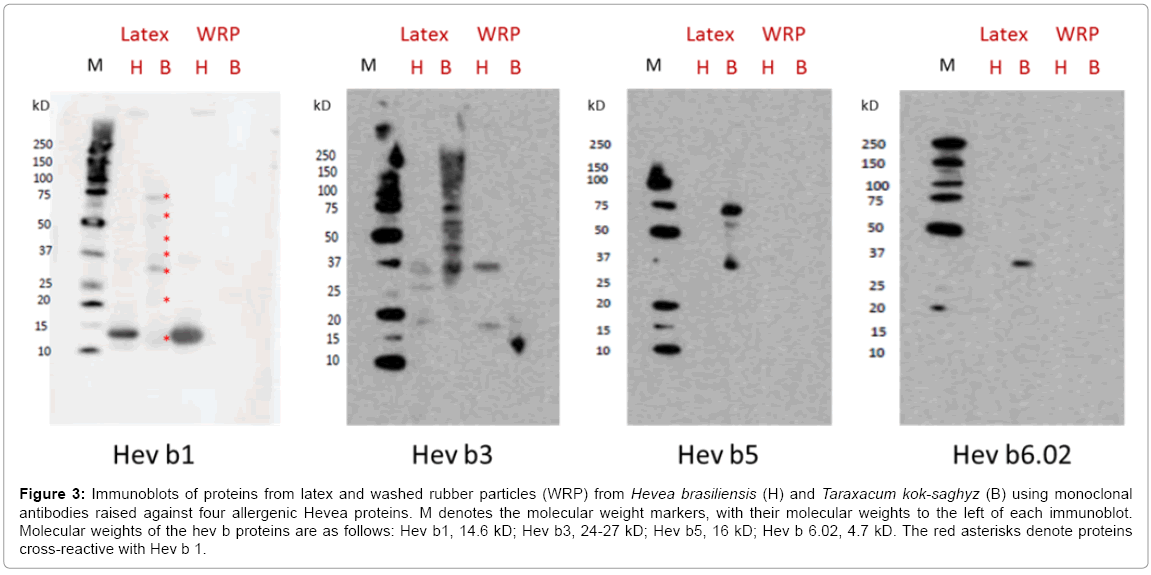
Multiple proteins from H. brasiliensis latex were recognized by the polyclonal antibodies, indicating that these were antigenic in rabbits. As has been seen previously [18,21], a number of immunogenic proteins (Hev b1 and Hev b3, for example) are attached to the H. brasiliensis rubber particles and are not solely detected in the soluble fraction of the cytosol (the C-serum). Similar results were obtained with mouse anti- HNRL latex protein polyclonal antibodies (immunoblot not shown). Of the many H. brasiliensis immunogenic latex proteins, 15 have been identified as being clinically significant in human Type I latex allergy [22]. Monoclonal antibodies were raised against recombinant versions of these allergenic proteins and four of them are included in the FIT kit (Icosagen). ASTM D7427 describes a standard ELISA method to uses four of these antibodies to assess cross-reactivity. These antibodies were used to identify cross-reactive T. kok-saghyz latex and WRP proteins in immunoblots in direct comparison with H. brasiliensis latex and WRP proteins (Figure 3). Hev b1 is the 14.6 kD “rubber elongation factor” and is clearly seen in both the H. brasiliensis latex and WRP. This antibody also recognizes structurally-related proteins, including one at 58kD.
Figure 3: Immunoblots of proteins from latex and washed rubber particles (WRP) from Hevea brasiliensis (H) and Taraxacum kok-saghyz (B) using monoclonal antibodies raised against four allergenic Hevea proteins. M denotes the molecular weight markers, with their molecular weights to the left of each immunoblot. Molecular weights of the hev b proteins are as follows: Hev b1, 14.6 kD; Hev b3, 24-27 kD; Hev b5, 16 kD; Hev b 6.02, 4.7 kD. The red asterisks denote proteins cross-reactive with Hev b 1.
The Hev b1 specific antibody clearly cross-reacts with at least seven proteins in T. kok-saghyz latex, although no proteins were visualized for the WRP preparation (Figure 3). Hev b1 is the H. brasiliensis 14.6 kD REF (“rubber elongation factor”) protein, which often forms higher molecular weight aggregates, and shares sequence similarity with Hev b3, and a soluble CPT (cis-prenyl transferase, 37 kD). CPT also quite strongly associates with the rubber particle surface through hydrophobic interactions, but can be washed away without losing particle-bound rubber transferase activity. Multimers of CPT are also sometimes seen on SDS-PAGE gels. Hev b3 is the 24-27 kD SRPP “small rubber particle protein” and cross-reactive proteins to its monoclonal antibodies are detected in the H. brasiliensis latexand WRP, and in the T. kok-saghyz latex. This antibody cross-reacts with larger and smaller molecular weight proteins in both latex and WRP in H. brasiliensis, including with the 37 kD CPT. We hypothesize that the sequence similarity of these proteins also leads to epitopic similarity in the translated proteins. However, in T. kok-saghyz, multiple latex proteins contain epitopes which are recognized by the Hev b3 specific monoclonal antibody. It seems likely that some of these are multimers or aggregates of SRPP and or CPT. Hev b5 is the 16 kD acidic protein and although the immunoblot was not sufficiently sensitive to visualize this in the H. brasiliensis latex lane, the monoclonal antibodies to this protein quite strongly cross-reacted with three proteins in T. kok-saghyz latex (specific identities not known at this time), suggesting that these proteins might pose a risk of triggering allergic reaction in latex-allergic patients. A similar issue arises with the anti Hev b6.02 immunoblot (Figure 3). Although Hev b6.02 is a very small protein, the 4.7 kDa antifungal hevein, cross-reactive epitopes of at least three other proteins in T. kok-saghyz latex were visualized, especially a protein of about 30 kD. The much fainter higher molecular weight bands suggest aggregates or multimers of this protein. However, hevein is a protein derived from prohevein, with is 21.9 kD. It seems possible that the cross-reactive T. kok-saghyz 30 kD latex protein could be related to this larger progenitor.
Clearly, significant cross-reactivity was detected between T. koksaghyz proteins and anti-HNRL antibodies, in both the soluble protein fraction and the rubber particle bound fractions. However, since these results were obtained with rabbit polyclonal and mouse monoclonal antibodies, the potential impact on humans, specifically Type I latex allergic patients, is only inferred.
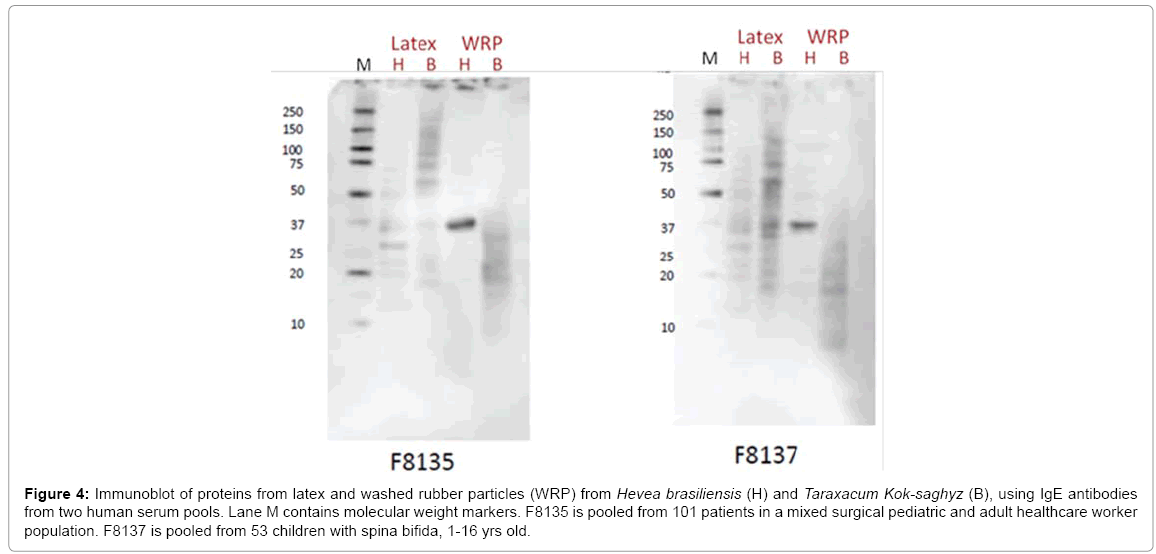
We directly examined this issue, by using human IgE antibodies from two groups of Type I HNRL allergic patients. One group consisted of a mixture of children sensitized during surgery and HNRL allergic healthcare workers while the other consisted strictly of spina bifida patients. The IgE from both groups cross-reacted with multiple proteins in T. kok-saghyz latex and WRP (Figure 4). Interestingly, the most predominant reactive protein in H. brasiliensis latex is around 37 kD in size, and so is likely to be CPT. This protein is not yet implicated in clinically symptomatic Type I latex allergy, but seems quite clearly to not be large enough to be the Hev b13, 43 kD esterase – the closest in size on the allergen list.
Figure 4: Immunoblot of proteins from latex and washed rubber particles (WRP) from Hevea brasiliensis (H) and Taraxacum Kok-saghyz (B), using IgE antibodies from two human serum pools. Lane M contains molecular weight markers. F8135 is pooled from 101 patients in a mixed surgical pediatric and adult healthcare worker population. F8137 is pooled from 53 children with spina bifida, 1-16 yrs old.
It should be noted, that even if we do not fully understand the basis for the observed cross-reactions, the fact that they exist at all, may pose a significant of allergic reaction in Type I latex-sensitized humans.
The presence of cross-reactive proteins does not mean that T. kok-saghyz latex and rubber cannot be used effectively in commercial products. They should just be used in the same way that H. brasiliensis latex and rubber products are currently used. Moreover, most of the rubber in soil-grown T. kok-saghyz roots has been irreversibly coagulated before harvest, and is not in latex form [23]. It thus seems unlikely that latex production from such plants will present a viable commercial opportunity to serve as a substitute for Hevea latex, and that efforts should concentrate on solid rubber applications.
Conclusion
Rubber and latex from T. kok-saghyz contain multiple proteins which share epitopes with antigenic and allergenic proteins from H. brasiliensis latex. This does not mean that the latex and rubber cannot be used commercially. The application of good manufacturing practices currently in use, should serve to keep protein levels low and prevent products from sensitizing people. However, as is the case for products made with H. brasiliensis, latex and rubber, the proteins in T. kok-saghyz products pose a risk for inducing allergic reactions in previously Hevea latex sensitized patients. Thus, while T. kok-saghyz rubber can substitute for H. brasiliensis rubber, it is not a Hevea latex allergy-safe alternative, a position still uniquely held by guayule.
Acknowledgements
We thank Abdul Ghaffar Muhammad Akbar for providing the H. brasiliensis latex used in this work. This research is partly supported by the USDA National Institute of Food and Agriculture, Hatch project 230837. This research is integral to the TIRE program within the Center for Applied Plant Sciences (CAPS), and to the Program of Excellence in Natural Rubber Alternatives (PENRA).
References
- Mooibroek H, Cornish K (2000) Alternative sources of natural rubber. ApplMicrobiolBiotechnol 53: 355-365.
- Mekkriengkrai D, Sando T, Hirooka K, Sakdapipanich J, Tanaka Y, et al. (2004) Cloning and characterization of farnesyldiphosphate synthase from the rubber-producing mushroom Lactariuschrysorrheus. BiosciBiotechnolBiochem 68: 2360-2368.
- Norimasa Ohya, Junko Takizawa, Seiichi Kawahara, Yasuyuki Tanaka (1998) Molecular weight distribution of polyisoprene from Lactariusvolemus. Phytochemistry 48: 781-786.
- Norimasa Ohya, Yasuyuki Tanaka, Kyozo Ogura, Tanetoshi Koyama (1997)Isopentenyldiphosphateisomerase activity in Lactarius mushrooms. Phytochemistry 46: 1115-1118.
- Katrina Cornish, Crittenden J. Ohlemacher, WenshuangXie, David Kostyal, David Shintani, et al. (2012) Alternative natural rubber sources to Hevea natural rubber – quality and allergenicity. Proceedings of the 181th Technical Meeting of the Rubber Division of the American Chemical Society, Cincinnati, Ohio.
- Nguyen KC, Williams JL, Wavrin JL, Cornish K (2008) Effect of the cure temperature, time, and film thickness on Yulex latex. Proceedings 11th International Latex Conference, Independence, Ohio.
- Katrina Cornish, Jali Williams, Julie L Hall, Raymond G McCoy III (2008) Production and Properties of Yulex (R) - the Natural Solution to Latex Allergy. Rubber Chemistry and Technology 81: 709-722.
- Cornish K (2012) Assessment of the risk of Type I latex allergy sensitization or reaction during use of products made from latex derived from guayule and other alternate rubber-producing species. Rubber Science 25: 139-155.
- Slater JE, Chhabra SK (1992) Latex antigens. J Allergy ClinImmunol 89: 673-678.
- Sussman GL, Beezhold DH, Kurup VP (2002) Allergens and natural rubber proteins. J Allergy ClinImmunol 110: S33-39.
- Turjanmaa K, Kanto M, Kautiainen H, Reunala T, Palosuo T (2002) Long-term outcome of 160 adult patients with natural rubber latex allergy. J Allergy ClinImmunol 110: S70-74.
- Yeang HY (2004) Natural rubber latex allergens: new developments.CurrOpin Allergy ClinImmunol 4: 99-104.
- Yunginger JW (2003) Natural Rubber Latex Allergy. In: Middleton’s Allergy Principles and Practice. Mosby.
- Liss GM, Sussman GL, Deal K, Brown S, Cividino M, et al. (1997) Latex allergy: epidemiological study of 1351 hospital workers. Occup Environ Med 54: 335-342.
- Merritt TG, J Merritt, RGO Kekwick (1995) Prevalence of latex specific IgE antibodies in the UK. Ann Allergy Asthma Immunol 2:74-50.
- Sussman GL, Beezhold DH, Liss G (2002) Latex allergy: historical perspective. Methods 27: 3-9.
- Long OE, Yip E, Fah LP (1995) Residual extractable proteins and allergenicity of natural rubber products. Proc. Int. Conf. on Latex Protein Allergy: the latest position. Paris, France, 1995.
- Palosuo T, Antoniadou I, Gottrup F, Phillips P (2011) Latex medical gloves: time for a reappraisal. Int Arch Allergy Immunol 156: 234-246.
- Hamilton RG, Cornish K (2010) Immunogenicity studies of guayule and guayule latex in occupationally exposed workers. Industrial Crops and Products 31: 197-201.
- Cornish K, Xie W (2012) Natural rubber biosynthesis in plants: rubber transferase. Methods Enzymol 515: 63-82.
- Siler DJ, Cornish K, Hamilton RG (1996) Absence of cross-reactivity of IgE antibodies from subjects allergic to Heveabrasiliensis latex with a new source of natural rubber latex from guayule (Partheniumargentatum). J Allergy ClinImmunol98: 895-902.
- Raulf M (2015) Latex allergy - allergens, diagnosis, management. In: Handbook of Molecular Allergology. Matricardi P, Kleine-Tebbe J, Ollert M (Eds.), European Academy of Asthma, Allergy Immunology.
- Cornish K, Bates GM, McNulty SK, Kopicky SE, Grewal S, et al. (2013) Buckeye Gold storage: A study into rubber production in Taraxacumkok-saghyzwith an emphasis on post-harvest storage.USATire Technology Intern ational 10: 36-38.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 11883
- [From(publication date):
December-2015 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 10832
- PDF downloads : 1051