Immunohistochemical Expression of Androgen and Estrogen Receptors
Received: 12-Oct-2018 / Accepted Date: 30-Oct-2018 / Published Date: 09-Nov-2018 DOI: 10.4172/2476-2024.100014
Abstract
Urothelial carcinoma is the most common malignancy affecting the urinary tract. It is at least three times more common in males than females suggesting the role of sex hormones in initiation and progression of bladder cancer. The purpose of this study is to detect the immunohistochemical expression of androgen receptors (AR) and estrogen receptor-β (ERβ) in urothelial carcinoma and correlate their expression with the known prognostic parameters of urothelial carcinoma. Seventy cases of urothelial carcinoma from radical cystectomy (15 specimens) and transurethral resection of the tumor (TURT) (55 specimens) were collected retrospectively. They were stained for AR and ERβ. The relationship between their expression and the available clinicopathological features were evaluated. AR/ERβ was positive in 62.9%/52.9% respectively of the studied cases. Significantly lower expression of AR/higher expression of ERβ were found in high-grade tumors (52.4%/66.7% respectively) and with muscle-invasive tumors (48.4%/71% respectively). AR and ERβ expression were significantly correlated with the tumor grade and degree of invasion suggesting the suitability of AR and ERβ as prognostic markers of urothelial carcinoma. High AR expression was associated with favorable prognosis of urothelial carcinoma in contrast to high ERβ expression which was associated with bad prognosis of urothelial carcinoma.
Keywords: Androgen; Estrogen; Receptor; Bladder cancer; Urothelial; Immunohistochemistry; Prognosis
Introduction
Bladder cancer (BC) is the most common malignancy in the urinary tract and urothelial carcinoma (UC) is the predominant histological type. Urothelial carcinoma is responsible for death of 165000 persons annually worldwide according to the International Agency for Research on Cancer and the World Health Organization, with the highest mortality rates among Egyptian males [1]. It is more common in middle and old age [2]. Females presented with the disease at older age than males [3]. Urothelial carcinoma is the 4th most common cancer in men and the 9th in women worldwide [4]. It is at least three times more common in men than women [1]. Excessive exposure to carcinogens, e.g. cigarette smoke and industrial chemicals, has been suggested to be a cause of higher incidence of bladder cancer in males [5]. Even, after controlling these carcinogens, men still have a higher risk than women [6].
In the light of the previous facts, existence of a relationship between sex hormones and cancer bladder is suggested. Data from animal and human studies suggested that sex hormones have important physiological effects on the lower urinary tract [7]. Sex steroids act by binding to their receptors in target cells including androgen receptors (ARs) and estrogen receptors (ERs) [8]. These receptors have been detected in normal bladder urothelium [9]. The AR and ER signaling pathways affect bladder cancer development and progression [8]. Bladder cancer management has remained essentially unchanged, with no new effective treatment options approved in the past few decades [10]. Treatment of non-muscle invasive bladder cancer involves transurethral resection of the tumor followed by intravesical chemo- or immunotherapy but muscle-invasive cancer requires radical cystectomy and systemic chemotherapy [11]. Many patients receiving BCG develop either local or systemic side effects [10]. The effects of sex hormones on bladder cancer cells need to be studied, which might help in development of prognostic biomarkers and new therapies. The purpose of this study is to detect the expression of (AR) and (ERβ) in urothelial carcinoma and correlate their expression with the known prognostic parameters of urothelial carcinoma to illustrate their prognostic and possible therapeutic roles. Multiple evidence have shown that CSC in PDAC can be detected by specific markers, including CD24, CD44, CD133, CXCR4, epithelial cell adhesion molecule (EpCAM; epithelial-specific antigen), nestin, and combinations of these markers [5-8]. However, definitive CSC markers for PDAC and their clinical relevance are still controversial.
CSC exhibit resistance to standard cytotoxic drugs due to many causes as it may remain in a quiescent state. Also, the group of CD133+CXCR4+ CSCs is related to tumor metastasis in pancreatic cancer. Therefore, the isolation and characterization of CSC from PDAC suggested this type of cells is to be blamed for the failure of classical therapy in pancreatic tumors [4].
There is substantial evidence that PDAC does not arises de novo , but rather progresses through a multistep model involving noninvasive precursor lesion known as pancreatic intraepithelial neoplasias (PanINs), and culminating in invasive cancer [9]. However, the relationship between CSCs and PanIN lesions remains unclear.
Materials and Methods
This study was carried out on 70 cases of urothelial carcinoma of the urinary bladder (56 males and 14 females, age ranged between 28 and 88 years). These cases were collected retrospectively from the archives of Pathology Department, Faculty of Medicine, Tanta University during the period of the research from 2015 to 2017 and patients data were obtained from the records. Approval from research ethics committee (REC), Faculty of Medicine, Tanta University, was taken antecedent to conducting study. Tissue specimens were in the form of radical cystectomy (15 specimens) and transurethral resection of the tumor (TURT) (55 specimens). After histopathological evaluation, Tumors were graded according to the WHO 2016 of urothelial neoplasia, and they were classified as low grade and high grade urothelial carcinoma. Tumors were staged according to American Joint Committee (AJCC), TNM pathologic staging of urinary bladder [8]. Immunohistochemical staining was performed on 10% formalin fixed, paraffin embedded tissue blocks for evaluation of AR and ERβ expression. Sections were immunohistochemically labeled, using primary antibodies to AR (EPR3778 clone, rabbit monoclonal antibody, 0.1 ml, dilution 1:50; Dako, Egypt) and ERβ (AR441 clone, mouse monoclonal antibody, 0.1 ml, dilution 1:300; Abcam, Egypt). AR and ERβ staining was detected as brownish nuclear staining and all these stains were manually scored by the German Immunoreactive Score based on multiplying percentage of immunoreactive cells {0%=(0), 1-10%=(+1), 11-50%=(+2), 51-80%=(+3), 81-100%=(+4)} by staining intensity {Negative (+0), weak (+1), moderate (+2), strong (+3)}. Scores (range 0-12) were considered negative (0; 0-1), weakly positive (+1; 2-4), moderately positive (+2; 6-8) and strongly positive (+3; 9-12) [8]. Chi-square test and Spearman’s correlation coefficient test were used as tests of significance to evaluate the association between categorized variables and p-value <0.05 was considered statistically significant.
Results
Clinicopathological criteria of the studied cases
Most of the patients were more than 50 years old (81.4%), most of them were male (80%).most of the tumors were less than 3 cm (78.6%) with occasional more than one mass is detected (48.6% of the cases were multiple masses). Grading, staging, muscle invasion, nodal metastasis, lymphovascular and prineural invasion were evaluated (Table 1).
| Clinicopathological features | No. | % |
|---|---|---|
| Age | ||
| <50 | 13 | 18.6 |
| ≥ 50 | 57 | 81.4 |
| Gender | ||
| Male | 56 | 80 |
| Female | 14 | 20 |
| Size | ||
| <3cm | 55 | 78.6 |
| ≥ 3cm | 15 | 21.4 |
| Multiplicity | ||
| Single | 36 | 51.4 |
| Multiple | 34 | 48.6 |
| Grade | ||
| Low | 28 | 40 |
| High | 42 | 60 |
| Degree of muscle invasion | ||
| NMI (Ta,T1) | 39 | 55.7 |
| MI (T2,T3,T4) | 31 | 44.3 |
| Level of invasion in cystectomy specimens (n=15) | ||
| pT2a | 1 | 6.7 |
| pT2b | 6 | 40 |
| pT3a | 2 | 13.3 |
| pT3b | 1 | 6.7 |
| pT4a | 4 | 26.6 |
| pT4b | 1 | 6. 7 |
| LN metastasis at cystectomy specimens (n=15) | ||
| Without | 11 | 73.3 |
| With | 4 | 26.7 |
| Perineural invasion | ||
| Without | 61 | 12.9 |
| With | 9 | 87.1 |
| Lympho-vascular invasion | ||
| Without | 62 | 88.6 |
| With | 8 | 11.4 |
Table 1: Clinicopathological classification of the studied cases.
AR and ERβ expression and their correlation with the clinicopathological characteristics
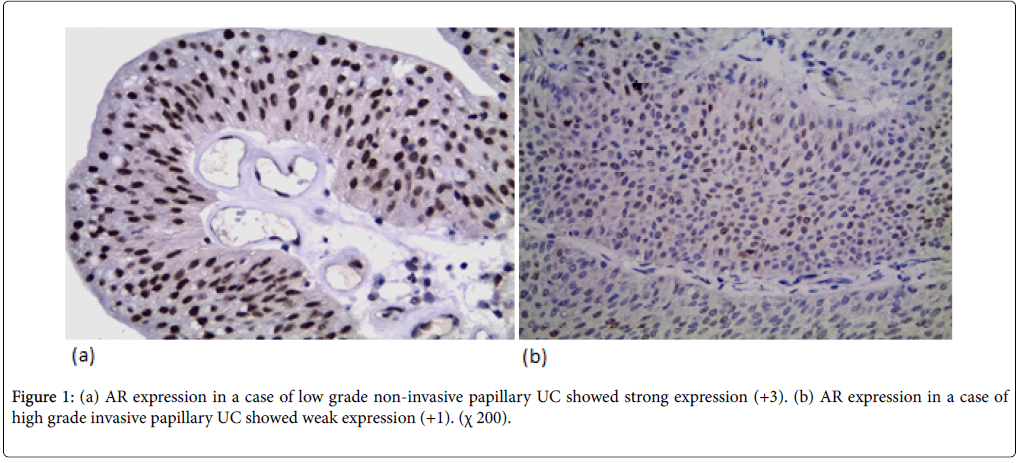
AR expression was detected in 44 cases (62.9%) (12 weak, 18 moderate, 14 strong). AR expression was not significantly correlated with the age and gender but there was a significant correlation between AR expression and tumor size (p-value=0.039). AR expression was not significantly correlated with the number of urinary bladder masses. AR expression was significantly correlated to tumor grade (p-value=0.026). AR expression was higher in low grade cases (78.5%) (Figure 1a) than high-grade cases (52.4%) (Figure 1b). AR expression was significantly correlated with degree of muscle invasion (p-value=0.026) with higher AR expression in NMI tumors (pTa, pT1) (74.4%) in contrast to only 48.4% of MI (pT2, pT3, pT4). In cystectomy specimens, there was a significant association between AR expression and the level of invasion (p-value=0.046) with negative correlation coefficient (rs) denoting loss of AR expression in more invasive tumors. AR expression wasn’t significantly correlated with lymph node metastasis in cystectomy specimens. There was a significant correlation between AR expression and perineural invasion (p-value=0.049). AR expression wasn’t significantly correlated with lympho-vascular invasion.
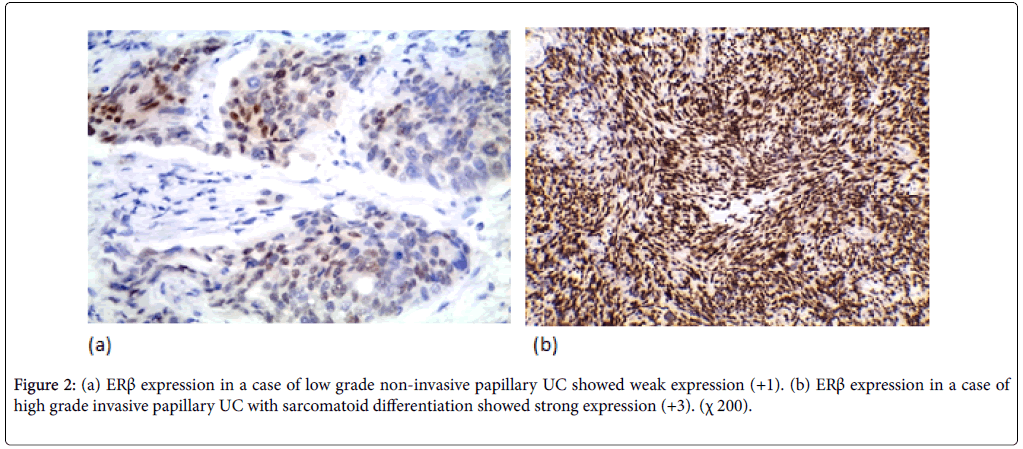
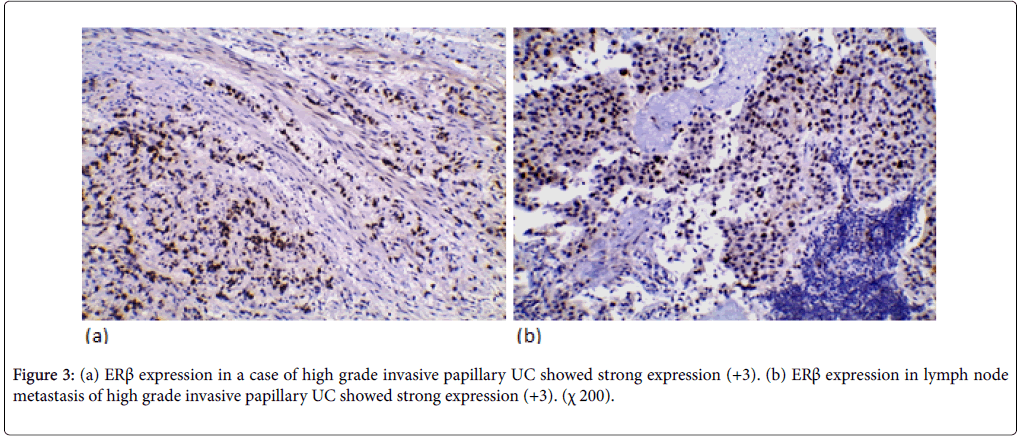
ERβ expression was positive in 37 cases (52.9%) (11 weak, 20 moderate, 6 strong). ERβ expression wasn’t significantly correlated with the age and gender. ERβ expression was significantly correlated with tumor size and multiplicity (p-value=0.018, 0.016 respectively). ERβ expression was significantly correlated to the tumor grade in the studied cases (p-value=0.005). Higher positive ERβ in high-grade cases (66.7%) than low-grade cases (32.1%) (Figure 2). ERβ expression was significantly correlated with degree of muscle invasion (p-value=0.007) with lower ERβ expression in NMI tumors (38.5%) opposite to 71% of MI (Figure 3a). In cystectomy specimens, there was a significant association between ERβ expression and invasion level (p-value=0.033) with positive correlation coefficient (rs) denoting high ERβ expression in more invasive tumors. In cystectomy specimens with lymph node metastasis, three cases (75%) showed a positive expression, but it did not reach the statistically significant level (p-value=0.929) (Figure 3b). ERβ expression was significantly correlated with perineural and lympho-vascular invasion (P-value=0.033, 0.041 respectively) (Table 2).
| Variables | AR | ERβ | ||||
|---|---|---|---|---|---|---|
| - ve (n=26) | + ve (n=44) | p-value | - ve (n=33) | + ve (n=37) | p-value | |
| Age | ||||||
| < 50 (13) | 6 (46.2%) | 7 (53.8%) | 0.456 | 6 (46.1%) | 7 (53.9%) | 0.529 |
| ≥ 50 (57) | 20 (35.1%) | 37 (64.9%) | 31 (54.4%) | 26 (45.6%) | ||
| Gender | ||||||
| Male (56) | 20 (35.7%) | 36 (35.7%) | 0.621 | 27 (48.2%) | 29 (51.8%) | 0.119 |
| Female (14) | 6 (42.9%) | 8 (57.1%) | 10 (71.4%) | 4 (28.6%) | ||
| Size | ||||||
| <3cm (55) | 17 (30.9%) | 38 (69.1%) | 0.039* | 30 (54.6%) | 25 (45.4%) | 0.018* |
| ≥3cm (15) | 9 (60%) | 6 (40%) | 3 (20%) | 12 (80%) | ||
| Multiplicity | ||||||
| Single (36) | 12 (33.3%) | 24 (66.7%) | 0.497 | 22 (61.1%) | 14 (38.9%) | 0.016* |
| Multiple (34) | 14 (41.2%) | 20 (58.8%) | 11 (32.4%) | 23 (67.6%) | ||
| Grade | ||||||
| Low (28) | 6 (21.4%) | 22 (68.6%) | 0.025* | 19 (67.9%) | 14 (32.1%) | 0.005* |
| High (42) | 20 (47.6%) | 22 (52.4%) | 9 (33.3%) | 28 (66.7%) | ||
| Degree of invasion | ||||||
| NMI (39) | 10 (25.6%) | 29 (74.4%) | 0.026* | 24 (61.5%) | 15 | 0.007* |
| MI (31) | 16 (51.6%) | 15 (48.4%) | 9 (29%) | 22 (71%) | ||
| Invasion level (15) | ||||||
| T2 (7) | 4 (57.1%) | 3 (42.9%) | 0.047* | 3 (42.9%) | 4 (57.1%) | 0.042* |
| T3 (3) | 2 (66.6%) | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | ||
| T4 (5) | 4 (80%) | 1 (20%) | 0 (0%) | 5 (100%) | ||
| L.N metastasis (15) | ||||||
| Without (11) | 7 (63.6%) | 4 (36.4%) | 0.679 | 3 (27.3%) | 8 (72.7%) | 0.929 |
| With (4) | 3 (75%) | 1 (25%) | 1 (25%) | 3 (75%) | ||
| Perineural invasion | ||||||
| Without (61) | 20 (32.8%) | 41 (67.2%) | 0.049* | 31 (50.8%) | 30 (49.2%) | 0.033* |
| With (9) | 6 (66.7%) | 3 (33.3%) | 2 (22.2%) | 7 (77.8%) | ||
| L-V invasion | ||||||
| Without (62) | 21 (33.9%) | 41 (66.1%) | 0.115 | 31 (50%) | 31 (50%) | 0.041* |
| With (8) | 5 (62.5%) | 3 (37.5%) | 2 (25%) | 6 (75%) | ||
Table 2: AR and ERβ expression correlated with Clinicopathological characteristics.
Discussion
AR and ERβ are members of nuclear receptor superfamily [3]. Androgen/androgen receptor signaling pathway up-regulates the expression of active β-catenin also affects p53 tumor suppressor functions [12]. A retrospective study involving patients who received androgen deprivation therapy for prostate cancer showed a significantly lower incidence of subsequent BC [13]. Higher AR expression associated with low grade tumour, non-muscle invasive tumour, size less than 3 cm and absent perineural invasion. Loss of AR expression was reported in cases with lymph node metastasis, but it did not reach the statistically significant level. From that, higher AR expression was associated with better prognosis of urothelial carcinoma which was in agreement with other studies [8,14,15]. In contrast to a study reported that AR expression had no prognostic value [16] and another one reported bad prognosis in AR-positive patients with higher rate of invasion and metastasis [17]. High AR expression was significantly correlated with low-grade cases (pvalue= 0.026) which was in agreement with Miyamoto, et al. (pvalue= 0.023) [8]. In contrast, Mashhadi, et al. reported a significant association between AR expression and high-grade tumors (pvalue= 0.024) [17]. High AR expression was significantly correlated with NMI cases (p-value=0.026) which was in agreement with Miyamoto, et al. (p-value=0.018) [8]. In contrast to Mir, et al. who concluded loss of AR expression in NMI tumors (p-value= 0.048) [16].
ERβ is the dominant estrogen receptors expressed in urothelium [10]. Estrogen affects cell cycle progression through up-regulation of both cyclin D1 and cyclin E [18]. Higher ERβ expression in the current study was significantly correlated with high grade tumors, more invasive tumors, size more than 3 cm, multiple lesions in the urinary bladder, tumors with perineural and lympho-vascular invasion. High ERβ expression was detected in cases with lymph node metastasis and risk of recurrence, but it did not reach the statistically significant level. From that, high ERβ expression was associated with bad prognosis of urothelial carcinoma which was in agreement with most studies [8,19]. In contrast Bangmin, et al. [20] reported favorable prognosis in ERβ positive patients. High ERβ expression was significantly correlated with high-grade tumors (p-value=0.005) which was in agreement with Miyamoto, et al. (p-value<0.001) [8]. In contrast, Bangmin, et al. reported a significant association between ERβ expression and lowgrade tumors (p-value=0.037) [20]. High ERβ expression was significantly correlated with MI cases (p-value=0.007) which was in agreement with Miyamoto, et al. (p-value<0.001) [8]. In contrast to Kontos et al. who reported higher positive ERβ expression in NMI tumors (p-value=0.001) with good prognosis [21].
Conflict of Interests
The author declares that there is no conflict regarding the publication of this paper.
References
- Sebastien A, Jacques F, Isabelle S (2017) Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. European Urology 71: 96-108.
- Scosyrev E, Noyes K, Feng C (2009) Sex and racial differences in bladder cancer presentation and mortality. Cancer 115: 68-74.
- Harun F, Joshua A, Eugene K (2011) Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol 29: 457-463.
- Elshikh E, Anan I, Ebaid A (2015) The National Cancer Registry in Egypt: A retrospective Cross Sectional Epidemiological Study. Value Health 18: 437.
- Cumberbatch M, Rota M, Catto J (2016) The role of tobacco smoke in bladder and kidney carcinogenesis: A comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol 70: 458-466.
- Burger M, Catto J, Dalbagni G (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63: 234-241.
- Ali Y, Mete C, Muhammet K (2013) Can Reproductive Characteristics Predict Bladder Cancer in Women with Haematuria? Asian Pac J Cancer Prev 14: 5107-5110.
- Miyamoto H, Yao J, Chaux A (2012) Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int 109: 1716-1726.
- Chavalmane A, Comeglio P, Morelli A (2010) Sex steroid receptors in male human bladder: expression and biological function. J Sex Med 7: 2698-2713.
- Guilherme G, Georgios G, Carolyn L (2016) Effects of Androgen and Estrogen Receptor Signaling Pathways on Bladder Cancer Initiation and Progression. Bladder Cancer 2: 127-137.
- Babjuk M, Burger M, Zigeuner R (2013) EAU guidelines on non-muscle invasive urothelial carcinoma of the bladder: Eur Urol 64: 639-653.
- Hsu J, Hsu I, Xu D (2013) Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am J Pathol 182: 1811-1820.
- Shiota M, Yokomizo A, Takeuchi A (2015) Secondary bladder cancer after anticancer therapy for prostate cancer: reduced comorbidity after androgen-deprivation therapy. Oncotarget 6: 14710-14719.
- Jong N, Sung P, Sang L (2014) Prognostic Value of Sex-Hormone Receptor Expression in Non-Muscle-Invasive Bladder Cancer. Yonsei Med J 55: 1214-1221.
- Williams E, Higgins J, Sangoi A (2015) Androgen receptor immunohistochemistry in genitourinary neoplasms. Int Urol Nephro 47: 81-85.
- Mir C, Shariat S, Van der Kwast TH (2011) Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU Int 108: 24-30.
- Mashhadi R, Pourmand G, Kosari F (2014) Role of steroid hormone receptors in formation and progression of bladder carcinoma: A case-control study. Urol J 11: 1968-1973.
- Jian T, Zun Y, David F (2008) Roles of estrogen receptor a and b in modulating urothelial cell proliferation. Endocr Relat Cancer 15: 351-364.
- Kauffman B, Robinson M, Downes M (2011) Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol Carcinog 50: 931-944.
- Bangmin H, Di C, Yifeng J (2014) Estrogen receptor b [ERb) is a novel prognostic marker of recurrence survival in non-muscle-invasive bladder cancer potentially by inhibiting cadherin switch. World J Urol 32: 149-155.
- Kontos S, Kominea A, Melachrinou M (2010) Inverse expression of estrogen receptorβ-and nuclear factor-kB in urinary bladder carcinogenesis. Int J Urol 179: 801-809.
Citation: Al-Nandy M, Alshenay A (2018) Immunohistochemical Expression of Androgen and Estrogen Receptors. Diagn Pathol Open 3: 147. DOI: 10.4172/2476-2024.100014
Copyright: © 2018 Al-Nandy M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4754
- [From(publication date): 0-2018 - Oct 20, 2025]
- Breakdown by view type
- HTML page views: 3782
- PDF downloads: 972