Immunohistochemical Evaluation of 5-Hydroxymethylcytosine (5-hmC) in Breast Phyllodes Tumors
Received: 17-Feb-2022 / Manuscript No. dpo-22-52109 / Editor assigned: 21-Feb-2022 / PreQC No. dpo-22-52109(PQ) / Reviewed: 03-Mar-2022 / QC No. dpo-22-52109 / Revised: 07-Mar-2022 / Manuscript No. dpo-22-52109(A) / Accepted Date: 07-Mar-2022 / Published Date: 14-Mar-2022 DOI: 10.4172/2476-2024.S10.1000001
Abstract
Phyllodes Tumors (PTs) pose a significant diagnostic challenge in breast pathology as histological criteria and cutoffs for grading are complex and arbitrary. Nevertheless, the clinical behavior of PTs varies widely and correlates, in part, with the histological grade. Methylation signatures have gained interest as diagnostic and prognostic tools in a variety of neoplasms. In particular, 5-hydroxymethylcytosine (5-hmC) has been found to be a useful biomarker. Herein, we assess 51 PTs of varying grades and 15 cellular fibroadenomas (cFAs) for stromal expression of 5-hmC by immunohistochemistry. We demonstrate that nuclear 5-hmC decreases as PT grade increases, correlating with histologic predictors of adverse behavior, while it shows comparable levels in benign PTs and cFAs. We identify thresholds at which reduced 5-hmC levels help to distinguish borderline and malignant PT from their benign counterparts. Our results indicate that 5-hmC may serve as a valuable adjunct in classifying PTs in diagnostically difficult cases.
Keywords: Breast; Phyllodes tumor; 5-hydroxymethylcytosine; Immunohistochemistry; Fibroepithelial lesion; Fibroadenoma; Methylation; Epigenetics
Introduction
Phyllodes tumors (PTs) are a heterogeneous group of fibroepithelial lesions of the breast. They are graded based on multiple morphologic criteria and classified as benign, borderline, or malignant [1]. The features incorporated in the grading scheme include tumor borders, stromal cellularity, atypia, mitotic activity, and presence or absence of stromal overgrowth or malignant heterologous elements. These tumor characteristics are evaluated in combination; however, it is not uncommon for a PT to exhibit features from more than one category. Some parameters, such as stromal cellularity or cytologic atypia, are subjective. Furthermore, there may be marked intralesional heterogeneity and prioritization of certain criteria by a pathologist, e.g., brisk mitotic activity (≥ 10 mitotic figures per 10 high power fields), prompting classification as higher grade [2]. To further add to the confusion, benign PTs show overlapping features with another fibroepithelial lesion, cellular fibroadenoma (cFA). Nevertheless, the accurate categorization of PTs, is clinically important. The risk of local recurrence increases with histologic grade with overall recurrence rates in the literature of 10-17%, 14-25%, and 23-30% for benign, borderline, and malignant PTs, respectively [2]. Whereas distant metastases have not been reported in benign PTs and are only rarely seen in borderline PTs (<1%), malignant PTs spread hematogenously to other organs in up to 22% of cases. Most patients with metastatic malignant PT behave aggressively and have a dismal outcome [2,3].
Reversible epigenetic methylation of genomic DNA is essential for the regulation of gene expression. Suppression proceeds through methylation of DNA base cytosine, forming 5-methylcytosine (5-mC), while increased gene expression follows demethylation, converting 5- mC to 5-hydroxymethylcytosine (5-hmC). Diverse human malignancies are characterized by altered methylation pathways, one of which involves the reduced conversion of 5-mC to 5-hmC and a consequent decrease in nuclear 5-hmC [4]. Invariably reduced levels of 5-hmC have been reported in tumors of the lung, colon, brain, breast, liver, prostate, kidney, etc., compared to its respective normal tissues [5]. Reduction in nuclear 5-hmC is reportedly detectable by immunohistochemistry (IHC) [6]. Nuclear loss of 5-hmC staining has proven useful in determining benign from malignant lesions in melanoma, mesothelioma and breast carcinoma, among others [6-8]. Previous studies have shown different methylation patterns in borderline and malignant PTs compared to benign PTs and fibroadenomas [9,10]. However, to date, the utility of immunohistochemistry for methylation-related proteins such as 5-hmC has not been established in fibroepithelial lesions.
Considering the shortcomings of the current grading system, we hypothesized that 5-hmC IHC could assist in the classification and risk stratification of breast PTs. Herein; we examined 5-hmC levels in the stroma of PTs in relation to the clinicopathologic characteristics. Since cFA comes into a differential diagnosis of benign phyllodes, it was also included in the study.
Materials and Methods
Following institutional review board approval, 66 representative cases of breast fibroepithelial lesions were selected. These included 30 benign, 11 borderline, 10 malignant PTs, and 15 cFA. From these cases, a formalin-fixed, paraffin-embedded representative tissue section was stained with hematoxylin and eosin (H&E). All H&E-stained slides were reviewed by five pathologists blinded to the diagnosis in order to confirm the original diagnosis and to document the criteria used to classify the fibroepithelial lesion.
The 5-hmC immunohistochemical stain was performed on representative tissue sections using a Leica Bond RX automatic stainer with a 15-min antigen retrieval (epitope retrieval solution I, Leica Biosystems, AR9961) and the 25-min DAB-modified protocol with the primary anti-5-hmC antibody (1:1500; Active Motif, Inc., Carlsbad, CA). Antigen-antibody binding was detected with Bond polymer refinedetection (Leica Biosystems, DS9800).
The intensity and percent positivity of lesional stromal cell nuclei were assessed by the same five pathologists. 5-hmC staining of benign breast stromal and epithelial cells and lymphocytes present on the same slide served as an internal control. A final score was calculated by multiplying the intensity (1=weak, 2=moderate, 3=strong) and proportion (1=0- 25%, 2=26-50%, 3=51-75%, 4=76-100%) of 5-hmC staining in tumor nuclei as compared to internal controls, with a minimum score of 1 and a maximal score of 12. Linear regression, Fisher’s exact test, and ROC curve were used for statistical analysis (Microsoft Inc, Redmond, CA).
Results
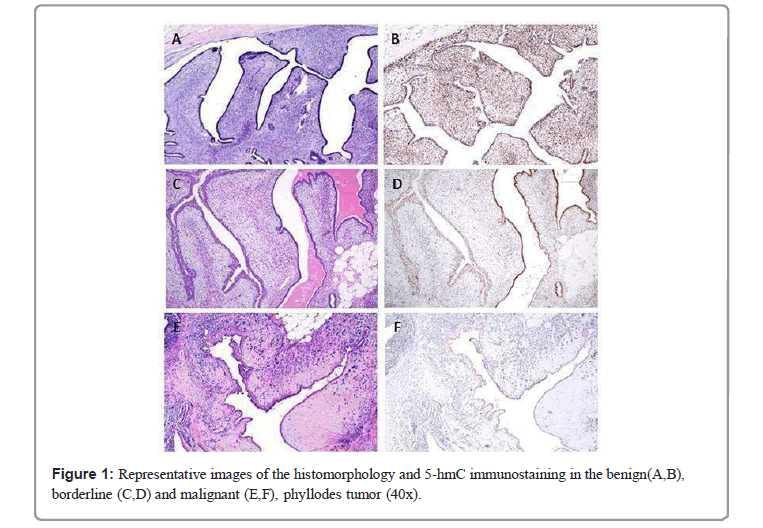
The mean quantitated expression of 5-hmC showed a significant gradual reduction as PT grade increased (p<0.001) (Table 1 and Figure 1). Mean quantitated 5-hmC loss was significantly greater in malignant PT (mean 5-hmC score 2.3 ± SD 2.1) as compared to borderline (mean 5-hmC score 6.3 ± SD 3.5) and benign (mean 5-hmC score 11.4 ± SD 1.6) tumors (p=0.001). However, the 5-hmC expression did not differ significantly between cFA and benign PT stroma (5-hmC scores of 11.6 ± SD 1.5 and 11.4 ± SD 1.6, respectively). In addition, 5-hmC levels significantly decreased with the presence of many adverse histologic features. 5-hmC staining showed a statistically significant decrease in lesions with larger tumor size, infiltrative margin, marked stromal cellularity, marked stromal cell atypia, increased stromal mitoses, as well as with the presence of stromal overgrowth and malignant heterologous elements (p<0.001). Other qualities of the lesions, such as prominent leaf-like fronds and heterogeneity in stromal cell distribution, also showed association with 5-hmC levels, although less significant (p<0.05). Predominant tumor architecture (pericanalicular, intracanalicular, or mixed) and heterogeneous gland- to-stroma ratio were not associated with the mean 5-hmC score. Defining borderline PT by an IHC score of ≤ 9, 5-hmC showed a sensitivity of 90.5% and specificity of 91.1% for identifying borderline category. For malignant PT defined as score ≤ 3, 5-hmC IHC achieved a sensitivity of 90% and specificity of 96.4%.
| Parameters | Cellular FA, N=15 | Phyllodes tumors, N=51 | 5hmC (mean ± SD) | p-value | |||
|---|---|---|---|---|---|---|---|
| Benign, N=30 (58.8%) | Borderline, N=11 (21.6%) | Malignant, N=10 (19.6%) | |||||
| Tumor size (cm, mean ± SD) | 2.4 ± 1.3 | 2.7 ± 1.7 | 4.8 ± 3.0 | 9.5 ± 8.5 | 9.2 ± 4.0 | 0.001 | |
| 5hmC IHC score (mean ± SD) | 11.6 ± 1.5 | 11.4 ± 1.6 | 6.3 ± 3.5 | 2.3 ± 2.1 | |||
| Tumor margin | Circumbscribed | 15 | 29 | 5 | 1 | 10.7 ± 2.9 | <0.001 |
| Infiltrative | 1 (focal) | 6 (focal) | 9 | 4.7 ± 3.9 | |||
| Architecture | Intracanalicular | 3 | 17 | 7 | 7 | 8.6 ± 4.3 | 0.367 |
| Pericanalicular | 10 | 6 | 2 | 1 | 10.2 ± 3.2 | ||
| Mixed | 2 | 7 | 2 | 2 | 9.4 ± 4.7 | ||
| Prominent leaf-like fronds | Absent | 15 | 15 | 5 | 4 | 10.1 ± 3.6 | 0.043 |
| Present | 15 | 6 | 6 | 8.0 ± 4.4 | |||
| Gland-to-stroma ratio | Uniform | 13 | 8 | 3 | 1 | 10.3 ± 3.3 | 0.083 |
| Variable | 2 | 22 | 8 | 9 | 8.5 ± 4.4 | ||
| Stromal cellularity | Mild | 5 | 21 | 1 | 11.5±1.5 | <0.001 | |
| Moderate | 10 | 9 | 10 | 1 | 9.2 ± 3.7 | ||
| Marked | 9 | 2.3 ± 2.2 | |||||
| Stromal cell distribution | Uniform | 11 | 6 | 1 | 10.9 ± 2.8 | 0.039 | |
| Variable | 4 | 24 | 11 | 9 | 8.6 ± 4.3 | ||
| Stromal cell atypia | No/Mild | 14 | 26 | 1 | 11.4 ± 1.8 | <0.001 | |
| Moderate | 1 | 4 | 10 | 1 | 7.4 ± 3.8 | ||
| Marked | 9 | 2.3 ± 2.2 | |||||
| Stromal mitosis/10 HPFs | 0 to 4 | 13 | 29 | 3 | 11.0 ± 2.4 | <0.001 | |
| 5 to 9 | 1 | 1 | 5 | 1 | 7.8 ± 4.2 | ||
| ≥ 10 | 1 | 3 | 9 | 3.7 ± 3.4 | |||
| Stromal overgrowth | Absent | 15 | 30 | 7 | 4 | 10.0 ± 3.5 | <0.001 |
| Present | 4 (focal) | 6 | 5.0 ± 4.5 | ||||
| Heterologous elements | Absent | 15 | 30 | 11 | 6 | 9.7 ± 3.6 | 0.006 |
| Present | 4 | 1.3 ± 0.5 | |||||
Table 1: 5hmC expression and tumor characteristics.
Discussion
Phyllodes tumors show a broad spectrum of clinical behavior. Careful morphological evaluation remains the mainstay of diagnosis and classification of these tumors; however, histological criteria and cutoffs for grading are complex and quite arbitrary. A number of immunohistochemical markers, including Ki-67, p53, CD117 (c-kit), vascular endothelial growth factor (VEGF), and epidermal growth factor receptor (EGFR), have been proposed to assist in the workup of PTs, with variable sensitivity, specificity, and uptake into routine pathology practice [11].
Mounting evidence exists that the pathway to cancer leads through a gradual accumulation of interacting epigenetic and genetic events over time. Most studies to date have concentrated on the genetic alterations in PTs, e.g., chromosomal imbalances or somatic gene mutations (e.g., MED12 exon 2 or TERT promoter mutations) [12-15]. Few investigators have evaluated PT methylation status. Huang et al. showed significantly elevated methylation of RASSF1A and TWIST1 promoters in PTs compared with fibroadenomas [9]. Kim et al. demonstrated a trend in increasing methylation frequency of five genes (GSTP1, HIN-1, RAR-beta, RASSF1A, and TWIST1) according to the histologic grade of PTs [10]. The same authors reported that methylation profiles segregated PTs into two distinct groups: the benign and the combined borderline/ malignant. These studies have brought attention to epigenetic mechanisms as possible diagnostic and/or prognostic tools in PTs.
Here we report that IHC expression of an epigenetic marker 5-hmC in the stroma of PTs is associated with the histologic grade. Our data provide both a statistically significant and diagnostically relevant difference in the extent of 5-hmC loss between benign, borderline, and malignant phyllodes. Interestingly, benign PT and cFA show comparably high levels of 5-hmC. These data support previous studies on similarities between the two neoplasms [2].
We observe a strong correlation of 5-hmC levels with morphologic predictors of adverse behavior, including larger tumor size, infiltrative margin, marked stromal cellularity, marked stromal cell atypia, increased stromal mitoses, presence of stromal overgrowth, and presence of malignant heterologous elements. Other qualities of the lesions that we routinelyevaluate, like prominent leaf-like fronds and heterogeneity in stromal cell distribution, are also associated with 5-hmC levels. Predominant tumor architecture or heterogeneous gland-to-stroma ratio are not correlated with 5-hmC expression. At specific thresholds of IHC score, the reduced 5-hmC expression performs as a sensitive and specific marker for borderline and malignant PT, which is the most clinically important distinction.
Conclusion
In summary, our pilot study shows a promising role for 5- hmC immunohistochemistry in the diagnostic workup of challenging cases of phyllodes tumors. Additional studies on larger cohorts are needed to validate the findings and to reproduce our data obtained in resection specimens in small biopsy samples. Finally, work examining additional 5-hmC-related epigenetic and metabolic markers (including 5-mC, ten-eleven translocation enzymes, isocitrate dehydrogenase, succinate dehydrogenase, or fumarate dehydrogenase) may further elucidate the interplay of genetic and epigenetic alterations in the pathogenesis of these rare and poorly understood neoplasms.
Declarations
Ethics approval and consent to participate: This study is approved by the University of Chicago Institutional Review Board. Waiver of Consent Process and Consent Documentation as well as a waiver of HIPAA authorization, were obtained. The University Federalwide Assurance number is FWA00005565. The current expiration date of the FWA is 3/14/2022. The University of Chicago IORG number is IORG0000201.
Consent for publication
The Authors hereby consent to the publication of the Work in The Journal of Diagnostic Pathology.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests
Funding
The study was supported internally by the Department of Pathology at the University of Chicago Medical Center.
Authors’ contributions
AB and TK conceived and designed the project. AB, JV, and LH performed a selection of study cases and reviewed clinicopathologic parameters. AB, LH, JV, JM, and HS reviewed H&E sections, confirmed diagnoses, and analyzed and quantified 5-hmC immunohistochemical staining. SD, RL, ZZ, and WZ performed a statistical analysis of the data.
Acknowledgments
We thank Dr. Terri Lee and the Human Tissue Resource Center for performing immunohistochemical staining. We thank Ryan McGary and the Histology Section for the preparation of tissue samples.
References
- Tan P H, Ellis I, Allison K, Brogi E, Fox, SB, et al. (2020) The 2019 World Health Organization classification of tumours of the breast. Histopathology 77:181-185.
[Crossref] [Google Scholar] [Pubmed]
- Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, et al. (2016) Phyllodes tumours of the breast: A consensus review. Histopathology 68:5-21.
[Crossref] [Google Scholar] [Pubmed]
- Krings G, Bean GR, Chen YY (2017) Fibroepithelial lesions; The WHO spectrum. Semin Diagn Pathol 34:438-452.
[Crossref] [Google Scholar] [Pubmed]
- Vasanthakumar A, Godley LA (2015) 5-hydroxymethylcytosine in cancer: significance in diagnosis and therapy. Cancer Genet 208:167-77.
[Crossref] [Google Scholar] [Pubmed]
- Mariani CJ, Madzo J, Moen EL, Yesilkanal A, Godley LA (2013) Alterations of 5-hydroxymethylcytosine in human cancers. Cancers (Basel) 5:786-814.
[Crossref] [Google Scholar] [Pubmed]
- Chapel DB, Husain AN, Krausz T (2019) Immunohistochemical evaluation of nuclear 5-hydroxymethylcytosine (5-hmC) accurately distinguishes malignant pleural mesothelioma from benign mesothelial proliferations. Mod Pathol 32:376-386.
[Crossref] [Google Scholar] [Pubmed]
- Lian CG, Xu Y, Ceol C, Wu F, Larson A, et al (2012) Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150:1135-46.
[Crossref] [Google Scholar] [Pubmed]
- Tsai KW, Li GC, Chen CH, Yeh MH, Huang JS, et al. (2015) Reduction of global 5-hydroxymethylcytosine is a poor prognostic factor in breast cancer patients, especially for an ER/PR-negative subtype. Breast Cancer Res Treat 153:219-34.
[Crossref] [Google Scholar] [Pubmed]
- Huang KT, Dobrovic A, Yan M, Karim RZ, Lee CS, et al. (2010) DNA methylation profiling of phyllodes and fibroadenoma tumours of the breast. Breast Cancer Res Treat 124:555-65.
[Crossref] [Google Scholar] [Pubmed]
- Kim JH, Choi YD, Lee JS, Lee JH, Nam JH (2009) Borderline and malignant phyllodes tumors display similar promoter methylation profiles. Virchows Arch 455: 469-75.
[Crossref] [Google Scholar] [Pubmed]
- Schnitt SJ, Collins, LC (2013) Biopsy interpretation of the breast. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
- Laé M, Vincent-Salomon A, Savignoni A, Huon I, Fréneaux P, et al. (2007) Phyllodes tumors of the breast segregate in two groups according to genetic criteria. Mod Pathol 20:435-44.
[Crossref] [Google Scholar] [Pubmed]
- Lv S, Niu Y, Wei L, Liu Q, Wang X, et al. (2008) Chromosomal aberrations and genetic relations in benign, borderline and malignant phyllodes tumors of the breast: a comparative genomic hybridization study. Breast Cancer Res Treat 112:411-8.
[Crossref] [Google Scholar] [Pubmed]
- Ng CC, Tan J, Ong CK, Lim WK, Rajasegaran V et al. (2015) MED12 is frequently mutated in breast phyllodes tumours: a study of 112 cases. J Clin Pathol 68:685-91.
[Crossref] [Google Scholar] [Pubmed]
- Yoshida M, Ogawa R, Yoshida H, Maeshima A, Kanai Y, et al. (2015) TERT promoter mutations are frequent and show association with MED12 mutations in phyllodes tumors of the breast. Br J Cancer 113:1244-8.
[Crossref] [Google Scholar] [Pubmed]
Citation: Vickery J, Han L, Dzul S, Zhang Z, Zhang Z (2022) Immunohistochemical Evaluation of 5-Hydroxymethylcytosine (5-hmC) in Breast Phyllodes Tumors. Diagnos Pathol Open S1:001. DOI: 10.4172/2476-2024.S10.1000001
Copyright: © 2022 Biernacka A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 3218
- [From(publication date): 0-2022 - Nov 05, 2025]
- Breakdown by view type
- HTML page views: 2503
- PDF downloads: 715