Research Article Open Access
Immunohistochemical Analysis of mTOR Pathway Expression in Gastric Neuroendocrine Tumors
Arsenic Ruza1*, Konstantin Griniak2, Lohneis Phillip1, Stephan Felder2, Frank Ulrich Pape3 and Dietel Manfred11Institute of Pathology, Charité, University Hospital Berlin, 10117 Berlin, Germany
2Medical students at the University Hospital Charité, Germany
3Department for Gastroenterology, University Hospital Berlin, 13353 Berlin, Germany
- *Corresponding Author:
- Dr. Ruza Arsenic
Institute of Pathology Charité
University Hospital Berlin, 10117 Berlin, Germany
Tel: 0049 30 450 536 018
E-mail: ruza.arsenic@charite.de
Received date: March 27, 2014; Accepted date: April 24, 2014; Published date: April 26, 2014
Citation: Ruza A, Griniak K, Phillip L, Felder S, Pape FU, et al. (2014) Immunohistochemical Analysis of mTOR Pathway Expression in Gastric Neuroendocrine Tumors. J Clin Exp Pathol 4:173. doi:10.4172/2161-0681.1000173
Copyright: © 2014 Ruza A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction H2O in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Background: The mammalian target of rapamycin (mTOR) is an important regulator of cell proliferation and protein translation and is activated in various malignancies. Expression of mTOR cascade components in gastric neuroendocrine tumors (NETs), however, has not yet been fully explored.
Aims: The goal of the present study was to assess the activation of mTOR and its upstream and downstream components in gastric NETs using immunohistochemistry and to investigate the relationship between expression and clinicopathological data.
Methods: The expression of phosphorylated mTOR (p-mTOR) and its major target the eukaryotic initiation factor 4E-binding protein 1 (p4EBP1), phospho-phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (pPIK3CA), phospho-protein kinase B (pAkt), phospho-phosphatase and tensin homolog (pPTEN), and phosphotuberous sclerosis 2 (pTSC2) were examined in a series of 35 gastric NETs.
Results: All samples from the 35 patients showed expression of the PI3K catalytic subunit PIK3CA and the mTOR inhibitor TSC2. The p-mTOR was expressed in 88.57%, pPTEN in 97.14%, and pAkt in 65.7% of the examined tumors. The mTOR effector p4E-BP1 was expressed in 88.57% of cases. In addition, the p-mTOR positive rate correlated with Ki-67 expression. In fact, patients with Ki-67 ≤ 2 had higher p-mTOR positive rates (p=0.032); however, no significant correlations between p-mTOR positivity and selected clinicopathological characteristics were observed.
Conclusions: In conclusion, these data demonstrate high mTOR activation in gastric NETs, suggesting that mTOR pathway inhibition may be a possible therapeutic strategy for treatment of gastric NETs.
Keywords
mTOR pathway; Neuroendocrine tumor; Molecular therapy
Abbreviations
4EBP1: Eukaryotic Initiation Factor 4E-Binding Protein 1; Akt: Protein kinase B; ENETS: European Neuroendocrine Tumor Society; MEN 1 (or 2): Multiple Neuroendocrine Neoplasia Type 1 (or 2); mTOR: Mammalian Target of Rapamycin; NEC: Neuroendocrine Carcinomas; NET: Neuroendocrine Tumor; PDK-1- 3: Phosphoinositide Dependent Protein Kinase; PIK3CA: Phosphatidylinositol-4,5-Bisphosphate 3-Kinase, Catalytic Subunit Alpha; PTEN: Phosphatase and Tensin Homolog; RBS6K: Ribosomal Protein S6 Kinase; TSC2: Tuberous Sclerosis 2; WHO: World Health Organization
Introduction
The incidence of gastric neuroendocrine tumors (NET) has increased rapidly over the last decade, likely due to the rising number of gastroscopies [1]. Four different biologically relevant types of gastric NET have been described [2,3]. Type 1 comprises about 70-80% of gastric NETs and is associated with atrophic gastritis. Patients show multiple small tumors in the mucosa. Type 2 is rare and is associated with a duodenal gastrinoma in patients with the diagnosis of multiple neuroendocrine neoplasia type 1 (MEN 1) syndrome. Therefore, the gastric mucosa in these patients does not exhibit atrophy but displays hyperplasia of chief cells and parietal cells. Type 3 tumors are sporadic gastric NETs that are mostly larger single tumors at the time of diagnosis with an increased risk of lymph node and liver metastasis [4]. Poorly differentiated neuroendocrine carcinomas (NEC) of the stomach are considered to be a forth type of NET, although these tumors have not been included in the original classification by Rindi [5]. Gastric NET and NEC are currently treated with multidisciplinary approaches, including a combination of surgery, chemotherapy, and targeted molecular therapy [5,6].
The mTOR inhibitor everolimus and the multikinase inhibitor sunitinib have previously gained approval from the FDA for the treatment of unresectable, locally advanced or metastatic pancreatic NET; however, little is known about the mTOR pathway in gastric NETs. In this respect, a detailed protein expression map of mTOR-pathway components in gastric NETs is a good starting point to define specific expression patterns that are predictive of clinical response, as suggested for other malignancies [7]. Additionally, this approach could facilitate investigation of the prognostic implications of these molecules.
The mTOR protein is a Ser/Thr protein kinase that is formed by two different protein complexes, mTORC1 and mTORC2. This complex is activated via phosphorylation by an upstream signaling cascade. Activation of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) via different receptors results in phosphorylation of 3-phosphoinositide-dependent protein kinase-1 (PDK-1) at Tyr 373/376 and Ser24. Then, pPDK1 phosphorylates Akt, and pAKT phosphorylates mTOR, while the tumor suppressor proteins tuberous sclerosis (TSC1), TSC2, and phosphatase and tensin homolog (PTEN) inhibit phosphorylation of mTOR. The known downstream targets of p-mTOR are the eukaryotic translation initiation factor 4E-binding protein (4EBP1) and the ribosomal protein S6 kinase (RBS6K), which mediate an increase in protein synthesis and cell growth [8,9].
In the present study, we used immunohistochemistry to investigate the expression of p-mTOR, its downstream target p4EBP1, the upstream regulators pAkt and pPIK3CA, and also the suppressor proteins pTSC2 and pPTEN. We examined these expression data in the context of clinicopathological variables.
Patients, Materials, and Methods
Patient characteristics
A total of 35 patients who received treatment for gastric NETs at the Charite University Hospital were included in this study. Of these, 19 were female (54.3%), and the median age for all 35 patients was 48.5 years. In 24 patients, NETs were associated with chronic atrophic gastritis (type 1 NET). Three of these patients had a known MEN1 syndrome (type 2 NET), and four patients had sporadic gastric NET (type 3 NET). Another four patients with the diagnosis of gastric NEC (type 4 tumors) were included. All tumors were graded and staged according to the latest WHO (WHO 2010) and ENETS classification (Table 1).
| Case | Age/Sex | Grading | Type |
|---|---|---|---|
| 1 | 50/F | G1 | 2 |
| 2 | 50/F | G1 | 1 |
| 3 | 47/F | G2 | 3 |
| 4 | 54/M | G1 | 1 |
| 5 | 59/M | G1 | 1 |
| 6 | 69/M | G3 | 4 |
| 7 | 74/M | G1 | 2 |
| 8 | 57/M | G1 | 1 |
| 9 | 72/F | G2 | 1 |
| 10 | 65/F | G1 | 1 |
| 11 | 57/M | G1 | 1 |
| 12 | 46/F | G1 | 1 |
| 13 | 57/M | G3 | 4 |
| 14 | 60/F | G1 | 1 |
| 15 | 57/M | G2 | 3 |
| 16 | 56/F | G1 | 1 |
| 17 | 73/M | G2 | 3 |
| 18 | 60/M | G1 | 1 |
| 19 | 75/F | G3 | 4 |
| 20 | 63/F | G3 | 1 |
| 21 | 54/F | G2 | 1 |
Table 1: Clinicopathological characteristics of the gastric NET patients. M-male, F-female, G1- well differentiated neuroendocrine tumor, G2- moderately differentiated neuroendocrine tumor, G3- poorly differentiated neuroendocrine tumor
Construction of tissue microarrays
For immunohistochemical analysis of mTOR pathway expression, tissue microarrays were constructed using a semiautomatic tissue arrayer (TMA Grandmaster, 3DHistech, Budapest, Hungary). A representative tumor-bearing paraffin block of formalin-fixed tissue was selected for each case. Hematoxylin and eosin-stained slides were prepared from the tissue blocks, and tumor-bearing areas were marked on the slides. Both the selection of blocks and the marking of tumor-bearing areas were conducted by a pathologist specialized in NET pathology (RA). Subsequently, one or two tissue cores with a diameter of 1 mm were punched from the tumor-bearing areas and transferred to a donor paraffin block.
Immunohistochemistry
Tissue microarray sections (2-3 um thick) were cut and incubated with antibodies directed against p-mTOR (Ser2448) (clone 49F9, 1:100; Cell Signaling Technology, Danvers, MA, USA), pTSC2 (polyclonal, 1:100; Cell Signaling Technology), pAkt (polyclonal, 1:200; Abcam, Cambridge, MA, USA), p4EBP1 (clone 235B4, 1:100; Cell Signaling Technology), pPTEN (monoclonal, 1:100; Cell Signaling Technology), and pPIK3CA (1:100; Cell Signaling Technology). Omission of the primary antibody served as a negative control. Ki-67 staining (clone M7240, 1:100; Dako, Glostrup, Denmark) was accomplished on whole slides. Staining was performed in a Benchmark XT autostainer (Ventana, Tuscon, AZ, USA), according to the manufacturer’s protocol. Samples were considered positive when at least 50% of tumor cells showed a moderate or strong expression of the detected protein.
Statistical analysis
Statistical analyses were conducted using SPSS 20 statistical software (SPSS Inc., Chicago, IL, USA). The associations between immunohistochemical marker expression and clinicopathological variables were determined by a chi-squared test. The correlation of the mTOR pathway components expression scores with each other and with proliferation indices was examined by Spearman's rank order correlation. All tests were two-tailed, and the results were considered significant when p<0.05. In addition, survival analysis was performed using the Kaplan-Meier log-rank test.
Results
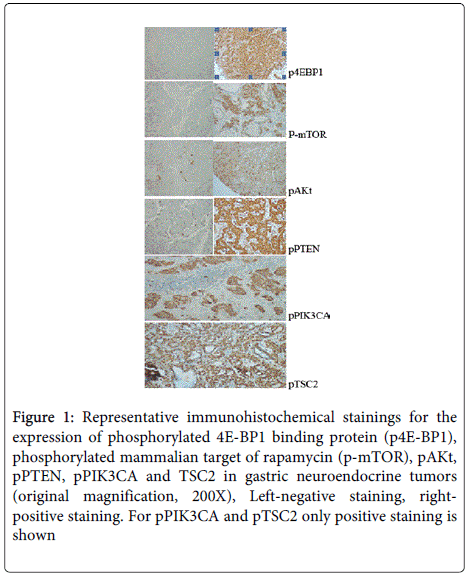
Expression of mTOR pathway components in gastric NET
We analyzed the expression of six proteins that are known to play important roles in the mTOR pathway. Samples from all 35 patients exhibited expression of the pPIK3CA and pTSC2. In addition, p-mTOR was expressed in 88.57%, pPTEN in 97.14%, and pAKT in 65.7% of the examined tumors. The mTOR effector p4EBP1 was expressed in 88.57% of cases (Table 2 and Figure 1).
| Case | p-mTOR | p-Akt | pBP1 | PTEN |
|---|---|---|---|---|
| 1 | + | + | + | + |
| 2 | + | + | + | + |
| 3 | + | + | + | + |
| 4 | + | + | + | + |
| 5 | + | + | - | + |
| 6 | + | - | + | + |
| 7 | + | - | + | + |
| 8 | + | - | + | + |
| 9 | - | - | + | + |
| 10 | + | - | + | + |
| 11 | + | - | + | + |
| 12 | + | + | + | + |
| 13 | - | - | - | - |
| 14 | + | - | + | + |
| 15 | - | + | - | + |
| 16 | + | + | + | + |
| 17 | + | - | + | + |
| 18 | + | + | + | + |
| 19 | + | + | + | + |
| 20 | + | + | + | + |
| 21 | + | - | + | + |
| 22 | + | + | + | + |
| 23 | + | + | + | + |
| 24 | + | + | + | + |
| 25 | - | + | - | + |
| 26 | + | + | + | + |
| 27 | + | - | + | + |
| 28 | + | + | + | + |
| 29 | + | + | + | + |
| 30 | + | - | + | + |
| 31 | + | + | + | + |
| 32 | + | + | + | + |
| 33 | + | + | + | + |
| 34 | + | + | + | + |
| 35 | + | + | + | + |
Table 2: Immunohistochemical results of gastric NET
Figure 1: Representative immunohistochemical stainings for the expression of phosphorylated 4E-BP1 binding protein (p4E-BP1), phosphorylated mammalian target of rapamycin (p-mTOR), pAKt, pPTEN, pPIK3CA and TSC2 in gastric neuroendocrine tumors (original magnification, 200X), Left-negative staining, rightpositive staining. For pPIK3CA and pTSC2 only positive staining is shown
Correlation between mTOR pathway components in gastric NETs
We next examined the correlation between the mTOR pathway components. As expected there was a positive, albeit weak, linear correlation between the expression of pAkt and p-mTOR (p=0.496) as well as a strong linear correlation between the expression p-mTOR and the effector protein p4E-BP1 (p=0.001). We could not, however, test the associations between expression of pPTEN and pPIK3CA or expression of pTSC2 and p-mTOR, because all samples were positive for pPIK3CA and pTSC2.
Correlation of mTOR pathway components with tumor type, grading, tumor stage, and metastasis
No statistically significant differences were detected in the p-mTOR expression between the Type 1, Type 2, Type 3, and Type 4 NETs (p=0.082). In addition, no statistically significant differences in p-mTOR expression in metastatic versus non-metastatic tumors were found (p=0.238). The rate of p-mTOR positivity correlated with Ki-67 staining, and more specifically, the patients with Ki-67≤2 had higher p-mTOR positive rates (p=0.032; Tables 2 and 3). No statistically significant differences were detected with respect to p-mTOR expression and ENETS stage classification (p=0.119).
| n | p-mTOr | p-Akt | p-4BEP1 | p-PTEN | |||||
|---|---|---|---|---|---|---|---|---|---|
| positive | P | positive | P | positive | P | positive | P | ||
| Tumortyp | |||||||||
| Typ1 | 24 | 22 (91.7) | 0.082 | 17 (70.8) | 0.438 | 24 (100) | 0.001 | 24 (100) | 0.092 |
| Typ2 | 3 | 3 (100) | 2 (66.7) | 3 (100) | 3 (100) | ||||
| Typ3 | 4 | 2 (50) | 3 (75) | 2 (50) | 2 (50) | ||||
| Typ4 | 4 | 3 (75) | 1 (25) | 3 (75) | 3 (75) | ||||
| Ki67 | |||||||||
| <=2 | 21 | 21 (100) | 0.032 | 15 (71.4) | 0.423 | 20 (95.2) | 0.242 | 21(100) | 0.004 |
| 2-20 | 11 | 8 (72.7) | 7 (63.6) | 9 (81.8) | 11(100) | ||||
| >=20 | 3 | 2 (66.7) | 1 (33.3) | 2 (66.7) | 2 (66.7) | ||||
| Metastasis | |||||||||
| yes | 9 | 7 (77.7) | 0.238 | 4 (44.4) | 0.119 | 6 (66.7) | 0.017 | 8 (88.9) | 0.085 |
| no | 26 | 24 (92.3) | 19 (73.1) | 25(96.1) | 26(100) | ||||
| ENETS | |||||||||
| Stage 0 | 15 | 14 (93.3) | 0.119 | 12 (78.5) | 0.222 | 15(100) | 0.002 | 15(100) | 0.186 |
| Stage 1 | 10 | 10 (100) | 5 (50) | 10(100) | 10(100) | ||||
| Stage 2 | 3 | 2 (66.6) | 3 (100) | 2 (66.7) | 3 (100) | ||||
| Stage 3 | 2 | 2 (100) | 1 (50) | 2 (100) | 2 (100) | ||||
| Stage 4 | 5 | 3 (60) | 2 (40) | 2 (40) | 4 (80) | ||||
Table 3: Relation between the expression of p-mTOR, pAkt, p4BEP1, pPTEN and clinico-pathological characteristics
Furthermore, no statistically significant differences in pPTEN expression were detected with respect to tumor type (p=0.092) or metastasis status (p=0.085), but a statistically significant difference was found in pPTEN expression with regard to Ki-67 level, higher positivity by lower Ki-67 levels (p=0.004). No statistically significant differences in pPTEN expression levels in association with ENETS stage classification (p=0.186). In an analysis of pAkt expression, we failed to find any statistically significant differences for any analyzed parameter (Table 3).
Higher p4EBP1 expression was found in Type 1 and Type 2 NETs compared to Type 3 and Type 4 NETs (p= 0.001). This expression was also higher in non-metastatic tumors than in metastatic tumors (p=0.017). We also detected statistically significant differences with respect to ENETS stage classification, with the lower stages exhibiting higher positivity (p=0.002). In contrast to other described markers, no statistically significant differences in expression of p4EBP1 were found with respect to Ki-67 levels (p=0.242). Both pTSC2 and pPIK3CA were positive in all cases. Additionally, we did not detect statistically significant differences in mTOR pathway expression with respect to survival.
Discussion
Expression of several mTOR pathway components has previously been assessed in pancreatic NET [10-14], but the functional activation of the PI3K/AKT/mTOR signaling pathway has never been extensively investigated in gastric NETs. Due to the development of selective inhibitors, clinical interest in the mTOR pathway has increased in recent years, and several preclinical trials have been conducted to test the efficacy of these inhibitors in different human malignancies, including NETs [15-17].
In this study, we report differential expression of pPIK3CA, pPTEN, pAkt, pTSC2, p-mTOR, and p4EBP1 in a cohort of gastric NETs. Compared with previous studies, our study included the largest number of patients for the investigation of the mTOR signaling pathway of gastric NETs. The immunohistochemistry analyses of gastric NETs identified the expression of p-mTOR in 88.57%, pPTEN in 97.14%, and pAkt in 65.7% of the examined tumors. The mTOR effector p4EBP1 was expressed in 88.57% of cases, while pTSC2 and pPIK3CA were expressed in all cases. The expression of p-mTOR was different with respect to Ki-67 status and p4EBP1 expression according to tumor type, metastasis status, and ENETS stage classification. Activation of the mTOR pathway would be expected to be associated with more aggressive clinical behavior. The proliferative marker Ki-67 has been widely used as a prognostic indicator for NETs. In contrary with observations in a previous study of gastroenteropancreatic NET [12], we did not find that expression of p-mTOR was associated with higher proliferative index in gastric NETs.
All samples were examined by immunohistochemistry, as this is the main technique used to assess protein expression or phosphorylation in paraffin-embedded tissues. To comprehensively assess mTOR signaling, future studies should expand on immunohistochemistry to include kinase assays, signaling blots, and transcriptional and translational assays to comprehensively assess mTOR signaling. Our study is important because mTOR pathway activation has not been adequately explored in gastric NETs. Thus, we provide compelling rationale for future studies of mTOR activation and mTOR inhibition as a therapeutic strategy in patients with gastric NETs.
In the present study, not all cases expressing p-mTOR co-expressed pAkt. This result suggests that the activation of mTOR in gastric NETs is regulated not only by the PI3K-Akt pathway but may also be transmitted through PDK1 without activation of Akt, as previously described in a study by Tan at al. [18]. Vasudevan at al. [19] found that PIK3CA may promote cancer through both Akt-dependent and Akt-independent mechanisms.
Previous reports on the prognostic role of mTOR pathway activation in human tumors have presented controversial findings. In some studies, activation of the mTOR pathway was found to be a favorable factor, such as for ovarian cancer [20], while others reported contradictory results in renal, biliary tract and breast carcinoma [21-24]. Discrepant results may be due to differences in various factors related to study design. For example, sample size, non-uniform treatment, insufficient follow up, or different thresholds for positivity may vary among studies, as these factors were determined by the authors of each report. Here, we also did not detect any statistically significant differences in mTOR pathway expression with respect to survival. The most likely reason for this finding was the small number of cases. A small sample size reduces the probability of achieving statistically significant results. However, our results provide the rationale for performing additional studies on mTOR activation in a larger population of patients.
In summary, despite differences in expression and correlations between expression of different components and clinicopathological data, our results demonstrate that the components of the mTOR pathway are highly expressed in gastric NETs and may, therefore, provide a potential therapeutic target for treatment of these tumors. Although the sample size in this study was limited, this is the first study to determine the expression of activated mTOR in gastric NETs. A high expression rate of p-mTOR pathway components in gastric NETs suggests that the recently described mTOR inhibitors may be effective therapeutic agents for treatment of this group of tumors. Additional research, including larger numbers of patients and clinical trials to investigate the mTOR inhibitors, should be considered in the future.
References
- Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, et al. (2001) Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect. A histopathological and immunohistochemical study. BMC Cancer 1:7-16.
- Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182: 4499-4506.
- Mantovani A (2007) Inflammation and cancer: the macrophage connection. Medicina (Buenos Aires), 67 (Suppl II):32-34.
- Terabe M, Berzofsky JA (2008) The role of NKT cells in tumor immunity. Adv Cancer Res 101: 277-348.
- Baskic D, Acimovic L, Samardzic G, Vujanovic NL, Arsenijevic NN (2001) Blood monocytes and tumor-associated macrophages in human cancer: differences in activation levels. Neoplasma 48: 169-174.
- Sanchez-Torres C, Garcia-Romo GS, Cornejo-Cortes MA, Rivas-Carvalho A, Sánchez-Schmitz G (2001) CD16+ and CD16- human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. IntImmunol 13:1571-1581.
- Hajto T, Hostanska K, Gabius HJ (1989) Modulatory potency of the beta-galactoside-specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res 49: 4803-4808.
- Hostanska K, Hajto T, Spagnoli G.C, Saller R(1996-97) A plant lectin, Viscum album agglutinin-I (VAA-I), stimulates cellular parameters of natural immunity in vivo and induces cytokine gene expression and apoptosis in cultures of peripheral blood mononuclear cells in vitro. Nat Immun 15:196-201.
- Hajto T, Hostanska K, Frei K, Rordorf K, Gabius HJ (1990) Increased secretion of tumor necrosis factor-alpha, interleukin-1 and interleukin-6 by human mononuclear cells exposed to beta-galactoside-specific mistletoe lectin. Cancer Res 50:3322-3326.
- Hajto T, Hostanska K, Weber K, Zinke H, Fischer J, et al. (1998) Effect of a recombinant lectin, Viscum album agglutinin on the secretion of interleukin-12 in cultured human peripheral blood mononuclear cells and on NK-cell-mediated cytotoxicity of rat splenocytes in vitro and in vivo. Nat Immun 16: 34-46.
- Hajtó T, Fodor K, Perjési P, Németh P (2011) Difficulties and perspectives of immunomodulatory therapy with mistletoe lectins and standardized mistletoe extracts in evidence-based medicine. Evid Based Complement Alternat Med 2011: 298972.
- Müthing J, Meisen I, Bulau P, Langer M, Witthohn K, et al. (2004) Mistletoe lectin I is a sialic acid-specific lectin with strict preference to gangliosides and glycoproteins with terminal Neu5Ac alpha 2-6Gal beta 1-4GlcNAc residues. Biochemistry 43: 2996-3007.
- Ghoneum M(1998) Enhancement of human natural killer cell activity by modified arabinoxylan from rice bran (BioBrab/MGN-3). Int J Immunotherapy, 14:89-99.
- Ghoneum M, Matsuura M (2004) Augmentation of macrophage phagocytosis by modified arabinoxylan rice bran (MGN-3/biobran). Int J ImmunopatholPharmacol 17: 283-292.
- Hidvégi M1, Rásó E, Tömösközi-Farkas R, Szende B, Paku S, et al. (1999) MSC, a new benzoquinone-containing natural product with antimetastatic effect. Cancer BiotherRadiopharm 14: 277-289.
- Szent-Györgyi A (1982) Biological oxidation and cancer. Int J Quant Chem Quant BiolSymp9: 27-38.
- Jakab F, Mayer A, Hoffmann A, Hidvégi M (2000) First clinical data of a natural immunomodulator in colorectal cancer. Hepatogastroenterology 47: 393-395.
- Boros LG1, Nichelatti M, Shoenfeld Y (2005) Fermented wheat germ extract (Avemar) in the treatment of cancer and autoimmune diseases. Ann N Y AcadSci 1051: 529-542.
- Fajka-Boja R, Hidvégi M, Shoenfeld Y, Ion G, Demydenko D, et al.(2002) Fermented wheat germ extract induces apoptosis and downregulation of major histocompatibility complex class I proteins in tumor T and B cell lines. Int J Onc, 20:563-570.
- Kirsch A, Hajto T (2011) Case reports of sarcoma patients with optimized lectin-oriented mistletoe extract therapy. J Altern Complement Med 17: 973-979.
- Hajto T, Kirsch A (2013) Case reports of cancer patients with hepatic metastases treated by standardized plant immunomodulatory preparations.J Cancer Res Update 2:1-9.
- Kirsch A (2007) Successful treatment of metastatic malignant melanoma with Viscum album extract (Iscador M). J Altern Complement Med 13: 443-445.
- Bock PR, Friedel WE, Hanisch J, Karasmann M, Schneider B, 2004. Efficacy abd safety of long-term complementary treatment with standardized European mistletoe extract (Viscum album L) in addition to the conventional adjuvant oncologic therapy in patients with primary non-metastasized mammary carcinoma. Results of a multi-center, comparative, epidemiological cohort study in Germany and Switzerland. Drug Res 54: 456-466.
- Tsunekawa H(2004) Effect of long-term administration of immunomodulatory food on cancer patients completing conventional treatments. ClinPharmacolTher 14: 295-302.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14969
- [From(publication date):
June-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10368
- PDF downloads : 4601