Immunity to Bacterial Infections: A Review
Received: 05-May-2023 / Manuscript No. jvmh-23-96137 / Editor assigned: 08-May-2023 / PreQC No. jvmh-23-96137 / Reviewed: 22-May-2023 / QC No. jvmh-23-96137 / Revised: 25-May-2023 / Manuscript No. jvmh-23-96137 / Accepted Date: 30-May-2023 / Published Date: 31-May-2023 DOI: 10.4172/jvmh.1000183 QI No. / jvmh-23-96137
Abstract
Bacterial infections have a significant impact on animal and human immunity. Bacteria must encounter with host in order to produce infection and inflammation. Pathogenic microorganisms can be transmitted through a variety of mechanisms. Bacterial infections are induced by two main processes fist bacteria cause inflammation, which leads to tissue destruction at the infection site. Second, bacteria produce toxins. Collectins, pentraxins, and ficolins are soluble PRRs. Complement proteins are required for the quick clearance of invading bacteria by innate defense mechanisms such as inflammation, opsonization, and bacteriolysis. Inflammation is triggered when bacteria or their products interact with molecules and cells of the innate immune system. Natural killer cells are a diverse population of innate immune cells capable of killing cells with low expression of MHC class I molecules. Bacterial super antigens are protein toxins that have the ability to cause significant immune system activation. Cell-Mediated Immunity effector mechanisms are mediated by cells rather than antibodies in cell-mediated immunity.
Keywords
Bacterial; Immunity; Infections
Introduction
Bacteria found everywhere on earth, including all water bodies, all landforms, soil, and plant and animal tissues. They are vital to the preservation of our living environment. Only a small percentage of bacteria on this planet cause infection . Some bacteria survive as infectious agents for multicellular animals, while phagocytosis was chosen as an effective technique to remove bacteria from host. Innate immune mechanisms, such as the presence of lysozyme or antimicrobial peptides, play a role in mucosal defense. When germs enter the internal environment, other cells such as complement, phagocytes, and natural killer cells (NK cells) respond. Although the evolutionary continuity of invertebrate and vertebrate immune mechanisms is not shown by the phylogeny of molecules such as Toll-like receptors. Pathogenic bacteria have been able to acquire genes encoding virulence factors, which allow them to colonize host tissues while simultaneously blocking innate defense mechanisms and allowing them to avoid specific reactions. Recognition of bacterial structures is at the heart of both innate and specific immune systems against bacteria. Because they recognize pathogen-associated molecular patterns (PAMPs), innate immunity receptors are known as pattern recognition receptors. PRRs are fixed in the genome (no rearrangement is required for expression), non-clonal in cell distribution, and only recognize foreign structures; on the other hand, specific immunity receptors are encoded in gene segments (rearrangement is required for expression), clonally distributed in B- and T-lymphocytes, and self-nonself discrimination is imperfect, resulting in tolerance [1,2].
Bacterial infections
Bacterial infections have a significant impact on animal and human immunity. Bacteria encounter with host in order to produce infection and inflammation in the host. Pathogenic microorganisms can be transmitted through a variety of mechanisms. By producing spores, bacteria such of the aerobic Bacillus and anaerobic Clostridium species may withstand environmental stress such as a lack of moisture, heat, pressure, salt, and depleted resources, and hence persist in habitats with compatible hosts. Others, such as the Shigella and Escherichia coli species, take advantage of situations when fecal contamination can lead to oral exposure and contact with host tissue. For example, Vibrio sp. and Klebsiella is a Gram-negative bacteria, typically, they can survive in situations with high fluid levels, such as water supplies, sewage, and even tainted medical fluids. Staphylococcus sp., a Gram positive bacterium, progresses and persists as a pathogenic microbe by adapting to cutaneous surfaces and causing infection in healthy and immune compromised individuals’ damaged skin or mucous membranes [3,4].
Immunity to bacteria infections
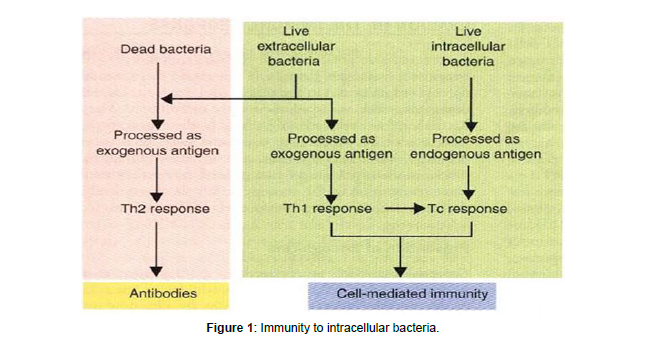
The extracellular infectious agents are able to replicate outside the host cell, they can replicate in the circulation, into the lumen of the respiratory and intestinal tract as well as in epithelia surfa. In the case of bacteria that inhabit the system circulatory, the phagocytosis is the main mechanism of innate immunity used to eliminate these infectious pathogens .The most important phagocytic cells are large cells represented by macrophages, neutrophils and dendritic cells.These cells engulf harmful microorganisms during which recognize pathogenassociated molecular patterns (PAMPs) through its pattern recognition receptors (PRRs). Once internalized by phagocytosis, the new formed vesicles gives rise the phagosome, which in turn merges into the lysosome originating the phagolysosome. During the maturation of these terminal vesicles, the elimination of infectious agents occurs by the action of enzymes and reactive oxygen intermediates and nitrogen present .While microbicidal mechanisms are constitutively present in phagocytes, these responses can be increased upon activation when the cells are exposed to type II interferon cytokines (IFN-γ), secreted by activated T lymphocytes. Extracellular bacteria are pathogenic in many different animals, and disease is induced by two main processes. To begin, these bacteria cause inflammation, which leads to tissue destruction at the infection site. Second, bacteria produce toxins that have a variety of pathogenic consequences) Endotoxins, which are components of bacterial cell walls, or exotoxins, which are produced by bacteria, are two types of toxins. Lipopolysaccharide (LPS), a gram negative bacterium endotoxin, is a potent activator of macrophages, dendritic cells, and endothelial cells. Exotoxins are cytotoxic in many cases, and others induce disease through a variety of mechanisms. Diphtheria toxin, for example, inhibits protein synthesis in infected cells, cholera toxin disrupts ion and water transport, tetanus toxin suppresses neuromuscular transmission, and anthrax toxin disrupts multiple important biochemical signaling pathways. Other exotoxins interfere with normal cellular functions without killing cells, and yet other exotoxins stimulate the production of cytokines that cause infections. Immunity against intracellular bacteria encompasses a wide range of effector mechanisms derived from cellular and humoral activities of the host against the invading microorganism. In order to prosper and continuous their lifecycles, the intracellular pathogens must invade the host cells. However, this event triggers several alarms able to recognize not only the microbial components but also the hostderived damage-associated molecular patterns (DAMPs) released from infected tissues. Among these signal alarms, are the innate receptors represented by the membrane-associated toll-like receptors (TLR) and the cytosolic nucleotide binding and oligomerization domain (nod) like receptors (NLRs) which are determinant to the inflammatory responses against the pathogens and the outcome of infection. The NLRs are part of an intracellular network mechanism that induces the assembly of multiprotein complexes called inflammasomes .These receptors once able to sense the microbe components lead to production of proinflammatory cytokines such as interleukin-1 and the induction of inflammatory cell death through the activation of caspase-1. The immune system recognizes foreign bodies and responds with the production of immune cells and proteins. All animals have innate immunity, a defense active immediately upon infection. Innate immunity is present before any exposure to pathogens and is effective from the time of birth. Innate immunity consists of external barriers plus internal cellular and chemical defenses [5,6].
Materials and Methods
Innate immunity
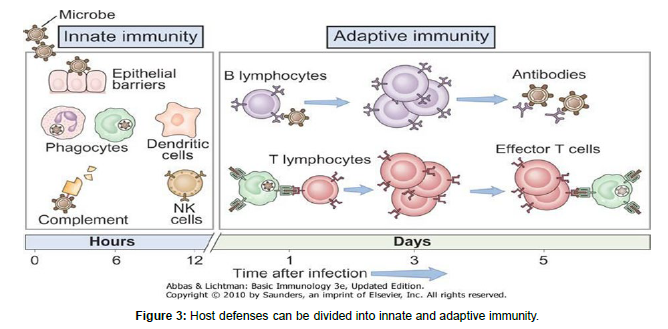
The term “innate immunity” was introduced to define the protective mechanisms against infection that operate in the absence of specific “adaptive” immunity. The immune system has been conceptually divided into innate and adaptive immunity. Innate immunity represents a rapid and stereotyped response to a large but limited number of stimuli. It is represented by physical, chemical, and biological barriers, specialized cells and soluble molecules, present in all individuals, irrespective of previous contact with offending agents or immunogens, and does not change qualitatively or quantitatively after contact. Resistance to bacterial infections is mostly mediated by innate immune mechanisms. Bacteria require specialized virulence factors to overcome innate defenses, and in these circumstances, the innate systems will strive to confine the infection until specific immunity is activated. The frequency and severity of opportunistic bacterial infections in patients with neutropenia, phagocytosis defects, or complement deficits demonstrates the significance of innate immunity. SCID mice that lack specific immunity, on the other hand, can survive in a clean environment (free of specific pathogens) due to innate immunity. Innate immune mechanisms, such as the presence of lysozyme or antimicrobial peptides, play a role in mucosal defense. When germs enter the internal environment, other cells such as complement, phagocytes, and natural killer cells (NK cells) respond.
Antibacterial peptides and proteins
Lysozyme is antimicrobial enzyme that can be found in mucosal secretions, tears, and saliva. The muramidase lysozyme degrades the peptidoglycan in bacterial cell walls. The peptidoglycan in Gram-positive bacteria is susceptible to lysozyme attack, but the outer membrane protects the peptidoglycan in Gram-negative bacteria. Other antimicrobial enzymes found in mucosal secretions are proteases and peroxidases. Lactoferrin, an iron-binding protein with antibacterial properties, is also found in mucosal secretions. Lactoferrin is involved in the hypoferremia due to the hepatic retention of iron and that is systemic nonspecific response to infection. Human phagocytic and secretory cells (such as Paneth cells) include LL-37 (a cathelicidin), defensins, and histatin-5 in their cytoplasmic granules. These short amphipathic peptides can distinguish between bacterial membranes with negatively charged lipids on their exterior surfaces and host cell membranes with neutral lipids on their outer leaflets. Antimicrobial peptides insert into bacterial membranes, subsequently disrupting the permeability barrier, mechanism of bacterial death. Moreover, some antimicrobial peptides are chemoattractants for inflammatory leukocytes. Although some Gram-negative and Gram-positive bacteria are naturally resistant to antimicrobial peptides, resistance transfer from a resistant to a susceptible strain appears improbable [7].
Collectins, Ficolins, and Pentraxins
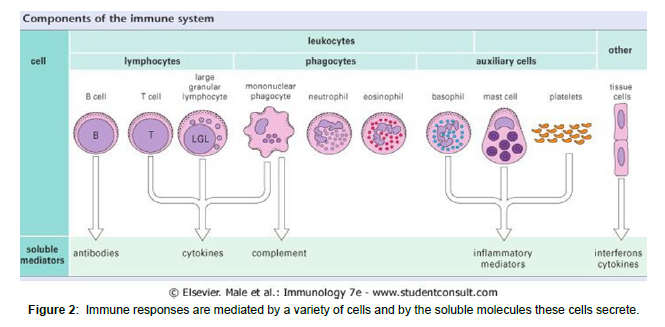
The first line of defense against bacterial infections includes lectins (sugar-binding proteins). Collectins, pentraxins, and ficolins are soluble PRRs that recognize microbial carbohydrates and activate defensive responses in reaction to them. Collectins are soluble proteins that belong to the C-type lectin superfamily (calcium-dependent lectins) (other C-type lectins are cell surface receptors). Collectins are polymers with a carbohydrate recognition domain (CRD) and a stalk that looks like collagen. Collectins bind to mannose, glucose, L-fucose, N- acetyl mannosamine, and Nacetyl-glucosamine, but not to other sugars [8]. In humans, three collectins have been identified: MBL (mannosebinding lectin), SP-A (surfactant protein A), and surfactant protein (SPD). MBL is a serum protein -produced by hepatocyte whose levels rise by 2-3 fold in response to an infectious stimulus. Staphylococcus aureus, Mycobacterium avium, Salmonella sp., Neisseria meningitidis, and Neisseria gonorrhoeae are among the Gram-positive and Gramnegative bacteria that MBL binds to MBL acts as an opsonin that facilitates the uptake of bacteria by phagocytes, although the exact identity of its receptor on the phagocyte surface is controversial MBL attachment to the bacterial surface activates the complement lectin pathway, resulting in opsonization, inflammation, and the development of a membrane attacks complex. The airway mucosal secretion contains SP-A and SP-D. Both collectins can interact with a variety of airway bacterial infections, to facilitate their uptake and killing by macrophages (Figure 1-3).
Complement system
Complement is a plasma protein network that is an important component of the innate immune system. Complement proteins are required for the quick clearance of invading bacteria by innate defense mechanisms such as inflammation, opsonization, and bacteriolysis. Complement proteins exist as inactive precursors in the blood and bodily fluids, but they are rapidly activated when they come into contact with bacterial cells. On the bacterial surface, an activated complement cascade produces a variety of responses that aid in the bacteria kill. The creation of ring-structured holes (the membrane attack complex, MAC) is the fastest response, killing Gram- negative bacteria within minutes. Nobel laureate Jules Bordet recognized bacteriolytic activity in 1895 when he discovered complement as a system in serum that allows antibodies in vaccinated animals to kill bacteria without the aid of immune cells. Complement is now known to be important not just for killing Gram-negative bacteria directly, but also for triggering a variety of additional innate processes such as the generation of chemo attractants, the marking of bacteria for phagocytosis, and intracellular killing by professional phagocytes. In most cases, proteolytic cleavage activates complement components, resulting in two fragments denoted by the letters “a” (small fragment) and “b” (the larger fragment, which usually acquires enzymatic activity).Complement activation can be initiated in three pathways.The alternative and lectin pathways are part of the innate immunity, whereas the classical pathway, that is evolutionarily more recent, requires in most cases a previous specific antibody response. All three activation pathways lead to a final lytic pathway. Spontaneous hydrolysis of complement component C3 leads to the formation of the bimolecular complex C3iB and the subsequent cleavage of factor B by factor D yields the complex C3iBb. C3iBb is a C3 convertase that initiates the alternative pathway. Alternative route activating surfaces include polysaccharide-containing bacterial cell walls. The lytic pathway begins with C5 cleavage.
Toll like receptor and nod like receptor
Innate immunity is the first line defense against bacterial infections. Activation of the innate immune system is triggered when pathogenassociated molecular patterns or damage-associated molecular patterns engage pattern recognition recepter (PRRs) on cells including epithelial cells, macrophages, and dendritic cells (DCs). Cells sensing infection using receptors that are predominantly expressed on sentinel cells referred to as ‘pattern recognition receptors (PRRs)’. PRRs are groups in to two these are nonsignaling PRRs that include soluble molecules such as collectins, pentraxins, and ficolins, and signaling PRRs that include transmembrane and cytosolic proteins which not only recognize microbial structures but also are able to trigger intracellular signaling pathways, eliciting inflammatory and antimicrobial responses. TLRs and Nod-like receptors (NLRs) are signaling PRRs involved in innate responses to bacterial pathogens. Toll is a receptor involved in the defensive response of Drosophila against fungal infections. Mammalians homologous of Toll are designated as TLRs and belong to the superfamily of interleukin-1 receptor. Humans have been found to contain ten functioning TLRs, six of which sense bacterial structures. TLR1/TLR2 recognizes triacyllipopeptides; TLR2/TLR6 recognizes lipoteichoic acid from Gram-positive bacteria’s cell wall and diacyllipopeptides from Mycoplasma; TLR2 recognizes bacterial lipoproteins, peptidoglycan from bacterial cell wall, lipoarabinomannan from Mycobacterium, and porins from Neisseria; TLR4 recognizes LPS from Gramnegative. TLR1, TLR2, TLR4, TLR5, and TLR6 are cell surface receptors, while TLR9 is present in the endosome. All these TLRs are expressed in monocytes/macrophages, and, with the exception of TLR6, also in dendritic cell. TLRs are expressed in various cell types that participate in epidermal or mucosal responses to bacterial infections, in addition to innate immune cells. In keratinocytes, TLR1, TLR2, TLR4, TLR5, TLR6, and TLR9 have been identified. When these cells recognize microbial infection; they engulf it and trigger inflammatory reactions. Adaptive immunity, on the other hand, is extremely specific, long-lasting, and has an immunological memory, but it develops in the late stages of infection. Antigen specific receptors produced on the surface of T and B cells, which are created. as a result of gene rearrangements, are essential for adaptive immune specificity.
Inflammation and phagocytosis
Inflammation is triggered when bacteria or their products interact with molecules and cells of the innate immune system. Extravasation of plasma and leukocytes from capillaries causes inflammation, which mobilizes multiple molecular and cellular effector mechanisms in a tissue threatened by pathogens. Histamine, prostaglandins, leukotrienes, bradykinin, and other mediators act directly on endothelial cells, increasing vascular permeability and causing plasma protein leakage and edema. Endothelial cells express E-selectin to retain circulating leukocytes after being activated by proinflammatory cytokines (IL-1, TNF-) or other mediators.The inflammatory focus produces a variety of chemotactic agents, including bacterial metabolites such as N-formyl-peptides, the complement fragment C5a, and chemokines (IL-8). Pathogens and cell detritus are removed by phagocytosis, a complex process carried out by cells in most organ systems. Activation of the inflammatory process is frequently followed by phagocytosis, which enhances pathogen clearance and reduces pathogen development. Phagocytosis is a vital biological function that helps to eliminate pathogens and infected or injured cells, promote tissue regeneration, and maintain homeostasis. Particles larger than 0.5mm are recognized by phagocyte transmembrane surface receptors and ingested into phagosomes, which are membrane-derived vesicles. After fusing with lysosomes, these phagosomes digest the cargo completely. Phagocytic leukocytes leave the capillary in response to chemotactic stimuli and migrate toward the chemoattractant source in the inflammatory. Phagocytes are specialized cells that ingest microbes and kill them. Polymorphonuclear leukocytes (mostly neutrophils) and mononuclear phagocytes are the two types of phagocytic leukocytes seen in mammals (monocytes and macrophages).A multitude of receptors on the surface of phagocytic leukocytes mediate the attachment and ingestion of bacteria. Some of these receptors stimulate phagocytosis by activating intracellular signals, whereas others may simply improve the efficiency of internalization. Two receptors involved in this are SR-A and MARCO. Gram-positive and Gram-negative bacteria; SR-A binds cell wall components lipoteichoic acid and LPS, whereas the precise ligands recognized by MARCO remain undefined. The macrophage mannose receptor permits microbial components to be internalized and is thought to be involved in phagocytosis. Complement receptors and receptors that identify the Fc domain of IgG are the major receptors implicated in opsonin- dependent phagocytosis. Bacteria coated with MBL, C1q, C4b, or C3b bind to CR1 (CD35), whereas bacteria coated with iC3b bind to CR3 (CD11b/ CD18): both receptors are found on macrophages and neutrophils, making all of these complement fractions nonspecific poisonings, At site of inflammation: tissue damage and complement activation cause release of chemotactic molecules .Activated phagocytes migrate across vessel walls and along concentration gradient to inflammatory site.
Natural killer cells
NK cells are cytolytic innate immune cells that lack the T cell marker CD3 and are identified by the expression of CD56. NK cells are made up of up to 15% of peripheral blood mononuclear cells and come from the bone marrow, which is home to secondary lymphoid tissues. Two primary subpopulations of NK cells may be identified based on the density of CD56 and CD16 surface expression, namely the cytotoxic CD56dimCD16bright and the immune regulatory CD56brightCD16dim subsets. Natural killer cells (NK cells) are a diverse population of innate immune cells capable of killing cells with low expression of MHC class I molecules. NK cells contribute to intracellular pathogens being exposed to antibodies by eliminating infected cells. NK cells play a significant part in viral infection resistance, but they are also implicated in bacterial pathogen nonspecific immunity. IL-12 has the ability to activate NK cells. IFN-γ, the major macrophage activating cytokine, is produced by activated NK cells. NKT cells are a type of T cell that expresses NK cell receptors and recognizes microbial glycolipids provided by CD1d, which is a nonclassical antigenpresenting molecule. NKT cells, for example, detect a diacylglycerol produced by Borrelia burgdorferi.
Acquired immunity
Innate and specific immunity molecules and cells work together to protect the host from pathogenic bacteria. The initial step in the formation of a specific immune response is the capture of bacterial antigens by antigen-presenting cells (APCs) and their presentation to T cells. APCs are nonspecific cells that express MHC-II proteins, such as macrophages and dendritic cells; TH cells identify foreign peptides in the context of self MHC-II molecules. APCs have a significant impact on the sort of specific response. Specific immunity, on the other hand, makes use of innate immunity’s effector systems, such as complement and macrophages. The antibody response, which includes B-cell maturation and antibody production, is co-ordinated by activated TH cells and B cells. Furthermore, through cooperating with cytotoxic T lymphocytes (CTLs) and producing macrophage-activating cytokines, activated TH cells are required for cell-mediated immunity.
T helper antigen recognition
Recognition of pathogens by receptors of APCs is followed by a number of events such as phagocytosis, expression of costimulatory molecules, upregulation of MHC-II molecules, and cytokine secretion. The exogenous pathway processes bacterial proteins that have been synthesized outside of APCs and ingested by pinocytosis or obtained from phagocytosed bacteria: protein antigens are degraded by lysosome proteases, and some of the resulting peptides form MHC-IIpeptide complexes that are presented to T CD4+ (TH) cells. Contacts between T CD4+ cells and APCs are made possible by CD4 affinity for the non polymorphic portion of MHC-II. The T-cell receptor (TCR) recognizes the bacterial peptide and polymorphic component of MHC-II, which triggers T cell activation. In the area of contact between the two cells (the “immunological synapse”) other signals are generated which complete the activation process: for example, co stimulatory molecules CD80 and CD86 bind the T-cell surface ligand CD28. Activation of naïve T CD4+ cells (TH0) culminates in their differentiation into either TH1 or TH2 cells. TH1 cells produce IL-2, IFN-γ, and TNF-β, whereas TH2 cells produce IL-4, IL-5, IL-10, and IL-13(Collin and Kauffmann, 2002). Since IL-2 is required for CTL activation and IFN-γ is critical in the activation of macrophages to increase their microbicidal potential, the TH1 response is considered central in the control of infection by intracellular pathogens. IL-4 and IL-5 production, in turn, stimulates the differentiation of activated B cells into antibody-producing plasma cells, resulting in an antibody response that is effective in controlling pathogens in the extracellular environment. Several factors influence the development of these T-cell subsets, including antigen dose, MHC-II and peptide density, levels and types of co stimulatory molecules expressed, state of APC activation, and local microenvironment; but the most important determining factor is the cytokine present at the time of TH0 activation The generation of TH1 is promoted by IL-12, IL-18, and INF-α, whereas IL-4 and IL- 10 induce TH2 cells.
Results and Discussion
Antibody response
The B cell receptor (BCR), which comprises a membrane immunoglobulin, allows B cells to connect directly with native antigens (mIg). Antigens are endocytosed and processed once bound to the mIg: the B cell operates as an APC, presenting MHC-II-peptide complexes to a previously activated TH cell. On the surface of both B and TH cells, a number of receptors and ligands are involved in the creation of costimulatory signals, which are required to complete B cell activation and cause immunoglobulin isotype switching. The main reaction begins with IgM class antibodies, which are gradually replaced by IgG isotype antibodies. Once the immunological memory has been established, successive responses to the same antigen are fast and powerful, and antibodies are of higher affinity than those formed in the primary response. The immunoglobulin isotype switching depends on the cytokine profile of the involved TH cell: for example, IL-4 and IL-5 act on B cells promoting the production IgG1, IgA, and Ig. The different immunoglobulin isotypes possess distinct properties, and therefore protect against bacteria in different ways. High-affinity IgG and IgA antibodies that are specific for the receptor-binding portion of a bacterial toxin are able to prevent the binding of toxin to the surface of target host cells. Similarly, high-affinity antibodies that recognize adhesins in the bacterial surface block the ability of bacteria to attach and invade host cells. Alternatively, IgG- coated bacteria bind to FcγRs in the surface of phagocytes, facilitating their uptake (specific opsonization). Immuno complexes formed by antigen molecules and specific IgM, IgG1, IgG2, and IgG3 antibodies initiate the classical pathway of complement activation with the consequences already mentioned: generation of inflammatory mediators and formation of the MAC. In many instances, the first step of infection is colonization and invasion of a mucosal surface. The mucosa associated lymphoid tissue (MALT) is responsible for specific immunity through mucosal surfaces. Antigens that translocate a mucosal barrier are captured by APCs and transported to immune inductive sites of MALT: Peyer’s patches and mesenteric lymph nodes for antigens from the gut lumen, and cervical lymph nodes or mediastinal lymph nodes for antigens from the upper or the lower respiratory tract, respectively. T and B lymphocytes exit inductive sites after activation and enter the lymphatic and blood circulations. Finally, effector and memory cells return to MALT when they reach the lamina propria, either from the colon or from the airways.
Super antigens
Bacterial super antigens are protein toxins that have the ability to cause significant immune system activation in humans. Super antigens are not endocytosed or processed by APCs; instead, they attach directly to APC MHC-II and the β chain of the α/β T-cell receptor chain, providing a bridge between the two cells. As a result, a significant number of T cells and APCs are stimulated in a reciprocal manner. Toxic shock syndrome is caused by the sudden and massive release of cytokines in response to super antigenic exotoxins from S. aureus and S. pyogenes. Super antigens are produced by Mycoplasma arthritidis and Yersinia enterocolitica, and have been linked to immunopathological sequelae of infection with these bacteria.
Cell-mediated immunity
Effector mechanisms are mediated by cells rather than antibodies in cell-mediated immunity. TH1 and CD8+ T cells are responsible for cell-mediated responses. IFN γ activates macrophages, increasing their bactericidal effectiveness; TNF-α activates neutrophils; and IL-2 delivers co stimulatory signals to enhance differentiation of activated CD8+ T cells into CTLs (Green) The presence of multifunctional CD4 T cells that express IFN-γ, TN.-α and IL-2 is linked to protection against intracellular microorganisms in general. However, the importance of MHC class I-restricted CD8+ T cells is becoming more widely recognized. Pathogen-derived peptides complexed with MHC-I molecules on the surface of infected cells are recognized by CD8+ T lymphocytes. CD8+ T cells primarily defend against microorganisms that bring antigens into the cytosol of infected cells because most peptides bound by MHC-I molecules are derived from cytosolic proteins. This is the situation with L. monocytogenes which, reaches the cytosol of infected cells after escaping the vacuolar compartment of infected macrophages by secreting listeriolysin O .Other intracellular infections such as salmonellae and mycobacteria, on the other hand, remain in phagocytic vacuoles, either because they avoid phagosomelysosome fusion or because they are resistant to intracellular bactericidal processes. The ability of these vacuolar pathogens to elicit particular CD8+ T cell responses suggests that, despite their initial vacuolar location, bacterial proteins reach the infected cell’s cytoplasm.
Conclusion
The development of an infectious disease involves complex interactions between the pathogen and the host. The key events during infection include the entry of the infectious agent, invasion and colonization of tissues. Overall the control of infectious pathogens requires different specialized immune responses, depending on the host tissue they replicate and its size. During the development of host protective immunity, the innate and adaptive immune systems cooperate in order to efficiently eliminate the infectious agent. The first line of defense of our body against infectious agents consists of mechanisms that exist before infection, which are capable of rapid responses to microbes and reacting in essentially the same way to repeated infections. This line of defense is made by physical and chemical barriers, such as epithelia and antimicrobial substances produced in the epithelial surfaces. In addition to these barriers, the body has other mechanisms which hinder the entry of the infectious agent, and proliferation within the tissues, such as the production of various antimicrobial agents. Extracellular Bacteria are spread by the blood and lymphatic systems, and intracellular pathogens multiply by cell-cell interaction, or by direct transmission from a cell to another or for the release of extracellular fluid. Extracellular bacteria are normally susceptible to phagocytosis and some of them develop resistance. Encapsulated gram-positive bacteria growing in the extracellular space and resistant to phagocytosis by the presence of a polysaccharide capsule. Inside the host tissues, the innate response will continuously fight against the pathogen through the action of phagocytic cells and natural killer cells, blood proteins including members of the complement system and other mediators of inflammation, and proteins called cytokines that regulate and coordinate many of the activities of innate immunity cells.
References
- Alsan M (2015) The effect of the tsetse fly on African development. Am Econ Rev 105: 382–410.
- Swallow, BM (2000) Impact of trypanosomiasis on African agriculture. PAAT Technical and Scientific Series.
- Shaw APM, Wintd B GC, GRW, Mattiolie RC, Robinson TP, et al. (2014) Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Prev Vet Med 113:197–210.
- NTTICC (2004) National Tsetse and Trypanosomosis Investigation and Control Center.
- Franco JR, Cecchi G, Priotto G, Paone M, Diarra A, Grout L, et al. (2018) Monitoring the elimination of human African trypanosomiasis: Update to 2016. PLoS Negl Trop Dis 12: 1–16.
- Shaw A, Wint W, Cecchi G, Torr S, Waiswa C, et al. (2017) Intervening against bovine trypanosomosis in eastern Africa: mapping the costs and benefits. Food and Agriculture Organization of the United Nations PAAT Technical and Scientific Series.
- Meyer A, Holt HR, Oumarou F, Chilongo K, Gilbert W, et al. (2018) Integrated cost-benefit analysis of tsetse control and herd productivity to inform control programs for animal African trypanosomiasis. Parasites and Vectors 11:1–14.
- Tekle T, Terefe G, Cherenet T, Ashenafi H, Akoda KG, et al. (2018) Aberrant use and poor quality of trypanocides: a risk for drug resistance in south western Ethiopia. BMC Vet Res 14: 4.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Umer AA (2023) Immunity to Bacterial Infections: A Review. J Vet Med Health 7: 183. DOI: 10.4172/jvmh.1000183
Copyright: © 2023 Umer AA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1895
- [From(publication date): 0-2023 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 1525
- PDF downloads: 370