Immune Reconstitution Inflammatory Syndrome Presenting as Psoriasis After Initiating Antiretroviral Therapy: A Case-Report.
Received: 21-Oct-2018 / Accepted Date: 15-Nov-2018 / Published Date: 25-Nov-2018 DOI: 10.4172/2332-0877.1000387
Keywords: Psoriasis; IRIS; HIV; AIDS; Cart; ART; Autoimmune disease
Introduction
The treatment of human immunodeficiency virus (HIV) infection with antiretroviral therapy has effectively changed the course of the epidemic. The use of combination antiretroviral therapy (cART), on a broad scale, has globally reduced the burden of the disease and allowed for a decline in the mortality from HIV and AIDS (Acquired Immune Deficiency Syndrome) and morbidity from the opportunistic infections associated with them [1]. The primary target of combination Antiretroviral therapy cART is to reinstate the host immune function by suppressing the replication of HIV [2]. Although cART has several clinical benefits, multiple concerns related to drug toxicities and metabolic side effects emerged over time [3]. In the most recent years, the side effect profile of these agents improved significantly, and clinicians gained experience in the management of such complications [4]. A well-known complication of cART is the immune reconstitution inflammatory syndrome (IRIS) [5]. IRIS is a morbid complication that may ensue with the initiation or reintroduction of cART [6]. The concept of IRIS was introduced at the 12th World AIDS Conference in Geneva in 1999, under the name ‘‘Immune Restoration Disease,’’ following numerous reports of conditions that worsen after starting cART [7]. IRIS can be clinically grouped into two categories based on the underlying pathophysiology: it is either caused by a previously subclinical infection that was unmasked by the immune response following the initiation of ART (unmasking IRIS) or by the paradoxical relapse of a recently treated opportunistic infection (paradoxical IRIS) [8]. To this moment, there is still no consensus on a clinically useful definition of IRIS. Several definitions of IRIS have been used, all of which focus on the “need to have an inflammatory component occurring in the setting of immune reconstitution that cannot be explained by drug toxicity or a new opportunistic infection” [9-12]. IRIS has highly variable clinical manifestations and those vary according to the associated pathogen [13]. Besides, the absence of clear-cut diagnostic criteria blurs the differentiation between IRIS and a more typical presentation of an undertreated primary disease [13, 14]. The incidence of IRIS ranges between 7-30% depending on the study [15,16] with an estimated mortality of 4.5% [6]. Several risk factors for IRIS have been identified such as the presence of OIs at cART initiation as well as a short interval between starting treatment for OI and cART initiation [17]. In addition, a lower baseline CD4 cell count is associated with increased incidence of IRIS, with the highest risk being at a CD4 cell count of 50 cells/μl or less [6,17]. Dermatologic manifestations of IRIS account for roughly half of the cases; significantly more women are affected than men [18,19] with a wide range of both infective (bacterial, viral, and fungal) and non-infective (inflammatory, neoplastic, and autoimmune) manifestations [20]. The management constitutes of conventional therapy for the associated condition, although some may be refractory to conventional therapy [20].
Psoriasis in HIV
Psoriasis is a chronic inflammatory skin disease with pathogenesis related to the balance between cytokines, innate immunity, and adaptive immunity [21]. Infection with HIV, especially advanced immune suppression can manifest as cutaneous diseases, such as psoriasis [22]. A 10-year cohort by Garbe et al. showed that the incidence of psoriasis in HIV-infected individuals was 6.4% as compared to 2% in the general population [22]. Additionally, patients with pre-existing psoriasis might undergo severe worsening or flares upon infection with HIV, becoming more severe as HIV progresses to AIDS [23]. Many mechanisms are postulated to explain the worsening of psoriasis in the HIV setting. The first hypothesis is related to the imbalance in the CD8+ T-cells to CD4+ T-cells ratio associated with advanced HIV. This would establish a good ground for psoriasis, which is CD8+ T cell-induced. Another plausible mechanism is the HIV induced imbalance of T regulatory cells (Treg) the safeguard cell responsible for immune regulation. The loss of this regulatory response leads to the development of excessive immune and autoimmune reactions such as psoriasis. A third theory is the direct involvement of HIV which acts as a co-stimulatory factor to CD8+ T-cell leading to the exacerbation of the skin immune response and worsening of psoriasis. This last theory is supported by the fact that HIV DNA was found inside cutaneous dendritic cells in HIV patients with psoriasis flares [20].
Psoriasis associated with HIV usually has a progressive clinical course and, in many cases, is refractory to traditional treatments (including topical agents, phototherapy, and oral retinoids) explaining its association with substantial morbidity and mortality [5,12,22,24], cART has been shown to improve psoriasis in such patients, particularly in moderate and severe disease [23,25]. As such, treatment is tailored based on the degree of disease severity (mild, moderate, or severe) and the input from an infectious disease specialist. In case of first-line treatment failure, biologics can be considered especially in combination with cART, which has been shown to have a positive effect on CD4 and viral counts [23-25]. Steady monitoring of the CD4 count, HIV viral loads, and potential adverse events is required in addition to regular consultation with an infectious disease specialist [25].
Case Presentation
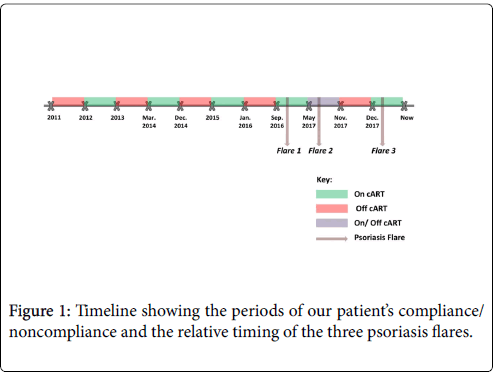
Here we present the case of a 39-year-old Lebanese man diagnosed with HIV towards the end of 2011; most likely contracted through same-sex intercourse. Our patient started treatment with emtricitabine, efavirenz and tenofovir-disoproxil-fumarate one year after his diagnosis. He was not compliant with his medications and attributed his noncompliance to the side effects of the medications (such as neuropathies) as well as to financial reasons. After initiating treatment, he became virally suppressed but with low CD4 counts ranging around 200 and an inverted CD4/CD8 ratio around 0.2 that failed to pick up. Table 1 depicts the patient's viral load, CD4 count, and CD4/CD8 ratio at different points in time (Figure 1).
| 2011 | 2013 | Mar | Dec | 2015 | Jan | Nov | Feb | Oct | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2016 | 2017 | ||||||||
| Viral Load (copies/ml) | 185000 | UD* | UD* | UD* | UD* | |||||
| CD4 Count (cells/μl) | 509 | 700 | 161 | 500 | 120 | 160 | 159 | 227 | 131 | |
| CD4/ CD8 Ratio | 0.2 | 0.19 | 0.23 | |||||||
Table 1: Table showing the patient's viral load, CD4 count, and CD4/CD8 ratio at different points in time, *UD= Undetectable
Our patient had three flares of psoriasis, all of which occurred upon reinitiating cART after periods of noncompliance. He had no previous history of psoriasis, no personal history of autoimmune diseases and no family history of psoriasis. His first flare occurred in mid- September 2016, after 6 months of being off cART. He had stopped all cART the CD4 count was 160 cells/μl. He was off therapy until September of the same year when he resumed the same antiretroviral regimens and noted a flare of psoriasis. At the time, he presented with confluent scaly silver plaques on the scalp, extensor and flexor surfaces of the legs and arms, hands, palms, and soles. A skin biopsy confirmed the diagnosis of psoriasis. Accordingly, a dermatologist-initiated methotrexate (three times weekly) in addition to cART (once daily). Psoriasis then resolved shortly after. The CD4 count 6 months before the flare while off cART was 160 cells/μl and 6 months after the flare and while on cART was 159 cells/μl. Noteworthy, he was virally suppressed at both times. Our patient reported taking cART inconsistently since May 2017, and he had his second flare of psoriasis in August 2017, after resuming the medication for a few days. His third flare was towards the end of December 2017, shortly after restarting cART earlier that month.
Discussion
Our patient is not compliant to cART with non-recovering CD4 counts since his diagnosis 8 years ago. He has presented with three flares of psoriasis each after re-initiation of cART as a picture of IRIS and resolved upon discontinuation of cART. He met one criterion of the proposed definition of IRIS that is the temporal relationship between ART initiation and the symptoms. On the other hand, he did not meet the viral load criteria; however, VL is not the best discriminator when tested by Haddow et al. and CD4 is to be used with caution [26]. The case we present here is the second reported case of IRIS manifesting as psoriasis. The first case reported in November 2015 was that of a 68-year-old man with a known history of psoriasis HIV and hepatitis C [27]. The patient presented one month after switching to (elvitegravir, tenofovir, cobicistat, emtricitabine) following noncompliance with his first cART regimen with picture highly suggestive of IRIS. Upon presentation Tripathi et al. describe confluent erythema affecting 10% of the patient’s skin diagnosed with psoriasis [27]. According to Seng et al., adherence to HIV treatment is fundamental to maximize the CD4/CD8 ratio recovery [28]. Similarly, a more recent study published in 2017 showed a decrease in the possibility of normalizing the CD4/CD8 ratio in the long term with treatment interruption [29]. This could explain the development of IRIS-psoriasis in our patient because of the imbalance in the CD4/CD8 ratio, as proposed by Lehloenya et al. [20]. Treatment noncompliance is the most plausible cause of this imbalance. Our patient was noncompliant with cART since his diagnosis with HIV in 2011, which lead to the question of whether IRIS is more likely to occur in the setting of treatment noncompliance. There is a scarcity of reports in the literature regarding a possible association. The studies that explored IRIS risk factors, such as the study, excluded the noncompliant patients [30].
Conclusion
We are describing a case of IRIS presenting as psoriasis in a patient with long-standing HIV infection with poor cART compliance over the past 8 years. To our knowledge, this is the second reported case in the literature. The patient has had three flares of psoriasis that coincided with the re-initiation of cART. We are proposing that this patient’s noncompliance with cART and the resulting low, nonrecovering CD4/CD8 ratio lead to IRIS presenting as psoriasis. Several sources have proposed that HIV-induced imbalance in the CD8/CD4 ratio is a possible cause of psoriasis. This may appear paradoxical since psoriasis is better described with advanced HIV and immunosuppression and its symptoms typically recede after the initiation of cART and immune restoration. However, data does elucidate to a dysfunction in regulatory T cells (Treg) that can probably account for psoriasis with IRIS similar to the dysfunction seen with HIV associated psoriasis. More research is needed in order to make the picture of these complex immune phenomena clearer.
References
- Sterne JA, Hernan MA, Ledergerber B, Tilling K, Weber R, et al. (2005) Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. The Lancet 366: 378-384.
- Hogg R, Lima V, Sterne JA, Grabar S, Battegay M, et al. (2008) Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. The Lancet 372: 293-299.
- Trickey A, May MT, Vehreschild JJ, Obel N, Gill MJ, et al. (2017) Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The Lancet HIV 4: e349-e356.
- Currier JS, Havlir DV (2017) CROI 2017: Complications and comorbidities of HIV disease and its treatment. Top antivir med 25: 77-83.
- Bartlett BL, Khambaty M, Mendoza N, Tremaine AM, Gewirtzman A, et al. (2007) Dermatological management of human immunodeficiency virus (HIV). Skin therapy lett 12: 1-3.
- Muller M, Wandel S, Colebunders R, Attia S, Furrer H, et al. (2010) Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet infect dis 10: 251-261.
- French M, Lenzo N, John M, Mallal SA, McKinnon EJ, et al. (2000) Immune restoration disease after the treatment of immunodeficient HIV infected patients with highly active antiretroviral therapy. HIV med 1: 107-115.
- Mayer KH, French MA (2009) Immune reconstitution inflammatory syndrome: a reappraisal. Clinic Infect Dis 48: 101-107.
- Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, et al. (2004) Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis 39: 1709-1712.
- French MA, Price P, Stone SF (2004) Immune restoration disease after antiretroviral therapy. Aids 18: 1615-1627.
- Lortholary O, Fontanet A, Mémain N, Martin A, Sitbon K, et al. (2005) Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. Aids 19: 1043-1049.
- Shelburne SA, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, et al. (2002) Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine 81: 213-227.
- Kestens L, Seddiki N, Bohjanen PR (2008) Immunopathogenesis of immune reconstitution disease in HIV patients responding to antiretroviral therapy. Curr Opin HIV AIDS 3: 419-424.
- Walker NF, Scriven J, Meintjes G, Wilkinson RJ (2015) Immune reconstitution inflammatory syndrome in HIV-infected patients. HIV/AIDS (Auckl) 7: 49-64.
- Grant PM, Komarow L, Andersen J, Sereti I, Pahwa S, et al. (2010) Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PloS one 5: e11416.
- Ratnam I, Chiu C, Kandala NB, Easterbrook PJ (2006) Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1–infected cohort. Clinic Infect Dis 42: 418-427.
- Shelburne SA, Montes M, Hamill RJ (2005) Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother 57: 167-170.
- Huiras E, Preda V, Maurer T, Whitfeld M (2005) Cutaneous manifestations of immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS 3: 453-460.
- Osei Sekyere B, Karstaedt A (2010) Immune reconstitution inflammatory syndrome involving the skin. Clin Exp Dermatol 35: 477-481.
- Lehloenya R, Meintjes G (2006) Dermatologic manifestations of the immune reconstitution inflammatory syndrome. Dermatologic clinics 24: 549-570.
- Lowes MA, Bowcock AM, Krueger JG (2007) Pathogenesis and therapy of psoriasis. Nature 445: 866-873.
- Mallon E, Bunker C (2000) HIV-associated psoriasis. AIDS patient care and STDs 14: 239-246.
- Morar N, Willis-Owen SA, Maurer T, Bunker CB (2010) HIV-associated psoriasis: pathogenesis, clinical features, and management. Lancet infect dis 10: 470-478.
- Nakamura M, Abrouk M, Farahnik B, Zhu TH, Bhutani T (2018) Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis 101: 38; 42; 56.
- Menon K, Van Voorhees AS, Bebo BF, Gladman DD, Hsu S, et al. (2010) Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol 62: 291-299.
- Haddow LJ, Easterbrook PJ, Mosam A, Khanyile NG, Parboosing R, et al. (2009) Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis 49: 1424-1432.
- Tripathi SV, Leslie KS, Maurer TA, Amerson EH (2015) Psoriasis as a manifestation of HIV-related immune reconstitution inflammatory syndrome. J Am Acad Dermatol 1: e35-e36.
- Seng R, Goujard C, Krastinova E, Miailhes P, Orr S, et al. (2015) Influence of lifelong cumulative HIV viremia on long-term recovery of CD4+ cell count and CD4+/CD8+ ratio among patients on combination antiretroviral therapy. Aids 29: 595-607.
- Raffi F, Le Moing V, Assuied A, Habak S, Spire B, et al. (2016) Failure to achieve immunological recovery in HIV-infected patients with clinical and virological success after 10 years of combined ART: role of treatment course. J Antimicrob Chemother 72: 240-245.
- De D, Sarkar RN, Phaujdar S, Bhattacharyya K, Pal HK (2011) Incidence and risk factors of immune reconstitution inflammatory syndrome in HIV-TB coinfected patients. Braz J Infect Dis 15: 553-559.
Citation: Cheaito MA, Khalifeh M, Jaafar B, Rizk N (2018) Immune Reconstitution Inflammatory Syndrome Presenting as Psoriasis After Initiating Antiretroviral Therapy: A Case-Report. J Infect Dis Ther 6: 388. DOI: 10.4172/2332-0877.1000387
Copyright: © 2018 Cheaito MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3696
- [From(publication date): 0-2018 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 2962
- PDF downloads: 734