Case Report Open Access
Immune Reconstitution Inflammatory Syndrome (IRIS) in a Child with Disseminated Tuberculosis
Ba ID*, Thiongane A, Ba A, Faye PM, Sow A, Deme/Ly I and Ba MDepartment of Pediatric Pulmonology, University Cheikh Anta Diop of Dakar, BP 16109 Dakar-Fann, Senegal
- *Corresponding Author:
- Idrissa Demba BA
M.D. Pediatric Pulmonology
University Cheikh Anta Diop of Dakar
BP 16109 Dakar-Fann
Senegal
Tel: (+221)777647125
E-mail: docteuridy@hotmail.com
Received date: August 31, 2015; Accepted date: September 25, 2015; Published date: October 5, 2015
Citation: Ba ID, Thiongane A, Ba A, Faye PM, Sow A, et. al (2015) Immune Reconstitution Inflammatory Syndrome (IRIS) in a Child with Disseminated Tuberculosis. Interdiscip J Microinflammation 3:134. doi: 10.4172/2381-8727.1000134
Copyright: © 2015 Ba ID et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
IRIS is a paradoxical reaction occurring in HIV-infected patient after initiation of highly active antiretroviral therapy (HAART). Incidence is estimated 12 to 19% of child starting HAART. IRIS with tuberculosis in non-HIV patients has not been reported in the literature. We report the case of an HIV negative 8 years-old girl who did develop IRIS and dilated cardiomyopathy during her treatment for severe disseminated TB (lungs, mediastinal lymph nodes, spleen, liver and kidneys). An appropriate antibiotic treatment was begun against the multiple sensitive mycobacterium tuberculosis strain retrieved: RHZE for 2 months followed by RH 10 months. The child appeared stable the 2 first weeks on treatment but not apyretic. She remained in the hospital for 6 months and experienced daily recurrent fever 39-40°C with rigors, diffuse bone pains, elevated transaminases and deterioration of chest infiltrates. Sequential abdominal CT and Gallium scans demonstrated a progressive involvement of liver, spleen, kidneys, medullar bones and abdomen lymph nodes for 2 weeks to 5 months. Then she gradually improved. Her complete immunological work-up at that point was considered normal. We concluded from an extensive review of the literature that this case highlights a paradoxical immune mediated reaction to Mycobacterium tuberculosis during an appropriate anti-TB treatment. IRIS results in clinical deterioration but is not a failure to treatment. Early recognition of IRIS could prevent unnecessary changes in anti-TB regimen.
Keywords
Immune reconstitution syndrome; Tuberculosis; HIV; Highly active antiretroviral therapy
Introduction
Immune reconstitution inflammatory syndrome (IRIS) is a paradoxical reaction initially described in HIV-infected patient after the initiation of an antiretroviral therapy (ART). IRIS is mostly seen in HIV-infected patients [1]. The incidence is estimated between 12 to 19% in children starting ART [2-4]. Tb associated IRIS is characterized by a clinical worsening after the start of ART in patients receiving Tb treatment. It can also be diagnosed as a new presentation of Tb that is «unmasked» in the weeks following the initiation of ART and the exclusion of other causes that could explain a deterioration (such as antimicrobial drug resistance, drug hypersensitivity reaction, or another opportunistic infection) [5]. IRIS in association with tuberculosis in non-HIV patients has not been reported in the literature. We reported the case of IRIS in a child-disseminated tuberculosis.
Case Report
An 8-old-year girl was referred to our hospital for fever and abnormal CXR, no medical investigation upon her arrival in Canada 3 years ago, unknown immunization status and no pets. Medical history noticed right thigh pain, weight loss over 2 months with sleepiness, fatigue and lack of energy. She coughed for 3 weeks presenting dyspnea on exertion. She suffered neither from any sputum, hemoptysis, thoracic pain nor wheezed any gastrointestinal symptoms.
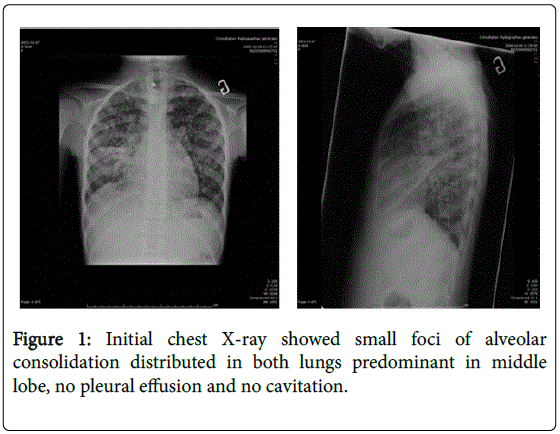
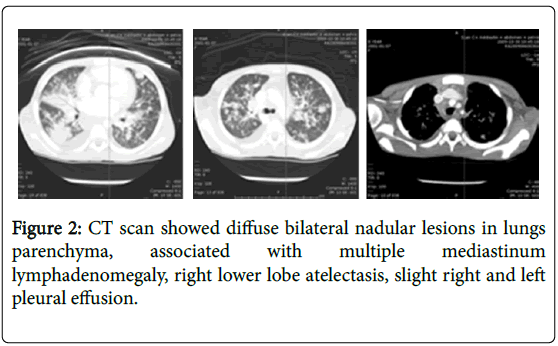
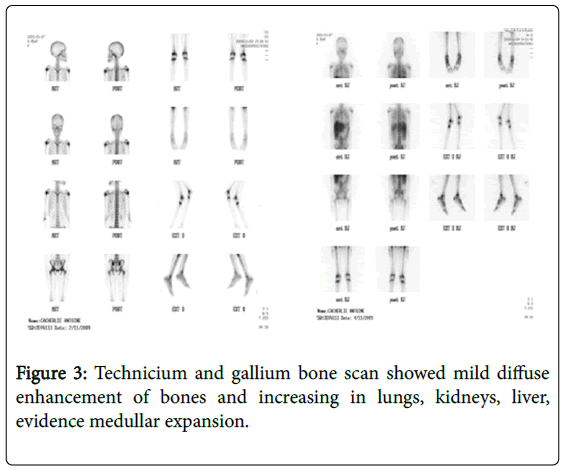
When arrived, the temperature was 39.1 degree centigrade, the weight below 3rd percentile and the height on the 10th percentile. Normal blood pressure and SpO2 98% on air room. On examination, she was cachectic, pale conjunctivae, no clubbing and peripheral oedema. The respiratory system exam found normal thorax, tachypnea (respiratory rate=28/min.), no indrawing, no grunting or stridor diffusing crackles lung bases (right>left). Cardio-vascular system objectived tachycardia (heart rate=172/min) and well perfused. Her abdomen was soft without any hepato-splenomegaly. She hadn’t any lymphadenopathy and neurological signs. The hemogram showed hypochromic microcytic anemia (Hb=59 g/L), leukocyte count of 5430/μl with polymorphonuclear predominance and platelet count normal. Serum electrolytes and creatinine were normal. C-reactive protein (CRP) was 60 mg/l; Liver transaminases were mildely elevated (ALT: 42 and AST: 78 IU/L, respectively); normal capillary gas. Mantoux test was non-reactive. The chest radiograph showed small foci of alveolar consolidation distributed in both lungs predominant in middle lobe, no pleural effusion or cavitation (Figure 1), right femur and pelvic X-ray normal. The chest computed tomographic (CT) (Figure 2) showed diffuse bilateral nodular lesions in the lung parenchyma, predominant in middle lobe associated with multiple mediastinum lymphadenomegaly, right lower lobe atelectasis, slight right and left pleural effusion. Abdomen and pelvis CT scan findings were heterogeneous spleen with multiple hypodensities, homogenous liver and pancreas, homogenous kidneys, ascites in mild to moderate. She still complained both persistent right thigh pain and diffuse bone one. So technitium and gallium bone scan (Figure 3) performed, showed mild diffuse enhancement of long bones, pelvis, skull and increasing activity in lungs, kidneys, liver, evidence medullar expansion. Gastric aspirate and sputum induction were positive for acid fast bacilli (AFB). PCR for Mycoplasma pneumonia and Chlamydia one were negative, blood culture isolator, Viral IF, Legionella antigen were also negative (urine). Eye exam was normal.
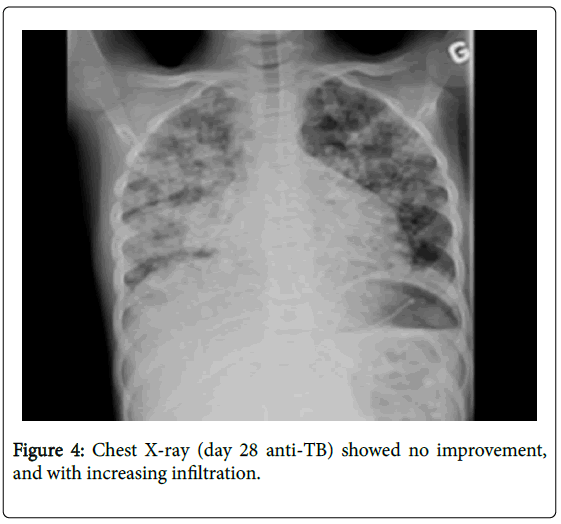
So, our diagnosis was disseminated TB (pulmonary, lymph nodes, liver, spleen, kidney localizations) with possible bone marrow involvement. Comorbidities were severe malnutrition (BMI=14 kg/ cm2) and severe anemia. HIV serologies were negative twice. An anti- TB treatment was initiated (RHZE 2 months; RH 10 months), adjuvant therapy (oral Pyridoxine) and nocturnal gavage (a total of 74 days), psychosocial and multidisciplinary fellow-up. She appeared stable the first week on anti-TB treatment, but not apyretic (38°-39°C), tachycardia, dyspnea at rest, gallop was noticed. Echo-cardiography showed left ventricular dilatation with diffuse ventricular hypokinesia and severe diastolic dysfunction; EKG found signs of left venticular dilatation. We conclude associated dilated myocardioathy probably multifactorial etiologies (tuberculosis, anemia and severe malnutrition). She benefited digoxin, diuretics, captopril, Steroids (Prednisone (1 mg/Kg/jour) and blood transfusion. Despite the start of the anti-TB treatment, she continued to have persistent fever (peak fever 40°C), both diffuse bone pains persisted and liver transaminases increased. Chest X-ray (day 28 anti-TB) (Figure 4) showed no improvement, and with increasing infiltration. Abdominal ultrasound (repeated) Liver enlarged (9.2 cm) and scattered by multiple nodules, Spleen stuffed micronodules, innumerable abdominal lymph nodes, Kidney lesions. Differential diagnosis on day was: multiple resistant TB, neoplasia (lymphoma), related anti-TB drug (drug toxicity), connective tissue diseases or other immune diseases. Performed bronchoscopy and bronchoalveolar lavage (BAL) found enlarged carina with a 20% reduction in calibrum of the main right brochus and inflammatory mucosae; culture isolated Hemophilus, klebsiella , Strepotococcal spicies in BAL specimen and no Mycobaterium, Legionella or Pneumocystis. We did further investigations including immunology and hematology assessment (both normal exhaustive immunologic work-up and normal bone marrow biopsy) and comprehensive assessement: infectious work-up (viral, parasites and fungi) negative, spleen biopsy (auramine negative), liver biopsy x 2 (auramine doubtful) and normal lumbar punction. At the end of the first month of the anti-Tb treatment, we decided to modify the anti-TB regimen by introducing second-line anti-TB drugs (IV Amikacin, Levaquin and Ethionamide, and Ethambutol discontinued). And steroid doses (Prednisone) increased up to 2 mg/Kg/day. Finally we got a strain susceptibility and the strain mycobaterium tuberculosis was very sensitive to R, I, Z, and E and the treatment was continued by RH for 10 months.
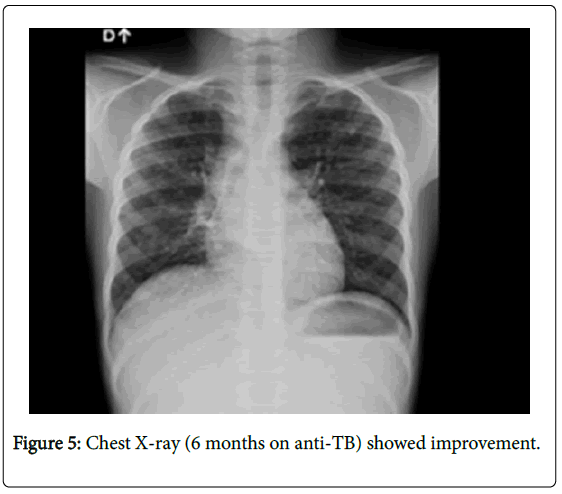
Our final diagnosis was immune reconstitution inflammatory syndrome (IRIS) in a negative HIV-patient. It took many weeks before IRIS manifestations decreased despite a good gain weight, resolution of CX-ray abnormalities and recovery of cardiac function. She was discharged after five months of hospitalization. Chest X-ray on 6 month-anti-TB (Figure 5).
Discussion
IRIS is a phenomenon observed in patients recovering from immunodeficiency. Generally, two clinical scenarios commonly occurred: an unmasking IRIS underlying unrecognized infection flares after starting ART; and parodoxical IRIS, resulting as the exacerbation of an often past known treated infection [5]. Pathogenesis of IRIS involve a combination of underlying antigenic burden, the degree of immune restoration following HAART, and host genetic susceptibility. These pathogenic mechanisms may interact and likely depend on the underlying burden of infectious or noninfectious agent [5-7].
In children, IRIS incidence is estimated between 12 and 19% of all children who initiate antiretroviral therapy [2-4] with a higher incidence in patients who have been diagnosed with an active opportunistic infection at the time of ART initiation [8,9]. Most data concerning IRIS originated from limited ressources settings. Risky factors for the development of IRIS include a high baseline HIV viral load before the initiation of ART, rapid decline in HIV viral load after initiation of ART, a lower baseline CD4 count before ART initiation, a high antigenic burden and disseminated opportunistic infection, initiation of ART concomitant with or soon after the initiation of the treatment of an acute infection, and lack of exposure to ART before initiating the current regimen [7]. IRIS causes in HIV-infected patients include infectious etiologies and non infectious causes [6]. Recent report also documented IRIS due to Mycobacterium bovis BCG in HIV-infected children in whom ART was initiated [10,11]. TB associated IRIS is characterized by a clinical worsening after the start of ART in patients receiving Tb treatment. It can also be diagnosed as a new presentation of TB that is «unmasked» in the weeks following the initiation of ART and the exclusion of other causes that could explain a deterioration [5].
TB is among the most frequent reported pathogen associated with IRIS. TB-IRIS can have a variety of clinical manifestations: fever, pulmonary disease, disseminated TB, systemic reaction inflammatory syndromes, acute renal failure [12]. Transient worsening of patient has been reported after the initiation of ART in severely immunocompromised patients [13,14] and paradoxical CNS TB reactions are well described in both children and adult HIV-negative patients [15,16]. No treatment has been prospectively studied in IRIS patients. Non-steroidal antiinflammatory drugs (NSAIDS) or corticostroids are commonly used to decrease inflammation in patients with severe IRIS [7,17].
Indeed, IRIS are not previously described in literature in negative HIV patients. We think that severe malnutrition is the main clinical risk causing immune impairement by developing IRIS in negative HIV patients. The mechanism is similar unlike in positive HIV by the restoration of the immune system. Genetic host susceptibility may certainly play a role in the development of this syndrome.
We concluded from an extensive review of the literature that this case highlights a paradoxical immune mediated reaction similar to the previously described IRIS in infected HIV patients but this time to Mycobacterium tuberculosis during an appropriate antibiotic treatment. Such an IRIS resulted in an apparent clinical deterioration but turned out not being a failure to treatment. In the future, early recognition of an IRIS in tuberculosis might prevent unnecessary changes in anti-TB regimen.
Acknowledgments
We thanks the Ste-Justine University Hospital Center, particularly the Pediatric Pulmonology staff and Infectious diseases staff, Dr. Guy Lapierre, Dr. Jacques-Edouard Marcotte, Dr. Denis Bérubé, Dr. Chantal Buteau, Dr. Sheila Jacob and Dr Mamadou Ba (Albert Royer Children’s Hospital, Dakar (Senegal), for all support during fellowship at Montreal.
References
- Shelburne SA, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, et al. (2002) Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 81: 213-227.
- Smith K, Kuhn L, Coovadia A, Meyers T, Hu C, et al. (2009) Immune reconstitution inflammatory syndrome among HIV-infected South Africans inititing antiretroviral therapy. AIDS 23:1097-1107.
- Thanyawee P, Peninnah O, Nuthapong U, Noppadon A, Suchart P, et al. (2006) Immune reconstitution syndrome from nontuberculous mycobacterial infection after initiation of antiretroviral therapy in children with HIV infection. Pediatr Infect Dis J. 25: 645–648.
- Wang M E., Castillo ME, Montano S M, Zunt J R. (2009) Immune Reconstitution Inflammatory Syndrome in Human Immunodeficiency Virus-Infected Children in Peru. Pediatr Infect Dis J. 28 : 900-903.
- French MA (2009) HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 48: 101-107.
- Murdoch M D, Venter DW, Van Rie A and Feldman C (2007) Immune reconstitution inflammatory syndrome (IRIS). Review of common infectious manifestations and treatment options. AIDS Res and Ther 4:9.
- Beatty GW (2010) Immune reconstitution inflammatory syndrome. Emerg Med Clin North Am 28: 393-407.
- Ratnam I, Chiu C, Kandala NB, Easterbrook PJ (2006) Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis 42: 418-427.
- Shelburne SA, Montes M, Hamill RJ (2006) Immune reconstitution inflammatory syndrome: more answers, more questions. J AntimicrobChemother 57: 167-170.
- Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, et al. (2006) BacilleCalmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis 42: 548-558.
- Hesseling AC, Schaaf HS, Hanekom WA, Beyers N, Cotton MF, et al. (2003) Danish bacilleCalmette-Guérin vaccine-induced disease in human immunodeficiency virus-infected children. Clin Infect Dis 37: 1226-1233.
- Lawn SD, Bekker LG, Miller RF (2005) Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 5: 361-373.
- Kiwanuka J, Graham SM, Coulter JB, Gondwe JS, Chilewani N, et al. (2001) Diagnosis of pulmonary tuberculosis in children in an HIV-endemic area, Malawi. Ann Trop Paediatr 21: 5-14.
- Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, et al. (2004) Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis 39: 1709-1712.
- Hejazi N, Hassler W (1997) Multiple intracranial tuberculomas with atypical response to tuberculostatic chemotherapy: literature review and a case report. Infection 25: 233-239.
- Teoh R, Humphries MJ, O'Mahony G (1987) Symptomatic intracranial tuberculoma developing during treatment of tuberculosis: a report of 10 patients and review of the literature. Q J Med 63: 449-460.
- Marais BJ, Graham SM, Cotton MF, Beyers N (2007) Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis 196 Suppl 1: S76-85.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 14635
- [From(publication date):
July-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 13611
- PDF downloads : 1024