Research Article Open Access
Immune Modulatory Function of Human Cytomegalovirus-Encoded UL128 in the Pathogenesis of Infection Related Hearing Loss
Zheng Qi1,2, Tao R1, Gao H1, Xu J1, Sun Y3, Zhao N1, Gu W4 and Shang S1*
1Zhejiang Key Laboratory for Diagnosis and Therapy of Neonatal Diseases, P.R. China
2Departments of Rheumatology and Immunology, Children's Hospital Affiliated to the Medical College, Zhejiang University, Hangzhou, China
3Department of Otolaryngology, Children's Hospital Affiliated to the Medical College, Zhejiang University, Hangzhou, China
4Department of Pathology, Children's Hospital Affiliated to the Medical College, Zhejiang University, Hangzhou, China
- *Corresponding Author:
- Shang S
Zhejiang Key Laboratory for Diagnosis and Therapy of Neonatal Diseases
3333# Binsheng Road Hangzhou, P.R. China
Tel: 86-571-87061007
Fax: 86-571-87033296
E-mail: shangshiq@163.com
Received date: June 02, 2017; Accepted date: June 27, 2017; Published date: June 30, 2017
Citation: Zheng Qi, Tao R, Gao H, Xu J, Sun Y, et al. (2017) Immune Modulatory Function of Human Cytomegalovirus-Encoded UL128 in the Pathogenesis of Infection Related Hearing Loss. J Infect Dis Ther 5:322. doi: 10.4172/2332-0877.1000322
Copyright: © 2017 Zheng Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Inflammatory cytokines such as tumor necrosis factor (TNF)-a, interleukin (IL)-6 were supposed to participate in the tissue damage of human cytomegalovirus (HCMV) infection related diseases. The pathogenesis of HCMV associated hearing loss is poorly understood mainly due to the lack of applicable virus infected model. We subjected cochlear epithelial cell line derived from human auditory progenitors to HCMV infection and examined the levels of cytokines released in the co-cultural supernatant of peripheral blood monocytes and HCMV pre-infected cochlear epithelial cells. Human cochlear epithelial cell line cultured in vitro is replicable and permissive for HCMV productive infection. Detection of the co-culture system revealed up-regulation of cytokines including IL-6 and TNF-α. Since HCMV UL128 protein interferes with virus entry into epithelial cells as well as eliciting primary antibody in vivo, transcription of HCMV UL128 gene was suppressed by short interference RNA in this experiment. Cytokine analysis revealed that UL128 deficient HCMV got impairment of inducing IL-6 and TNF-α production when interacted with host immune cells. We deduce that UL128 may serve as a viral immune modulator participating in the pathology of HCMV related hearing loss and UL128 gene can be a potential target for treatment of infection induced immune pathological damage.
Keywords
UL128; Auditory epithelial cell; Human cytomegalovirus; Hearing loss; Inflammation
Abbreviations
HCMV: Human Cytomegalovirus; GPCMV: Guinea Pig Cytomegalovirus; SNHL: Sensorineural Hearing Loss; cDNA: Complementary DNA; MEM: Modified Eagle's Medium; FCM: Flow Cytometry; PCR: Polymerase Chain Reaction; MIP-1α: Microphage Inflammatory Protein-1α; IE: Immediate-Early; PP65: Phosphoprotein 65; CPE: Cytopathic Effect; IL-6: Interleukin 6; TNF-α: Tumor Necrosis Factor-Alpha; FCS: Fetal Calf Serum; EGF: Epidermal Growth Factor; RNAi: RNA Interference; siRNA: Small Interfering RNA; PBMC: Peripheral Blood Monocyte.
Introduction
Sensorineural hearing loss (SNHL) is one of the most common birth defects in infants. Besides of the genetic factor, human cytomegalovirus (HCMV) infection is the most common pathogen giving rise to hearing loss [1]. Approximately 22%–65% of symptomatic HCMV infected children and 6%–23% of asymptomatic children will develop hearing loss [2,3]. Delayed hearing loss is associated with persistent HCMV shedding during childhood [4]. Recent studies display that virus-induced proinflammatory cytokines play an important role in the pathogenicity of HCMV infection, but the detailed mechanism remains unknown.
When encountered exogenous molecules, the inner ear can rapidly generate immune responses which may lead to cochlear cells degeneration. Since human sensory epithelial cells lack regenerative capability in vivo, degeneration of these cells results in permanent hearing loss [5]. Inflammation of the inner ear is mediated and amplified by the recruitment of circulating leukocytes to the sites of infection. This process is initiated by chemokines, a group of small molecules that can modulate functions of leukocyte as well as chemotaxis. Four kinds of chemokines classified as C, CC, CXC or CX3C are based on the arrangement of 1 or 2 N-terminal cysteine residues [6].
In order to establish lifelong infection, HCMV has interacted with its host by incorporating genes that are homologous to host cellular genes [7]. Virally encoded immune modulatory genes interrupt host immunity by disruption of the normal balance of immune system; therefore play an important role in immune-mediated hearing loss [8]. A specific viral immunomodulatory gene product (macrophage inflammatory protein 1 alpha (vMIP1α)) which functionally mimics of human CC chemokine is found to contribute to auditory pathologic findings in the guinea pig model. Guinea pigs infected by cytomegalovirus with deleted MIP-1α have significant better hearing than wild-type CMV infected ones [9]. Because of CMV species-specific infection pattern and difficulty in requirement of human auditory model, little is known about the influences of HCMV encoded chemokine on hearing loss and infectious related inflammation in inner ear. Recently, inspiring studies have shown that human fetal auditory stem cells (hFASCs) can be expanded and differentiated into functional auditory hair cell-like cells in vitro [10] which provided a cell model for our study.
When comparing immune modulators encoded by HCMV with those of guinea pig cytomegalovirus (GPCMV) and human CC chemokine, we found that amino acid sequences of HCMV UL128 were similar to human CC chemokine as well as GPCMV MIP-1α. UL128 protein has been proved to be a key factor in HCMV entry into epithelial and endothelial cells by binding with UL130-UL131 gene products in the virus envelope [11]. The trimer pUL128–131 was assumed to be immunodominant in eliciting a neutralizing antibody response in vivo, which confirm the significant role of UL128 protein in the interaction between virus and human immune responses [12]. In our previous study, we proved that recombinant UL128 protein could recruit peripheral blood monocytes [13] and promote PBMC proliferation through MAPK/ERK transduction pathway [14]. In the present study, we hypothesized that UL128 may play an important role in the initiation of HCMV related inner ear inflammation.
Materials and Methods
Cell preparation
Cochlear epithelial cells: The experimental protocol was approved by the ethics committee from the medical school of Zhejiang university. Informed consent from patients underlying labyrinthectomy was obtained before collection of cochlear tissues. The tissue was carefully harvested during surgery. Using an otologic drill, bones were removed from semicircular canals and the sensory epithelia were teased away from their neuronal attachments. Freed tissue was immediately placed in a specimen disk containing MEM (GIBCO) supplemented with 0.05 mg/ml ampicillin (MEM/Amp) and incubated overnight at 37°C as a preliminary step to increase cell viability. Epithelial patches were then lifted and dissociated by incubation with 0.125% trypsin in Phosphate Buffered Saline (PBS) at room temperature for 20-30 min. Cell clusters were underwent gently mechanical dissociation using a 1000-μl pipette tip and then, a 200-μl pipette tip. After that, the plate was tapped gently and the media containing the floating cells transferred to a fresh plate using high glucose DMEM with 10% FCS. After 24 h, the culture media was replaced with high glucose DMEM mixed with F12 (50:50) including N2 and B27 supplements (Invitrogen) and human recombinant EGF (20 ng/ml) was added conductive to growing of epithelial cells.
Purification of cochlear epithelial cells: Epithelial cells were enriched and repeated differential adhesion as well as enzyme digestion to minimize the contamination of mesenchymal fibroblasts. When digesting cells, the fibroblasts were much easier to be removed from the wall than epithelial cells. According to this difference, epithelial cells and fibroblasts are separated by multiple differential digestions. Fibroblast cells adhere faster than epithelial cells. Most fibroblast cells adhere in a short period of time (about 30 min), while epithelial cells were instability in a short time especially in serum-free medium. So suspension cells were inoculated in a culture dish and set aside for 30 min. After some cells adherent by observation under inverted microscope, we collected the suspension cells to a new culture dish and set aside for another 30 min. Repeated adherence and collecting suspension cells can purify epithelial cells. Suspended cells were then divided into different population, and low-passage epithelial cells were used in the following experiment.
Peripheral blood mononuclear cell (PBMC): PBMC from whole blood of healthy volunteers were isolated by standard density centrifugation (Ficoll Separation Solution: GIBCO, Germany). After centrifugation the mononuclear cells were collected and washed in phosphate-buffered saline (PBS, pH 7.2) two times. By adjusting to 1 × 106 cells/ml, the cells were re-suspended in MEM medium with 10% heated-activated fetal calf serum.
Co-culture of cochlear epithelial cells and PBMC in vitro: Cochlear epithelial cells (1 × 104 cells/well) transfected with UL128 specific siRNA were seeded in a 24-well plate 6 h for attaching, then culture media was changed with fresh medium without serum in it, and HCMV was seeded afterwards. The culture media was replaced by MEM+10% FCS two hours later and PBMC (2 × 105 cells/well) were co-cultured with epithelial cells simultaneously. Similar co-culture system had been proved to be useful in studying the interaction between microbial and host [15].
Virus infection
The HCMV strain Toledo was used throughout the whole experiment. The virus stocks were prepared cell-free. Wiled HCMV strain Toledo was kindly provided by Infectious Disease Laboratory of the First Affiliated Hospital of Zhejiang University School of Medicine. Virus titers were determined by plaque assays on primary HELFs. HCMV infected cochlear epithelial cells was identified through CPE and encoding of HCMV IE gene (IE-cDNA) and expression of virion particle–associated protein pp65 in the host cells. Monoclonal mouse anti-pp65 antibody (1:200, Novatragen) was used in cellular immunofluorescence assay.
Immunocytochemistry
Cells were transferred into 24-well plates with coverslips and cultured in DMEM supplemented with 10% FBS overnight, then fixed in PBS buffered 4% paraformaldehyde, and washed three times with PBS, then permeated and blocked in 0.1% Triton X-100 with 5% normal goat serum for 20 min. Coverslips were incubated with rabbit monoclonal anti-Nestin antibody (1:200, Millipore), rabbit monoclonal anti-SOX2 antibody (1:100, Epitomics), mouse polyclonal anticytokeratin 18 (CK18) antibody (1:100, Santa Cruz), mouse monoclonal anti-Math1 antibody (1:100, Epitomics), and rabbit monoclonal anti-p27Kip1 antibody (1:200, Millipore) at 37 for 2h. After washing with PBS (5 min × 3 times), coverslips were incubated with fluorescein isothiocyanate (FITC) – or cy3-conjugated secondary antibody (1:200, Epitomics) to reveal the cell markers, and DAPI (Beyotime, C1005) for nuclei visualization. Coverslips were mounted onto slides and examined under fluorescence microscope.
EDU incorporation
For quantitative detection of cell proliferation, chemiluminescent detection of EDU using a cell proliferation kit (C10310, Ribobio) was performed according to the manufacturer’s instruction. After a 72 h incubation, adherent cells were plated onto coverslips in a 24-well plate containing DMEM and 10% FBS. EDU labeling solution (final concentration of 10 mM) was added and incubated for 16 h. After labeling, cells were fixed and incubated with anti-EDU antibody peroxidase conjugate. Fluorescence FITC-tagged secondary antibody (1:200) was employed for visualization, and then washed three times by PBS to remove the excess antibody. The result was examined under fluorescence microscope.
RNA interference
Small-interfering RNA (siRNA) designed to UL128 (Genbank accession NO. ADE62343.1) were synthesized by Invitrogen. Negative control siRNA (invitrigen, StealthTM) was used in this experiment. SiRNA was transfected into epithelial cells using LipofectaminTM RNAiMAX (Invitrogen), according to the protocols provided by the manufacturer. In addition, a control duplex labeled with GFP was used to monitor transfection efficiency. A fluorescein-labeled, doublestranded RNA duplex with the same length, charge, and configuration as standard siRNA was used to assess the transfection efficiency. A fluorescein-labeled, doublestranded RNA duplex with the same length, charge, and configuration as standard siRNA was used to assess the transfection efficiency. The most efficient duplex of UL128 siRNA was selected as follows: 5'- UGAAUUCGCAACAUUCUUCUGCGCG-3'; 5'-CGCGCAG
AAGAAGUUGCGAAUUCA-3'. Quantitative detection of UL128 mRNA was measured by SYBR Premix Ex TaqTM (TaKaRa, cat.DRR420) using ABI 7500 real time PCR system. UL128 overlapping primers read as follows: forward primer 5’- GCTGAGATTCGCGGGATCGT -3’; reverse primer 5’- GTACTGCGCCTTGTCGTTCA -3’. A housekeeping gene hexose-6- phosphate dehydrogenase (GAPDH) was served as endogenous control: forward primer 5’-GAAGGTGAAGGGTCGGAGTC-3’, reverse primer 5’-GAAGATGGTGATGGGATTC-3’. PCR reactions were performed at 95 for 5 min followed by 35 cycles (94, 15 sec; 60, 30 sec). The relative changes in gene expression were calculated using the following formula: fold change in gene expression, 2-Ct=2-{Ct (siRNA) –Ct (control)}, Ct=Ct (UL128 or control) – Ct (GAPDH) and Ct represents threshold cycle number.
Flow cytometric (FCM) analysis
Culture medium (200 μl) from different time points was harvested and centrifuged at 1000 g for 5 min. Supernatant was collected for cytokines detection. Cytokines IL-2, IL-4, IL-6, IL-10, IFN-γ and TNF- α levels were determined using Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kit (BD Pharmingen, USA, Cat. 551809) according to the protocols. Data was collected from FCM through three independent experiments.
Statistical analysis
All data were reported as means ± SD. Comparison of cytokine level was using one-way ANOVA (Newman-keuls multiple comparison test). In all analyses, two tailed significance levels of p<0.05 were used.
Results
Human cochlear epithelial cell line cultured and expanded in vitro
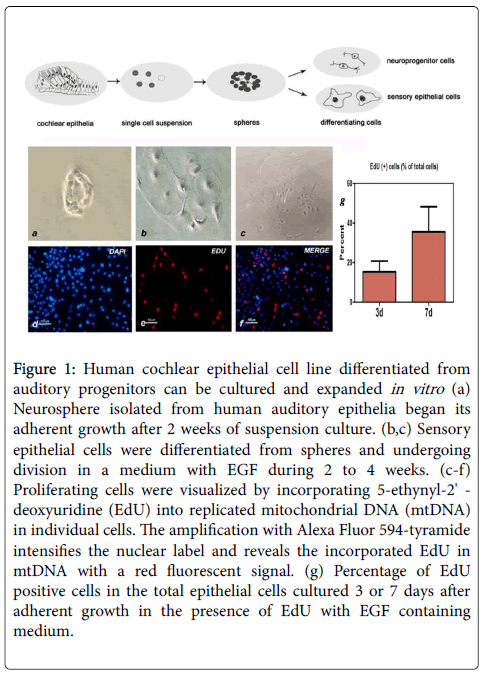
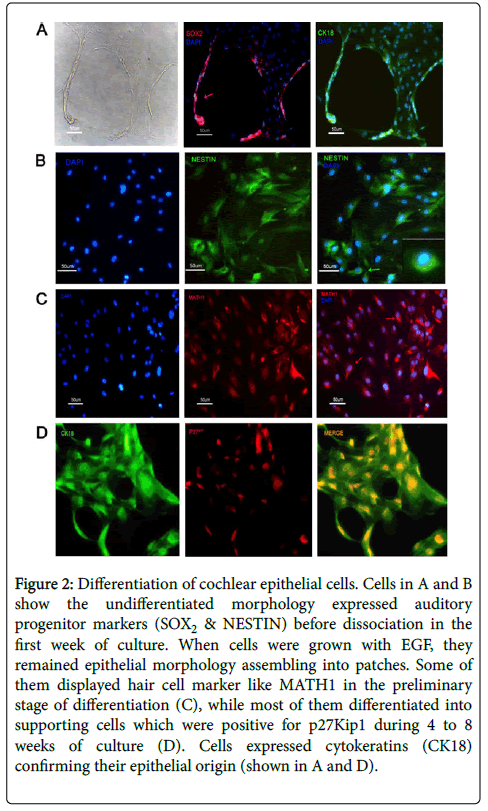
When cultured in a well-defined condition, the renewable stem cell system could differentiate and expand into two major sensory lineages, including cochlear epithelial cells and auditory neurons. To set up the condition that would support the selective expansion of cochlear epithelial cells, single-cell suspensions were plated in the presence of MEM plus F12 nutrient solution and epidermal growth factor (EGF, 20 ng/ml). As trypsin can induce neuronal differentiation in cultured condition, cell monolayer was dissociated by 0.02% EDTA and mechanical isolation. After 2 weeks culture in vitro, suspending cell line was able to grow into adherent monolayers and cellular immune fluorescence assay was conducted then. Mitosis proliferation was determined by labeling newly synthesized mitochondrial DNA using synthetic nucleotide EdU fluorescent cellular marker (Figure 1). Otic progenitor markers such as SOX2, NESTIN were expressed in isolated cell line and cytokeratin 18 was used to confirm its epithelial origin. P27 Kip label is restricted to the supporting cell types within the organ of Corti (Figure 2). Cells can be expanded for 25–30 population doublings and remained viable for at least 4 weeks after ceasing to proliferate.
Figure 1: Human cochlear epithelial cell line differentiated from auditory progenitors can be cultured and expanded in vitro (a) Neurosphere isolated from human auditory epithelia began its adherent growth after 2 weeks of suspension culture. (b,c) Sensory epithelial cells were differentiated from spheres and undergoing division in a medium with EGF during 2 to 4 weeks. (c-f) Proliferating cells were visualized by incorporating 5-ethynyl-2' - deoxyuridine (EdU) into replicated mitochondrial DNA (mtDNA) in individual cells. The amplification with Alexa Fluor 594-tyramide intensifies the nuclear label and reveals the incorporated EdU in mtDNA with a red fluorescent signal. (g) Percentage of EdU positive cells in the total epithelial cells cultured 3 or 7 days after adherent growth in the presence of EdU with EGF containing medium.
Figure 2: Differentiation of cochlear epithelial cells. Cells in A and B show the undifferentiated morphology expressed auditory progenitor markers (SOX2 & NESTIN) before dissociation in the first week of culture. When cells were grown with EGF, they remained epithelial morphology assembling into patches. Some of them displayed hair cell marker like MATH1 in the preliminary stage of differentiation (C), while most of them differentiated into supporting cells which were positive for p27Kip1 during 4 to 8 weeks of culture (D). Cells expressed cytokeratins (CK18) confirming their epithelial origin (shown in A and D).
HCMV infected cochlear epithelial cell model
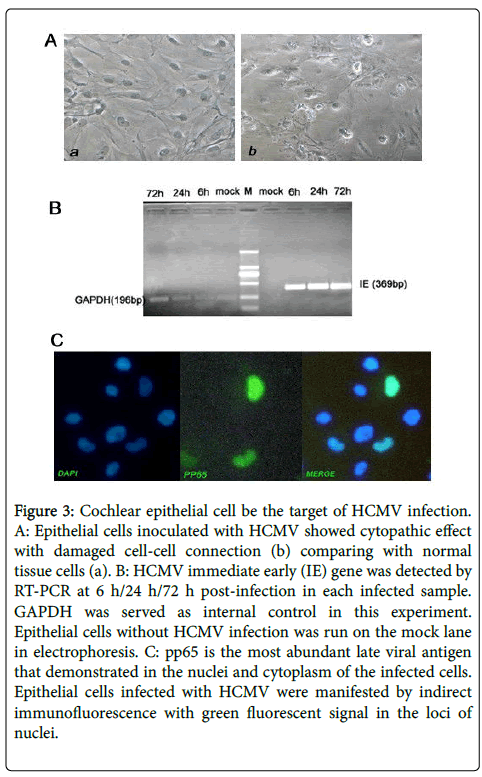
Replication of virus is dependent on host cell transcription factors and expression of all other HCMV genes requires transcription of IE genes. In this study, transcription of HCMV IE gene was detected earlier than 6 h post infection which meant that efficient replication of viral genes could be established in cochlear epithelial cells. Virally specific protein pp65 stained by bright green fluorescence was observed 24 h post infection in the nucleus of infected cells. Cytomegalic cells with mess morphological changes were apparently 96 h post infection and a small number of HCMV infected cells became structural disarray and segregated with neighboring cells (Figure 3). Remarkably, cochlear epithelial cell line isolated and analyzed in this study was permissive for HCMV replication in vitro.
Figure 3: Cochlear epithelial cell be the target of HCMV infection. A: Epithelial cells inoculated with HCMV showed cytopathic effect with damaged cell-cell connection (b) comparing with normal tissue cells (a). B: HCMV immediate early (IE) gene was detected by RT-PCR at 6 h/24 h/72 h post-infection in each infected sample. GAPDH was served as internal control in this experiment. Epithelial cells without HCMV infection was run on the mock lane in electrophoresis. C: pp65 is the most abundant late viral antigen that demonstrated in the nuclei and cytoplasm of the infected cells. Epithelial cells infected with HCMV were manifested by indirect immunofluorescence with green fluorescent signal in the loci of nuclei.
Enhanced pro inflammatory cytokines in the supernatant of HCMV infected cochlear epithelial cell/PBMC co-culture system
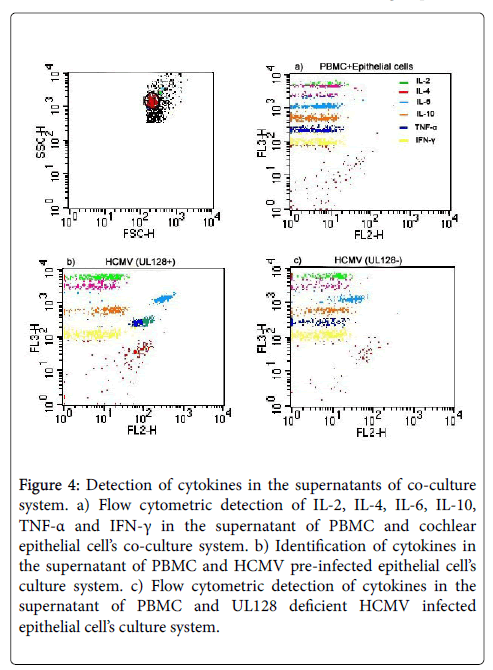
Before establishing co-culture system, epithelial cells were seeded with HCMV strain AD169 (TCID50) for 2 h and culture medium was replaced by fresh medium afterwards. As expected, cochlear epithelial cells could coexist with PBMC peacefully in vitro. Six cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ) were measured in the supernatants of co-culture system, while only level of IL-6 was found to be dramatically elevated, and level of TNF-α was slightly enhanced in this experiment. Level of IL-2 was a little bit higher in the HCMV infected group than the negative control group, but no significant difference was found according to statistical analysis. Expression of IL-6 elevated gradually and cumulatively by time, while TNF-α got its peak at 18 h after co-culturing, then dropped to a lower level at 24 h and experienced a small increase at following time. There was no significant change of the other cytokines detected between HCMV infected group and negative control group.
Suppression of HCMV UL128 transcription by RNA interference
SiRNA targeted HCMV UL128 gene could be successfully transfected into host cells. Suppression efficacy was evaluated by qRT-PCR at different concentration and different time. Data collected in one of the experiment that detected the UL128 mRNA levels at different time points were displayed (Table 1). The most efficient concentration of RNA duplex which depleted expression of UL128 genes was used before HCMV infection.
| Number | Gene | Time | CT value | Mean (CT) | ‚?≥CT | ‚?≥‚?≥CT | Ratio (2-‚?≥‚?≥CT × 100%) |
|---|---|---|---|---|---|---|---|
| 1 | UL128 | siRNA-12 h | 21.10 | - | - | - | - |
| 2 | UL128 | siRNA-12 h | 21.01 | - | - | - | - |
| 3 | UL128 | siRNA-12 h | 21.15 | 21.09 | -1.0 | 2.59 | 17% |
| 4 | UL128 | siRNA-24 h | 20.60 | - | - | - | - |
| 5 | UL128 | siRNA-24 h | 20.46 | - | - | - | - |
| 6 | UL128 | siRNA-24 h | 23.15 | 21.41 | -0.79 | 4.32 | 6% |
| 7 | UL128 | siRNA-48 h | 12.99 | - | - | - | - |
| 8 | UL128 | siRNA-48 h | 16.05 | - | - | - | - |
| 9 | UL128 | siRNA-48 h | 11.20 | 13.41 | -4.69 | -1.32 | 6% |
| 10 | UL128 | Control-12 h | 16.89 | - | - | - | - |
| 11 | UL128 | Control-12 h | 16.61 | - | - | - | - |
| 12 | UL128 | Control-12 h | 16.64 | 16.71 | -3.59 | - | - |
| 13 | UL128 | Control-24 h | 13.09 | - | - | - | - |
| 14 | UL128 | Control-24 h | 16.42 | - | - | - | - |
| 15 | UL128 | Control-24 h | 16.23 | 15.24 | -5.11 | - | - |
| 16 | UL128 | Control-48 h | 13.05 | - | - | - | - |
| 17 | UL128 | Control-48 h | 13.29 | - | - | - | - |
| 18 | UL128 | Control-48 h | 13.30 | 13.21 | -8.97 | - | - |
| 19 | GAPDH | siRNA-12 h | 22.00 | - | - | - | - |
| 20 | GAPDH | siRNA-12 h | 22.12 | - | - | - | - |
| 21 | GAPDH | siRNA-12 h | 22.14 | 22.09 | - | - | - |
| 22 | GAPDH | siRNA-24 h | 22.02 | - | - | - | - |
| 23 | GAPDH | siRNA-24 h | 22.00 | - | - | - | - |
| 24 | GAPDH | siRNA-24 h | 22.57 | 22.20 | - | - | - |
| 25 | GAPDH | siRNA-48 h | 18.00 | - | - | - | - |
| 26 | GAPDH | siRNA-48 h | 18.67 | - | - | - | - |
| 27 | GAPDH | siRNA-48 h | 17.63 | 18.10 | - | - | - |
| 28 | GAPDH | Control-12 h | 20.03 | - | - | - | - |
| 29 | GAPDH | Control-12 h | 20.09 | - | - | - | - |
| 30 | GAPDH | Control-12 h | 20.07 | 20.06 | - | - | - |
| 31 | GAPDH | Control-24 h | 20.10 | - | - | - | - |
| 32 | GAPDH | Control-24 h | 20.46 | - | - | - | - |
| 33 | GAPDH | Control-24 h | 20.50 | 20.35 | - | - | - |
| 34 | GAPDH | Control-48 h | 18.04 | - | - | - | - |
| 35 | GAPDH | Control-48 h | 18.02 | - | - | - | - |
| 36 | GAPDH | Control-48 h | 17.19 | 17.75 | - | - | - |
Table 1: UL128 mRNA levels detected at different time points.
Knock down of HCMV UL128 led to down-regulation of IL-6 and TNF- expression
Down-regulation of IL-6 and TNF-α was observed when knocking down HCMV UL128 genes in the co-culture system (Figure 4). Production of IL-6 and TNF-α in the HCMV (UL128-) group were 61.5% and 40.1% reduction respectively, comparing with HCMV (UL128+) group. CPE of infected cells and virus titer in the infected supernatant were also assessed, but no significant differences were found between HCMV (UL128+) and HCMV (UL128-) group.
Figure 4: Detection of cytokines in the supernatants of co-culture system. a) Flow cytometric detection of IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ in the supernatant of PBMC and cochlear epithelial cell’s co-culture system. b) Identification of cytokines in the supernatant of PBMC and HCMV pre-infected epithelial cell’s culture system. c) Flow cytometric detection of cytokines in the supernatant of PBMC and UL128 deficient HCMV infected epithelial cell’s culture system.
Discussion
At the present time, positive identification of inflammation during the acute phase of a hearing loss is technically not feasible. Moreover, species specificity of HCMV precludes the direct study of the virus in an animal model limits our understanding of HCMV associated deafness. Research conducted on animal models display that cochlear vascular, neural, and epithelial cells are likely targets of CMV infection [16]. In neonatal mouse model of profound SNHL, MCMV preferentially infected both cochlear perilymphatic epithelial cells and spiral ganglion neurons [17]. Since now, we are still lack of evidence to convince that HCMV can persistently infect human auditory organs because of the difficulty in requirement of human auditory cells. In this study, we established a new cell line based on human auditory stem cells for HCMV infection. Proliferating epithelial cells were differentiated from auditory progenitors under specified condition. CPE was observed when inoculating HCMV into the cochlear epithelial cells. Virus immediate early gene and structural late protein PP65 were also found highly expressed in HCMV infected cells. It was convinced that HCMV could efficiently multiplied in this new cell line in vitro. These results demonstrated cochlear epithelial cell model derived from the auditory progenitors was robust and informative, with the potential for providing a new tool for studying the mechanisms of HCMV-related hearing loss.
In a number of auditory pathologic models, immunomodulatory proteins and several proinflammatory cytokines have been proved to play a pathogenic role after foreign antigen inoculating into the inner ear [18-20]. CMV infection in inner ear can induce persistent inflammation which is deleterious to cochlear function [21]. A number of CMV genes appear to participate in the complex immune regulatory system, which includes homologs of cellular immune effector proteins such as chemokines, chemokine receptor-like G protein-coupled receptors, and MHC class molecules [22]. In our previous study, we revealed that recombinant UL128 protein had the function similar to human -chemokine MIP-1α, recruiting both monocytes and lymphocytes during the chemotaxis assay. MIP-1α is one of the four subgroup chemokine (CC chemokine) designations that involves in mitogenic, chemotactic, and inflammatory activities [23]. Animal models engineered to be deficient in MIP-1α showed attenuated inflammatory responses to CMV both in pulmonary inflammation and SNHL [9,24]. Recruitment of neutrophils and macrophages to the inflammatory sites is the major property of human chemokine that functions in tissue repair, cytokine production, and removal of cytopathic debri, which is also the primary innate defense against virus infection [25]. But over-activation of immune cells can be harmful to neighboring tissue cells and may cause lethality of spiral ganglion neurons and hair cells in the mammalian cochlear [26]. In CMV infected neonatal mouse, MCMV induced cochlear hair cell death by 21 days post-infection after the complete clearance of the virus from the cochlear 14 days post-infection, indicating a pivotal role of inflammation in MCMV-induced SNHL [27]. For the virus, mimic of immunomodulatory functions with increased inflammation can raise the efficiency of virus infection and benefit viral dissemination [28].
We constructed a co-culture system of PBMC and cochlear epithelial cells to mimic of the cellular micro environment of interactions between tissue cells and peripheral immune cells. Production of proinflammatory cytokines such as TNF-α and IL-6 were found elevating in the supernatants. TNF-α has been proved to be a key factor in immune-mediated cochlear injury since inhibition of TNF-α activity significantly attenuates local inflammation as well as hearing impairment in experimental models [29]. Cytokines TNF-α and IL-6 released by cochlear spiral ligament fibrocytes and peripheral immune cells can initiate programmed cell death in auditory hair cells [30,31]. This is the first study demonstrating the induction of proinflammatory cytokines in HCMV infected cochlear upon a cellular basis. HCMV encoded UL128 may participate in the pathogenesis of CMV-induced hearing loss during intercellular communications and UL128 was a potential target for antiviral therapy during HCMV infection related immune injury.
Acknowledgment
This work was supported by National Natural Science Foundation of China (NO. 81071337) and Natural Science Foundation of Zhejiang Province (NO.Z2110006).
Disclosure Statement
No competing financial interests exist.
References
- Fowler KB, Boppana SB (2006) Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol 35: 226-231.
- Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, et al. (1997) Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr 130: 624-630.
- Williamson WD, Demmler GJ, Percy AK, Catlin F (1992) Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics 90: 862-866.
- Michael JC, Terri BH, Schmid DS (2011) Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 21: 240–255.
- Chardin S, Romand R (1995) Regeneration and mammalian auditory hair cells. Science 267: 707–709.
- Barrett JR (1997) Chemokines. Blood 90: 909-928.
- Susan M (2004) Consequences of Human Cytomegalovirus Mimicry. Hum Immunol 65: 465-475.
- Powers C, De FV, Malouli D, Fruh K, (2008) Cytomegalovirus immune evasion. Curr Topics Microbiol Immun 325: 333–359.
- Scott AS, David KB, Mark RS, Jareen MD, John HG, et al. (2007) The Role of CMV Inflammatory Genes in Hearing Loss. Otol Neurotol 28: 964-969.
- Angelika D, Patricia W, Leea YS, Andrew G, Neil S (2006) Prospective identification and purification of hair cell and supporting cell progenitors from the embryonic cochlea. Brain Res 1091: 282-288.
- Brent JR, Marie CC, David CJ (2008) HCMV gH/gL/UL128–131 interferes with virus entry into epithelial cells: Evidence for cell type-specific receptors. PNAS 105: 14118-14123.
- Emilia G, Elena P, Antonella SM, Grazia R, Fausto B, et al. (2011) Serum antibody response to the gH/gL/pUL128–131 five-protein complex of human cytomegalovirus (HCMV) in primary and reactivated HCMV infections. J Clin Virol 52: 113-118.
- Gao H, Tao R, Zheng Q, Xu J, Shang SQ (2013) Recombinant HCMV UL128 expression and functional identification of PBMC-attracting activity in vitro. Arch Virol 158: 173-177.
- Zheng Q, Tao R, Gao H, Xu J, Shang SQ, et al. (2012) HCMV-Encoded UL128 Enhances TNF-a and IL-6 Expression and Promotes PBMC Proliferation Through the MAPK/ERK Pathway In Vitro. Viral Immunol 25: 98-105.
- Tiscornia I, Sánchez-Martins V, Hernández A, Bollati-Fogolín M (2012) Human monocyte-derived dendritic cells from leukoreduction system chambers after plateletpheresis are functional in an in vitro co-culture assay with intestinal epithelial cells. J Immunol Methods 384: 164-170.
- Li L, Kosugi I, Han GP, Kawasaki H, Arai Y, et al. (2008) Induction of cytomegalovirus-infected labyrinthitis in newborn mice by lipopolysaccharide: a model for hearing loss in congenital CMV infection. Lab Invest 88: 722-730.
- Yuehua Q, Longzhen Z, Kailin X, Lingyu Z, Lingjian M, et al. (2011) Inflammatory Lesions of Cochlea in Murine Cytomegalovirus-Infected Mice with Hearing Loss. Cell Biochem Biophys 62: 281-287.
- Shigehisa H, Peter B, Jeffrey PH, Gary SF, Elizabeth MK (2005) Innate Immunity Contributes to Cochlear Adaptive Immune Responses. Audiology and neuro-otology 10: 35-43.
- Hitoshi S, Gary SF, Peter BB, Jeffrey PH, Elizabeth MK (2002) Tumor Necrosis Factor-α, an Initiator, and Etanercept, an Inhibitor of Cochlear Inflammation. The Laryngoscope 112: 1627-1634.
- Charlotte A, Linda ML (2006) Immune-mediated inner-ear disorders in neuro-otology. Current Opinion in Neurology 19: 26-32.
- Schachtele SJ, Mutnal MB, Schleiss MR, Lokensgard JR (2011) Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neurovirol 17: 201-211.
- Edward S, Mocarski JR (2002) Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends in Microbiology 10: 332-339.
- Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF (1995) Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 269: 1583-1585.
- Weigt SS, Elashoff RM, Keane MP, Strieter RM, Gomperts BN, et al. (2008) Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant 8: 1512-1522.
- Clemens E, Cristiana SW, Lisa AB (2005) Chemokines- Key Players in Innate and Adaptive Immunity. J Invest Dermatol 125: 615–628.
- Wei L, Ding D, Salvi R (2010) Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience 168: 288-299.
- Juanjuan C, Yan F, Li C, Haizhi L, Ling W, et al. (2011) Murine model for congenital CMV infection and hearing impairment. Virol J 15: 70.
- Koedood M, Fichtel A, Meier P, Mitchell PJ (1995) Human cytomegalovirus (HCMV) immediate-early enhancer/promoter specificity during embryogenesis defines target tissues of congenital HCMV infection. J Virol 69: 2194-2207.
- Jeon EJ, Park YS, Choi YC, Yeo SW, Jung TT, (2001) Effect of inhibitor of tumor necrosis factor-alpha on experimental otitis media with effusion. Ann Otol Rhinol Laryngol 110: 917-921.
- Wakabayashi K, Fujioka M, Kanzaki S, Okano HJ, Shibata S, et al. (2010) Blockade of interleukine-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci Res 66: 345-352.
- Abi-Hachem RN, Zine A, Van De Water TR (2010) The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Pat CNS Drug Discov 5: 147-163.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 3006
- [From(publication date):
August-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 2191
- PDF downloads : 815