Immune Checkpoint Inhibitors and Atherosclerosis: Emerging Insights and Therapeutic Implications
Received: 26-Apr-2023 / Manuscript No. asoa-23-99812 / Editor assigned: 28-Apr-2023 / PreQC No. asoa-23-99812 (PQ) / Reviewed: 12-May-2023 / QC No. asoa-23-99812 / Revised: 18-May-2023 / Manuscript No. asoa-23-99812 (R) / Accepted Date: 24-May-2023 / Published Date: 25-May-2023 DOI: 10.4172/asoa.1000202
Abstract
As the use of immune checkpoint inhibitors (ICIs) expands in clinical practice, our understanding of their potential adverse effects continues to grow. Arising substantiation indicates a connection between ICI remedy and an increased threat of accelerated atherosclerosis and atherosclerotic cardiovascular (CV) events. In this discussion, we delve into the biological plausibility and clinical evidence supporting the impact of immune checkpoint inhibition on the development of atherosclerotic CV disease. Additionally, we offer insights on potential diagnostic and pharmacological strategies aimed at mitigating atherosclerotic risk in patients receiving ICI treatment. Although our comprehension of the pathophysiology of ICI-related atherosclerosis is in its early stages, further research is warranted to unravel the underlying mechanisms linking ICI therapy to atherosclerosis. Leveraging the valuable insights provided by ICI therapy into CV biology, it is crucial to develop robust approaches to effectively manage the growing population of patients who may face an increased risk of atherosclerotic CV disease.
Keywords
Atherosclerosis; Oncology; Inflammation; Cardiovascular disease; Checkpoint inhibitor
Introduction
The field of oncology has witnessed a transformative shift with the advent of cancer immunotherapy, particularly with the emergence of immune checkpoint inhibitors (ICIs) [1-4]. Immune checkpoints are molecules expressed by various immune regulators that play a role in activating or suppressing the immune system. Unfortunately, cancer cells can exploit these molecules to evade detection by the immune system. Presently, the Food and Drug Administration (FDA) has approved monoclonal antibody-based ICIs that function by blocking these immune checkpoints. This blockade aims to diminish the inhibitory signals while augmenting the stimulatory signals that regulate the recognition of tumor neoantigens by cytotoxic T cells.2 ICIs targeting pathways such as cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1) have emerged as key components in the treatment of various malignancies, either as standalone therapies or in combination regimens [5].
In addition to the well-established immune checkpoint inhibitors (ICIs) targeting CTLA-4, PD-1, and PD-L1, there is a growing focus on several other immune checkpoints for cancer therapy. Some of these checkpoints have more recently received FDA approval, such as lymphocyte-activation gene 3 (LAG-3), while others are in advanced stages of clinical development. These include targets like CD47, T cell immunoglobulin and mucin sphere containing- 3( TIM- 3), inducible T cell costimulatory (ICOS), T cell immunoglobulin and ITIM sphere( TIGIT), B7 homolog 3 protein( B7- H3), V-sphere immunoglobulin suppressor of T cell activation( outlook), and B and T lymphocyte attenuator( BTLA). Currently, the U.S. FDA has approved a total of 10 ICIs, including two CTLA-4-blocking antibodies (ipilimumab and tremelimumab), four PD-1-blocking antibodies (nivolumab, cemiplimab, dostarlimab, and pembrolizumab), three PD-L1-blocking antibodies (atezolizumab, avelumab, durvalumab), and one targeting LAG-3 (relatlimab). The remarkable efficacy of these therapies has led to a rapid increase in the number of FDA approvals for ICIs. As of June 2022, there were approximately 125 indications for ICIs, including their use in adjuvant and neoadjuvant settings, and even as first-line therapy, across more than 20 different cancer types [6]. The number of ICIs being tested in clinical trials exceeds 300, targeting a wide range of over 5600 different proteins. Consequently, the pool of eligible cancer patients for ICI therapy is estimated to be approximately 36% in the United States [7,8]. Since immune checkpoints also play a role in regulating autoreactivity, it is not surprising that ICIs can lead to immune-related adverse events (irAEs), where disinhibited cytotoxic T cells mistakenly target healthy tissues in various organs. These irAEs exhibit clinical diversity and can occur in up to 70% of patients, typically manifesting as mild and manageable events [9]. However, there are instances of highgrade irAEs, including cardiotoxicities associated with ICIs, which can be disabling or life-threatening and significantly impact cancer treatment and overall patient outcomes. Over time, our understanding of the spectrum and incidence of cardiotoxicity linked to ICI use has improved. Previously underestimated, recent studies have revealed an incidence of major adverse cardiac events of up to 10.3% in patients treated with ICIs [10-12]. Furthermore, our understanding of the range of toxicities associated with ICIs and their potential cardiovascular (CV) effects has expanded. Initially, ICI-related cardiac events were primarily focused on myocarditis. However, recent data have revealed a broader association that includes heart failure, cardiomyopathy, conduction abnormalities, venous thrombosis, pericardial disease, vasculitis, and, the focus of this review, atherosclerotic-related events [11-16]. Considering the significant success of ICIs in treating various cancers, the heightened risk of exacerbating atherosclerosis and atherosclerotic cardiovascular disease (ASCVD) in ICI-treated patients, particularly in adjuvant and neoadjuvant settings, holds considerable importance.
Key Points:
• Immune checkpoint inhibitors (ICIs) have transformed the landscape of oncology, establishing themselves as the standard treatment for numerous types of cancer.
• The increasing utilization of ICIs has uncovered a wide range of potential immune-related adverse events (irAEs), extending beyond the previously recognized rare cases of myocarditis to include various ICI-associated cardiac events.
An overview of immune checkpoint inhibitor (ICI) associated atherosclerotic cardiovascular disease (ASCVD)
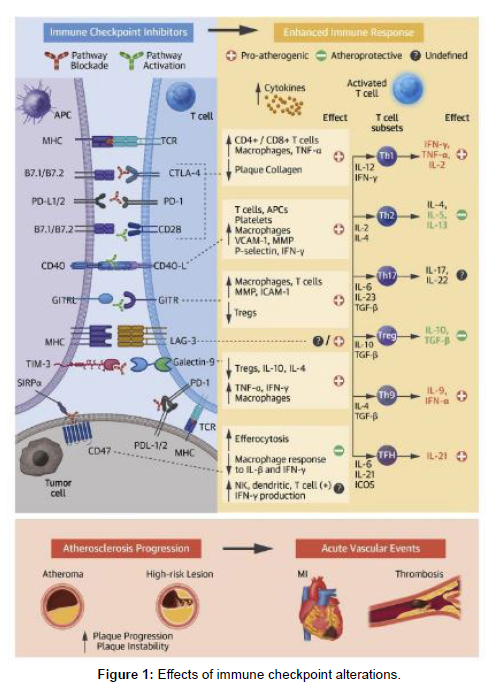
The association between established cancer therapies, such as radiation and targeted therapies, and an increased risk of atherosclerosis and related events has been well-documented [17,18]. Recent evidence suggests that FDA-approved immune checkpoint inhibitors (ICIs) may also contribute to the acceleration of atherosclerosis and the occurrence of atherosclerosis-related cardiovascular (CV) events, including acute myocardial infarction (MI), stroke, and peripheral arterial disease [19]. Heart disease stands as a leading cause of mortality among cancer survivors, with cancer and cardiovascular disease (CVD) sharing common risk factors such as aging, diabetes, hypertension, cardiometabolic dysfunction, physical inactivity, tobacco use, and chronic low-grade inflammation [20,21]. Moreover, cancer itself, along with shared risk factors and cancer therapies, is being investigated as a potential independent risk factor for the development of heart disease [22,23]. As the approval of ICIs continues to expand, particularly in adjuvant and neoadjuvant care, and the overall survival of patients receiving ICIs improves, it becomes crucial to consider the potential risk of CVD. This risk is supported by a profound understanding of the role of ICIs in pivotal atherosclerosis pathways, as depicted in the Central Illustration.
Extensive preclinical data utilizing cellular and animal models have demonstrated the crucial role of immune checkpoint proteins, such as CTLA-4, PD-1, LAG-3, and PD-L1, which are targeted by FDAapproved immune checkpoint inhibitor (ICI) therapies, as negative regulators of atherosclerosis [22-26]. Consequently, blocking these immune checkpoints may result in accelerated atherosclerosis by enhancing the responses of effector T cells, impairing the function of regulatory T (Treg) cells, and promoting their infiltration into the vascular endothelium [25-29]. The clinical evidence supporting these preclinical findings continues to grow, demonstrating that ICI therapy leads to the expedited progression of atherosclerotic plaques, thereby elevating the risk of atherosclerotic cardiovascular disease (ASCVD) [30]. This review focuses on the existing evidence concerning the impact of ICIs on coronary atherosclerosis and outlines the molecular mechanisms that link inflammation and cell-mediated immunity to atherogenesis within the context of ICI therapy. We also discuss potential diagnostic and therapeutic strategies to mitigate the impact of ASCVD in patients receiving ICI treatment for cancer, along with the clinical implications for optimal cardiovascular risk management and cardio-oncology care during and after immunotherapy.
Central illustration: impact of immune checkpoint modifications
Immune checkpoint inhibitors (ICIs) elicit an intensified immune response and persistent inflammation. The mechanisms involved in the exacerbation of atherosclerosis following ICI therapy implicate several pathways, with the most compelling evidence supporting the co-inhibitory blockade of cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1), as well as the co-stimulatory activation of CD40/CD40L and glucocorticoid-induced tumor necrosis factor familyrelated protein (GITR) pathways. Noteworthy costimulatory molecules include B7.1 (CD80) and B7.2 (CD86). Other relevant abbreviations include APC (antigen-presenting cell), GITRL (glucocorticoidinduced tumor necrosis factor family-related protein ligand), ICAM (intracellular adhesion molecule), ICOS (inducible costimulatory), IFN (interferon), IL (interleukin), LAG-3 (lymphocyte activation gene 3, TIM-3 (T cell immunoglobulin and mucin containing domain-3), TNF (tumor necrosis factor), Treg (regulatory T), and VCAM (vascular cell adhesion molecule) (Figure 1).
Involvement of inflammation in atherosclerosis
Decades of extensive research in basic, translational, and clinical fields have firmly established atherosclerosis as a chronic inflammatory disorder characterized by an imbalance in lipid metabolism, vascular function, and an aberrant immune response.33 At a broad level, the inflammatory process in atherosclerosis is initiated and sustained by activated M1 macrophages, while activated M2 macrophages are associated with inflammation resolution. M1 macrophages contribute to the accumulation of intracellular lipids and the secretion of proinflammatory molecules like tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. Conversely, M2 macrophages (stimulated by IL-4 and IL-13) promote lipid clearance and produce antiinflammatory factors such as IL-10 and collagen. The dynamic balance between M1 and M2 macrophage phenotypes plays a critical role in the development, progression, and vulnerability of atherosclerotic plaques, and this balance is evident in both human and mouse lesions. ICIs, similar to other established models of ASCVD such as HIV, may contribute to accelerated atherosclerosis and increased risk of ASCVD through immune activation and inflammation.
In-depth investigations have demonstrated that individuals with HIV, despite achieving undetectable viral loads, face an elevated risk of cardiovascular disease (CVD) that cannot be fully explained by traditional risk factors alone. This heightened risk is attributed to persistent immune activation and inflammation, which contribute to the progression of atherosclerosis. Notably, studies utilizing coronary computed tomography (CT) angiography have revealed a correlation between arterial inflammation and increased noncalcified plaque (NCP) as well as high-risk plaque (HRP) morphology in HIV patients receiving treatment, who possess a low Framingham Risk Score and no known history of CVD, when compared to control subjects. There is a noteworthy overlap between HIV and cancer in terms of immune checkpoints, as the same immune checkpoint proteins targeted in cancer therapy are also involved in the pathogenesis of HIV. Consequently, patients with HIV exhibit increased expression of CTLA-4 and PD-1 on T cells. Furthermore, HIV evades immune surveillance by inducing T cell exhaustion, a mechanism akin to how cancers evade immune eradication. Findings from notable studies like CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study), COLCOT (Colchicine Cardiovascular Outcomes Trial), and LoDoCo (Low-dose Colchicine) have provided evidence that targeting specific inflammatory and immune-related pathways could potentially decrease the occurrence of atherosclerosis-related cardiovascular (CV) events. Therefore, it is of utmost importance to comprehend the diverse mechanisms and immune dysregulation present at the site of plaque formation, as this knowledge can aid in the identification of potential immunotherapeutic targets for cardiovascular disease (CVD) treatment, extending beyond the conventional standard of care management.
Role of immunity in atherosclerosis
Although both B and T lymphocytes contribute to the development and progression of plaques, atherosclerosis is predominantly recognized as a T cell-mediated disease. Recent advancements in single-cell proteomic and transcriptomic analyses of atherosclerotic lesions in mice and humans have enabled the characterization of the immune cell repertoire and distinct molecular characteristics of innate and adaptive immune cells associated with stable and vulnerable plaques. Notably, studies utilizing single-cell RNA analysis of human carotid lesions from patients with symptomatic atherosclerosis have identified activated T cells, including CD8+ cytotoxic T lymphocytes and CD4+ lymphocytes (such as T helper 1 [Th1] cells, Th2 cells, Th17 cells, and Treg cells). Th1 cells, which are the predominant cell type found in plaques, have been linked to proatherogenic cytokines like interferon gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α). Experimental studies in mice have revealed that TNF-α is associated with advanced necrotic plaques, and inhibiting Th1 cells has shown atheroprotective effects by reducing IFN-γ levels within the plaques. Furthermore, Treg cells play a well-defined atheroprotective role by secreting transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), which promote an anti-inflammatory macrophage phenotype.
Furthermore, research has demonstrated that regulatory T (Treg) cells express the immune checkpoint molecule CTLA-4, and their abundance is inversely correlated with the size and vulnerability of atherosclerotic plaques. Additionally, the plaques of individuals treated with immune checkpoint inhibitors (ICIs) contain T cell subsets displaying signs of cellular exhaustion, such as high levels of PD-1 and perforin, as well as molecular markers associated with immune checkpoint blockade (PDCD1 and LAG-3). These plaques also exhibit macrophages with activated phenotypes, which are linked to plaque vulnerability. The presence of exhausted T cells expressing PD-1 within atherosclerotic plaques suggests that PD-1 inhibitors may activate these T cells within the plaques and potentially exacerbate atherosclerosis.
Exploring immune checkpoints in atherosclerosis: therapeutic targets and mechanisms
The therapeutic potential of immune checkpoints in atherosclerosis has been investigated extensively, with the blockade of CTLA-4 and the PD-1-PD-L1 dyad emerging as the first two strategies translated from laboratory research to clinical practice. Studies employing genetic knockout models and pharmacological modulation of PD-1, PD-L1, and CTLA-4 have shed light on the role of these co-inhibitory proteins in experimental atherogenesis. Mechanistically, reduced levels of the PD-1-PD-L1 dyad have been associated with increased coronary atherosclerotic plaque burden, as both PD-1 and PD-L1 suppress T cell-mediated inflammation and plaque progression. Gotsman et al. demonstrated the impact of the PD-L1/2 pathway on regulating proatherogenic T cell responses by comparing atherosclerotic lesion characteristics in hypercholesterolemic PD-L1/2-/-LDLR-/- mice and LDLR-/- control subjects. Their findings revealed that PD-L1/2 deficiency correlated with heightened atherosclerotic burden and increased CD4+ and CD8+ T cell presence in the lesions. Similarly, Bu et al. observed that PD-L1/2-/-Ldlr-/- mice developed larger lesions characterized by abundant CD8+ T cells and macrophages. Moreover, these studies demonstrated that PD cells were more susceptible to antigen-presenting cell-induced proliferation, exhibited an activated phenotype, and displayed elevated levels of proatherosclerotic cytokines such as IFN-γ and TNF-α. Flow cytometry analysis of human peripheral blood mononuclear cells further supported these findings, as it revealed significantly down-regulated expression of PD-1 and PD-L1 on T cells and myeloid dendritic cells in patients with coronary artery disease compared to healthy individuals. These observations underscore the crucial role of the PD-1/PD-L pathway in attenuating proatherogenic T cell responses and atherosclerosis by limiting antigen-presenting celldependent T cell activation.
Discussion
The advent of ICIs has revolutionized the field of oncology, leading to significant advancements in our understanding of cardiovascular (CV) biology and fostering the rapid growth of cardio-oncology as a specialized discipline over the past decade. While the use of ICIs has presented clinical challenges, particularly in terms of ICI-related cardiotoxicities, our knowledge regarding the spectrum of atherosclerotic-related events and the impact of ICI therapy on aggravated atherosclerosis is still in its early stages. To gain comprehensive insights into the link between ICI use and atherosclerosis, it is crucial to conduct more extensive longitudinal studies and include patients in systematic cardiooncology registries. This will not only enhance our understanding but also inform optimal management strategies for atherosclerotic risk, cardiovascular disease surveillance, and therapeutic interventions in patients undergoing ICI treatment and long-term cancer survivors. From a basic and translational standpoint, numerous questions remain unanswered regarding the underlying mechanisms of ICIassociated atherosclerosis, the identification of predictive biomarkers, the improvement of diagnostic approaches, and the development of effective treatments. Leveraging tools from immunology, genomics, bioinformatics, and imaging can assist in characterizing the effects of ICI therapies on atherogenesis and elucidating the mechanisms involved in the progression of atherosclerotic lesions triggered by these therapies.
Conclusion
To date, there is limited evidence available on the specific effects of ICI therapy on atherosclerotic cardiovascular disease (CVD) from clinical trials. It is crucial to incorporate routine assessment and systematic reporting of cardiac adverse events during and after novel cancer therapies in oncology trials in the future. Cardio-oncologists will continue to play a vital role in managing acute cardiotoxicity and minimizing the risk of long-term complications in patients receiving ICI treatment. Integrating current and emerging imaging and pharmacotherapeutic strategies can offer significant benefits to this unique patient population, helping to mitigate risk and improve overall outcomes. Furthermore, it is important to foster collaborations among cardio-oncologists, imaging specialists, oncologists, and pharmaceutical partners to expand clinical research efforts based on innovative insights from basic and translational experiments. Implementing these steps, among others, is necessary to enhance cardiovascular outcomes for cancer patients undergoing ICI therapy.
Acknowledgement
Not applicable.
Conflict of Interest
Author declares no conflict of interest.
References
- Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG (2021) The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity. J Am Coll Cardiol Cardio Onc 3:35-47.
- A Ribas, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350-1355.
- Haslam A, Gill J, Prasad V (2020) Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open 3:423.
- Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541:321-330.
- Tang J, Shalabi A, Hubbard-Lucey VM (2018) Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 29:84-91.
- Upadhaya S, Neftelinov ST, Hodge J (2022) Campbell Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov 21:482-483.
- Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, et al. (2018) Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov 17:854-855.
- Haslam A, Prasad V (2019) Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2:535.
- Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158-168.
- Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB (2018) Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 391:933.
- Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, et al. (2018) Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 19:1579-1589.
- Laenens D, Yu Y, Santens B, Jacobs J, Beuselinck B, et al. (2022) Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J Clin Oncol 40:3430-3438.
- Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, et al. (2020) Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 141:2031-2034.
- Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, et al. (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71:1755-1764.
- Gong J, Drobni ZD, Zafar A, Quinaglia T, Hartmann S, et al. (2021) Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer 9.
- Gong J, Drobni ZD, Alvi RM, Murphy SP, Sullivan RJ, et al. (2021) Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer 158:99-110.
- Yang EH, Marmagkiolis K, Balanescu DV, Hakeem A, Donisan T, et al. (2021) Radiation-induced vascular disease-a state-of-the-art review. Front Cardiovasc Med 8.
- Mitchell JD, Cehic DA, Morgia M, Bergom C, Toohey J, et al. (2021) Cardiovascular manifestations from therapeutic radiation. J Am Coll Cardiol Cardio Onc 3:360-380.
- Seijkens TPP, Lutgens E (2018) Cardiovascular oncology: exploring the effects of targeted cancer therapies on atherosclerosis. Curr Opin Lipidol 29:381-388.
- Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, et al. (2019) A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 40:3889-3897.
- Lutgens E, Seijkens TTP (2020) Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J Immunother Cancer 8:300.
- Florido R, Daya NR, Ndumele CE, Koton S, Russell S, et al. (2022) Cardiovascular disease risk among cancer survivors: The Atherosclerosis Risk In Communities (ARIC) Study. J Am Coll Cardiol 80:22-32.
- Joshu CE, Barber JR, Coresh J, Couper DJ, et al. (2018) Enhancing the infrastructure of the Atherosclerosis Risk in Communities (ARIC) study for cancer epidemiology research: ARIC cancer. Cancer Epidemiol Biomarkers Prev 27:295-305.
- Strauss L, Mahmoud MA, Weaver JD, Tijaro-Ovalle NM, Christofides A, et al. (2020) Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 5:1863.
- Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, et al. (2007) Lichtman Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest 117:2974-2982.
- Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir EAD, et al. (2019) Single-cell immune landscape of human atherosclerotic plaques. Nat Med 25:1576-1588.
- Bu DX, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, et al. (2011) Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 31:1100-1107.
- Lee J, Zhuang Y, Wei X, Shang F, Wang J, et al. (2009) Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J Mol Cell Cardiol 46:169-176.
- Matsumoto T, Sasaki N, Yamashita T, Emoto T, Kasahara K, et al. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler Thromb Vasc Biol 36:1141-1151.
- Amiri-Kordestani L, Moslehi J, Cheng J, Tang S, Schroeder R, et al. (2018) Cardiovascular adverse events in immune checkpoint inhibitor clinical trials: a U.S. Food and Drug Administration pooled analysis. J Clin Oncol 36:3009.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Xu L (2023) Immune Checkpoint Inhibitors and Atherosclerosis: EmergingInsights and Therapeutic Implications. Atheroscler Open Access 8: 202. DOI: 10.4172/asoa.1000202
Copyright: © 2023 Xu L. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1148
- [From(publication date): 0-2023 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 912
- PDF downloads: 236