Immediate Breast Reconstructions after Mastectomy due to Breast Cancers with the Use of Serasynth and Seragynbr Synthetic Meshes. Single-Oncological Center Experience, Analysis of Complications
Received: 02-Nov-2022 / Manuscript No. jmis-22-67818 / Editor assigned: 05-Nov-2022 / PreQC No. jmis-22-67818 / Reviewed: 12-Nov-2022 / QC No. jmis-22-67818 / Revised: 19-Nov-2022 / Manuscript No. jmis-22-67818 / Published Date: 29-Nov-2022
Abstract
Purpose: Mastectomies with immediate reconstruction are the standard of treatment method in patients with breast cancer who cannot be treated with conserving breast surgery. The use of meshes in reconstructive breast surgery has become a gold standard. The purpose of the study was to analyse the complications and own experience after mastectomies with immediate breast reconstruction with the use of Serasynth and SeragynBR synthetic meshes.
Methods: In the period from December 2017 to July 2020, 118 reconstructive surgeries of the breast were performed in the Department of Breast Cancer and Reconstructive Surgery in Maria Sklodowska – Curie Memorial Cancer Center and Institute of Oncology in Warsaw, Poland with the use of SeragynBR and Serasynth meshes in 93 patients operated for breast cancer. 78 Serasynth meshes (Group1) and 40 SeragynBR meshes (Group1I) were implanted.
Results: The most common complication was persistent seroma collection, which was reported in 17.9% of cases in Group1 and 25% in Group1I. Skin inflammation was reported in 7.6% and 17.5%, while infections in 2.5% and 5% of the surgically treated breasts of Group1 and Group2 patients. Reoperation was required in 5.1% and 5% of the patients in Group1 and Group2. The percentage of complications was lower when Serasynth rather than Seragyn BR meshes were implanted. The frequent incidence of the seroma collection did not contribute in any significant way to serious complications such as the need for removal of mesh/implant or infection. The complications, which developed following the implantation of both mesh types, were similar to those presented in other publications concerning mastectomy with a simultaneous breast reconstruction with synthetic meshes. The percentage of implant losses/explanations in the discussed group of patients was lower than that reported in literature.
Conclusion: Despite the complications, both types of meshes can be considered as safe additions to reconstructive breast surgeries.
Keywords
Breast implants; Breast neoplasms; Mammoplasty; Mastectomy; Surgical mesh
Introduction
Mastectomies with immediate reconstruction are the standard of treatment method in patients with breast cancer who cannot be treated with concervating breast surgery. Breast reconstructive surgery has evolved from subpectoral surgeries; which for many years have been considered as a classic way of breast reconstruction procedure to prepectoral breast reconstruction. In recent years; prepectoral breast reconstruction has dominated operating rooms [1]. For breast reconstruction after mastectomy; in many cases; meshes are used to stabilize the implant; strength the subcutaneous tissues around the implants or protection against implant rotation; both in subpectoral and prepectoral surgery. The use of meshes in reconstructive breast surgery has become a gold standard. There is a variety of meshes available on the market: synthetic and biological; partially; fully or nonabsorbable [2; 3].
All commercially available meshes designed for breast reconstruction have their advantages and disadvantages. Biological meshes (ADM) are fully absorbable and due to their thickness are suitable i.e. for patients with thin skin and subcutaneous tissue. This can be perceived as an advantage; but high price is a well-known disadvantage. Synthetic; non-absorbable meshes are commonly available because of a decent price; however; they can cause hardness of breast tissues after reconstruction.
There is no ideal mesh for breast reconstruction surgery; any solution may cause certain complications; which should be taken into consideration. The most common complication; regardless of the type of mesh used; is the accumulation of the seroma around the implant [4]. Other complications include: inflammation of the skin and subcutaneous tissue; infections; fistulas; skin necrosis or the need to remove the implants [5]. Despite the complications associated with the use of surgical meshes for breast reconstruction; it can be stated that their use is safe and the benefits outweigh the potential problems associated with their implantation.
In this article clinical data will be presented illustrating complications on the use of two types of synthetic meshes; one completely absorbable - Serasynth and the other partially absorbable - SeragynBR; both produced by SERAG-WIESSNER GmbH & Co. S. KG used for breast reconstruction in both subpectoral and prepectoral methods in patients after subcutaneous mastectomies performed due to breast cancer.
SeragynBR mesh is partly absorbable and made from bio component fibres - a polypropylene core covered with a layer of polyglycolic acid and caprolactone. Serasynth mesh is a fully absorbable synthetic mesh made of monofilament polydioxanone fibres. The complete absorption for this mesh is 180 -210 days.
This is the first study describing complications of these two types of meshes [6].
Methods
This is a quantitative retrospective electronic patient file/data study of the results of immediate breast reconstruction surgeries after subcutaneous mastectomy with the use of Serasynth and SeragynBR meshes; performed in the Department of Breast Cancer and Reconstructive Surgery in Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology in Warsaw; Poland.
The Medical and Health Research Ethics Committee of Maria Sklodowska -Curie Memorial Cancer Center and Institute of Oncology in Warsaw; Poland approved this study (ref no. 88/2021). Written informed consent was obtained from all the patients to access their medical data.
The study was made in the period from December 2017 to July 2020. The period of post-operative follow-up amounted to a mean of 20 months (10 to 36 months). Sample size was originally designed for 100 patients from which 93 patients ‘data was feasible for analysis.
In all cases data was collected from the hospital database; then it was grouped; analyzed and filtered based on respective criteria designed by the authors (demographic information; oncological diagnosis; surgery treatment; reconstruction information; complications and reoperation and follow-up.
The qualification for treatment in the author’s Department was performed in compliance with the standards specified in the 2nd Consensus of the Polish Society of Surgical Oncology ‘Surgical treatment of neoplastic breast changes’ and departmental processes. Qualified for subcutaneous mastectomy with nipple-areola-complex sparing (NSM) or without nipple-areola-complex sparing (SSM) or with nipple removal (ASM) with immediate breast reconstruction were both patients with breast cancer primarily treated surgically and after neoadjuvant chemotherapy [7].
Indications for breast reconstruction included: breast cancer not qualifying for breast conserving surgery (BCS) (including multifocal/ multicenter cancers; with extensive microcalcifications); diagnosed BRCA1/2 mutations; mastectomy rather than BCS as a patient’s decision.
The main disqualification criteria for NSM; SSM and ASM included primary local advanced stage of breast cancer (T4 and N3 features; for NSM – less than 2 cm from the nipple cancer location); unstable diabetes; BMI ≥ 35; active cigarette smoking; negative psychooncological consultation.
Particular caution was applied to the qualification for surgery of patients with diagnosed cT3N1; cT3N2 stage tumors; which were preoperatively systemically treated as well as patients after the prior thoracic cavity area radiation therapy. The patients with originally planned adjuvant postoperative radiation therapy were informed in detail about possible postoperative complications. Possible complications and the postoperative course were discussed with all the patients. They also had a routine consultation with a psycho-oncologist prior to the final qualification for the surgery [8].
Two synthetic types of meshes were used for the reconstruction. Operations with the use of SeragynBR were performed in the Department in the period from the end of 2017 to mid-2019. Since the end of 2018; Serasynth meshes were also available. SeragynBR meshes were used primarily in operations performed with the subpectoral method while Serasynth meshes were used in prepectoral operations.
NSMs were usually performed through an inframammary fold incision; with the whole mammary gland; together with the fascia of pectoralis major; being removed. Where the neoplasm was located at a distance closer than 2 cm from the nipple; the nipple areola complex was removed through an ellipsoid skin incision and it was from this incision that SSM was performed or only the nipple was removed and the areola left; and ASM was performed. In the case of large; pendulous breasts; skin-reducing mastectomy was performed from an inverted T incision. The epidermal flap left provided an additional protection of the lower pole of the implant. A sample from under the nipple was collected for histopathological examination; whenever the nipple was spared on mastectomy [9].
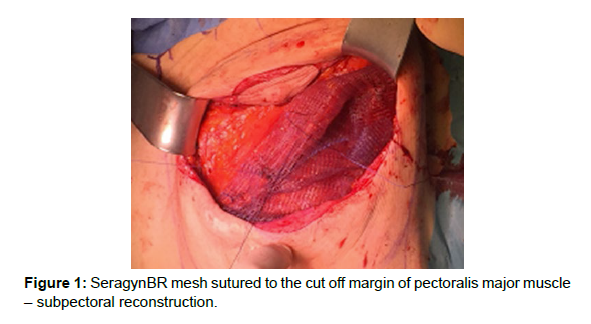
In the case of breast reconstruction with the use of the subpectoral method; the inferior attachment of the pectoral muscle and partly also its attachment to the sternum were cut off. SeragynBR mesh was sutured to the cut off margin with Vicryl 2.0. Its other end was folded under the implant and sutured to the inframammary fold so as to cover the inferior or medial pole of the implant [Figure 1].
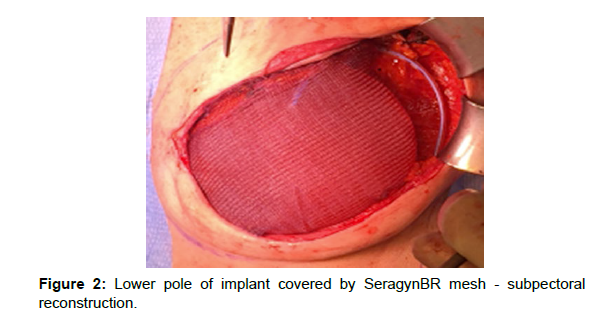
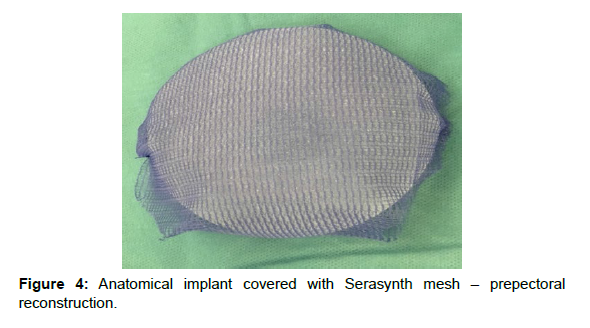
For breast reconstructions; meshes and Sebbin anatomical implants manufactured by Groupe Sebbin or MENTOR® anatomical implants manufactured by Johnson&Johnson were used. In cases of prepectoral reconstruction the implants were enveloped in the mesh (“ravioli method”); which was subsequently sutured with single Vicryl 2.0 sutures. The excess mesh was cut off so that it would not form excessive folds particularly on the margins of the implant. An implant enveloped in the mesh was placed in the skin pocket after mastectomy and the mesh was sutured to the inframammary fold and to the pectoralis major with at least three PDS 2.0 sutures. This form of attachment allowed for adequate protection of the implant against rotation in the postoperative period. To reduce the risk of infection; prior to the placement of the implant; the surgical wound was rinsed with saline while gloves and surgical draping were changed [Figure 2].
Biopsies of the sentinel node were made from a separate incision in the axillary fossa or; if the node was localized in a low position of the axillary fossa; from an access through the mastectomy incision. Axillary lymphadenectomies were performed through a separate incision in the axillary fossa to separate the surgical field of mastectomy from that of lymphadenectomy [10].
In all the cases; one or two Redon drains were left inserted. Redon drain was left till the daily drainage of the serum content amounted to ca. 20-30 ml. Until the moment of the drain removal; the patients received Cephalosprin 2x500 mg; which is recommended in other publications [11]. In cases of seroma collection after drain removal; additional rehabilitation procedures were applied. Therapeutic seroma punctures were performed when the thickness of the liquid over the implant exceeded 5 mm. Where the lymph accumulation persisted; lymph cultures were made to exclude infection [Figure 3, 4].
Statistical analysis
Differences in the distribution of variables between Group1 and Group2 were analyzed with the student test for numeric variables or chi-square tests and Fisher tests for categorical variables.
Results
In the period from December 2017 to July 2020; 93 patients underwent mastectomy with immediate breast reconstruction with an implant; with the use of synthetic meshes; in the author’s Department.
Demographic information is shown in [Table 1].
| Group1 | Group2 | p value | |
|---|---|---|---|
| No of patients | 56 | 37 | NS |
| Age (mean) | 45.63 years (26 to 64) | 46.54 years (33 to 67) | 0.6387 |
| BMI (mean) | 23.8 kg/m2 (16 to 33) | 22.4 kg/m2 (17 to 29) | 0.066 |
Table 1: Demographic information.
In 91 (97.8%) of the patients the indication for mastectomy was breast cancer. In G1; pT2 breast cancer dominated (37.1%) while in G2 pTis (DCIS - ductal carcinoma in-situ - 35%) and invasive pT1 breast cancer (30%); according to TNM. Clinically changed regional lymph nodes - cN0 - were not found in the majority of the patients (G1- 66.6% and G2 - 92.5%). Poorly differentiated cancers- G3 - prevailed among the invasive neoplasms diagnosed in the patients (in G1 - 52.3% and in G2- 50% respectively). Detailed information about specification of the cancers histopathology and TNM are presented in [Table 2, 3].
| Group1 | Group2 | p value | |
|---|---|---|---|
| Type of tumour | n-78 | n-40 | 0.011 |
| Invasive breast cancer | 42 (53.9%) | 22 (55.0%) | |
| carcinoma in situ | 13 (16.7%) | 14 (35.0%) | |
| Other neoplasms and benign lesions | 1 (1.3%) | 1 (2.5%) | |
| Absence of tumour – RRM, conversion from sub- to prepectoral | 22 (28.2%) | 3 (7.5%) | |
| Subtypes of in situ breast cancer | N=13 | N=14 | 0.481 |
| DCIS | 12 (92.3%) | 14 (100%) | |
| LCIS | 1 (7.7%) | 0 | |
| Other neoplasms and benign changes | 1 | 1 | NS |
| Phyllodes tumour | 1 | 0 | |
| Dysplasia | 0 | 1 | |
| Subtypes of invasive breast cancer | N=42 | N=22 | 0.536 |
| NST cancer | 37 (88.1%) | 19 (86.4%) | |
| Lobular carcinoma | 2 (4.8%) | 2 (9.1%) | |
| Mucinous cancer | 2 (4.8%) | 0 (0%) | |
| Tubular cancer | 1 (2.4%) | 0 (0%) | |
| Metaplastic cancer | 0 (0%) | 1 (4.6%) | |
| Biological subtypes of breast cancer | n-42 | n-22 | 0.298 |
| Luminal A | 8 (19.1%) | 8 (36.4%) | |
| Luminal B | 13 (31.0%) | 4 (18.2%) | |
| TNBC | 15 (35.7%) | 9 (40.9%) | |
| Her2(+) | 6 (14.3%) | 1 (4.6%) | |
| Histological grading- G feature | n-42 | n-22 | 0.682 |
| G1 | 6 (14.3%) | 5 (22.7%) | |
| G2 | 14 (33.3%) | 6 (27.3%) | |
| G3 | 22 (52.4%) | 11 (50%) |
Table 2: Specification of the cancers histopathology.
| Group1 | Group2 | p value | |
|---|---|---|---|
| Tumour size acc. to pTNM—(breast) | n- 78 | n-40 | 0.006 |
| TIS | 13 (16.6%) | 14 (35%) | |
| pT1 | 12 (15.3%) | 12 (30%) | |
| pT2 | 29 (37.1%) | 11 (27.5%) | |
| pT3 | 2 (2.5%) | 0 | |
| pT4 | 0 | 0 | |
| T0* | 22 (28.2%) | 3 (7.5%) | |
| Lymph nodes classification cN | n- 78 | n- 40 | 0,004 |
| cN0 | 52 (66.6%) | 37 (92.5%) | |
| cN1 | 3(3.8%) | 0 | |
| cN2 | 0 | 0 | |
| cNx* | 23 (29.5%) | 3(7.5%) | |
| Lymph nodes classification pN | n- 78 | n- 40 | 0.001 |
| pN0 | 42 (53.8%) | 35 (87.5%) | |
| pN1 | 13 (16.6%) | 2 (5%) | |
| pN2 | 0 | 0 | |
| pNx* | 23 (29.5%) | 3(7.5%) |
Table 3: TNM Classification.
In total; 118 breast reconstructions were made; 78 breasts were reconstructed with the use of Serasynth mesh (Group1). The prepectoral approach of breast reconstruction was applied in this group. SeragynBR mesh (Group2) was used for breast reconstruction in 40 patients. In this group of patients; dual-plane reconstruction was mostly used [12].
Originally; 92 surgeries were done within the lymph node drainage area. Biopsies of sentinel lymph nodes were performed in 52 cases (66.6%) in G1 and in 37 cases (92.5%) in G2. Lymphadenectomy (LND) was performed only in G1 in 3 cases (3.8%) lymphatic drainage due to primary metastases to the lymph nodes of the axillary fossa. Due to metastases to sentinel lymph nodes; LND was performed at the second stage in 12 patients; in 10 (12.8%) of patients from G1 and 2 (5%) from G2.
Three of the operated patients reported current cigarette smoking; 2 (3.5%) from G1 and 1 (2.7%) from G2, Diabetes was diagnosed only in 1 (2.7%) patient from G2. Since the number of the patients involved was small; they were not included in the analysis of possible complications entailed [13].
Due to the advanced stage and/or the biological subtype of the cancer; qualified for neoadjuvant chemotherapy (NAC) were 24 (42.8%) of the patients from G1; 15 of which underwent bilateral operations. In G2; 12 (32.4%) of the patients received preoperative treatment. Analysis of the preformed procedures and indications for surgery is presented in [Table 4].
| Group1 | Group2 | p value | |
|---|---|---|---|
| Therapeutic mastectomy | |||
| · unilateral | 52 patients (52 breasts) | 37 patients (37 breasts) | 0.002 |
| · bilateral | 2 patients (4 breasts) | 0 | |
| RRM | |||
| · unilateral | 2 patients (2 breasts) | 0 | |
| Conversion from sub- to prepectoral | 1 patient (1 breast) | ||
| RRM together with therapeutic mastectomies | |||
| 19 patients (19 breasts) | 3patients (3 breasts) | ||
| Bilateral surgeries (patients) | 22 (88.0%) | 3 (12.0%) | <0.001 |
| Types of mastectomies (no of breasts): | |||
| · SSM | |||
| · NSSM | 12 (15.4%) | 3 (7.5%) | 0.19 |
| · ASM | 62 (79.5%) | 37 (92.5%) | |
| 4 (5.1%) | 0 (0%) | ||
| Implant location (No of breasts): | |||
| · prepectoral | <0.001 | ||
| · subpectoral | 76 (97.4%) | 2 (7.3%) | |
| 2 (2.6%) | 38 (92.7%) | ||
| Removal of the drain | Day 13.6 (6 to 21) | Day 12.1 (4 to 19) | 0.0419 |
| Surgery within the lymph drainage region | N=78 | N=40 | 0.002 |
| SLNB, without LND | 42 (53.9%) | 35 (87.5%) | NS |
| SLNB, with later LND (pN+) | 10 (12.8%) | 2 (5%) | NS |
| Primary LND (cN+/pN+) | 3 (3.9%) | 0 (0%) | NS |
| No indications* | 23 (29.5%) | 3 (7.5%) | NS |
| Resection R0 | 74 (94.8%) breasts | 36 (90%) breasts | 0.441 |
| Resection R1 | 4 (5.2%) breasts | 4 (10 %) breasts | NS |
| Adjuvant RT | 10 (12.8%) breasts | 3 (7.5%) breasts | 0.539 |
| NAC** | 24 (42.8%) | 12 (32.4%) | 0.386 |
Table 4: Summary of the surgery and treatment per group
Seroma collection; which persisted after the removal of the drain; was the most common complication, The drain was removed; on average; on the 13 postoperative days when the drained quantity of the serum did not exceed 30 ml per day. Seroma collection developed in 14 (17.9%) breasts operated with the use of Serasynth mesh and in 10 (25%) breasts operated with the use of SeragynBR. Seroma punctures were performed mainly in patients who were primarily treated surgically (in G1 - 50%; in G2 - 80%). A repeated drainage to evacuate lymph was not required in any of the cases.
In 3 operated breasts; apart from the lymph accumulation; inflammation developed in the skin and in the subcutaneous tissue. In these cases; the lymph culture performed revealed bacterial infection requiring an additional; prolonged and modified antibiotic therapy. In G1; in one of the patients; improvement of the local condition and effective treatment of bacteria Klebsiella oxytoca infection was obtained with conservative treatment. In another patient; in spite of antibiotic treatment of Aerococcus viridans infection; cutaneous fistulae formed in both breasts; which required excision as well as removal of the implants and implantation of smaller-volume expanders 5 months after the surgical procedure. This particular patient had a history of radiation therapy for Hodgkin’s lymphoma [14].
In G2; in one patient; the accumulating lymph and Staphylococcus aureus infection caused a prolonged inflammation of the skin and development of an abscess in the first week of the radiation therapy. In that patient; the implant was removed in the course of radiotherapy. In G1; the inflammation of the skin and the infection in one patient were linked to a self-absorbing hematoma without seroma collection. The patient was diagnosed with Escherichia coli; ESBL strain; infection; which was successfully treated with an antibiotic. Lymphocele were accompanied by ischemia of the skin flap in 3; G1 patients; one of whom had a history of radiation therapy. In the remaining 9 patients; local inflammatory conditions of the skin and the subcutaneous tissue; without infections; were successfully treated in a conservative way and the effect of the operation was assessed by the authors as good or very good.
The inflammation of the skin and the subcutaneous tissue constitutes the second of the most common complications after reconstructive surgeries with the use of the meshes referred to. It developed in 6 (7.6%) of the operated breasts in G1 and 7 (17.5%) in G2. Once patients with a culture-confirmed bacterial infection which developed in two patients in each group (2.5% in and 5% of the operated breasts in G1 and in G2; respectively) are added to inflammatory and infectious complications developed in 8 (10.2%) of patients in the group with fully absorbable meshes against 9 (22.5%) patients in the group with partly absorbable meshes [15].
Another group of complications involves skin ischemia. The latter was observed in 8.9% (7 breasts) of breast surgeries performed with the use of Serasynth mesh and 20% (8 breasts) of breast surgeries with the use of SeragynBR mesh. However; only in 4 breasts; skin necrosis required reoperation. In G1; reoperation was necessary in 3 breasts (3.8%) because of fistulae which developed as a late effect of ischemia and skin inflammation. In one case; excision of the skin fistula with repeated suturing of the wound were possible (wound infection did not develop in that patient); while in another patient fistulae in both breasts were excised and implants replaced with smaller-size expanders to reduce skin tension. In G2; one patient (1 breast - 2.5%) required reoperation due to ischemia. Necrosis affected the full thickness of one “inverted T” flap. The remaining cases of skin ischemia involved marginal skin necrosis or superficial ischemia and did not require surgical intervention. This concerned 4 breasts (5.1%) in G1 and 7 breasts (17.5%) in G2; respectively. In all the cases; conservative treatment was successful [16].
Ischemic complications in G1 were most common in patients after non-adjuvant chemotherapy (6 breasts - 7.6%) while in G2 skin ischemia developed in patients after primary surgical treatment (6 patients - 15%). Specification of complications is shown in [Table 5].
| Complications | Group1 | Group2 | p value |
|---|---|---|---|
| No of breasts operated on | n-78 | n-40 | |
| Prolonged seroma collection (breasts) | 14 (17.9%) | 10 (25%) | 0.479 |
| Haematoma | 1 (1.2%) | 1 (2.5%) | 1 |
| Inflammation | 6 (7.6%) | 7 (17.5%) | 0.216 |
| Infection (+ culture results) | 2 (2.5%) | 2 (5%) | 0.611 |
| Skin ischemia (breasts) | 7 (8.9%) | 8 (20%) | 0.148 |
| • superficial | 4 (5.1%) | 7 (17.5%) | NS |
| • full-thickness | 3 (3.8%) | 1 (2.5%) | NS |
Table 5: Complications
Reoperations due to complications in the perioperative period (within 30 days from the operation) in both study groups were necessary; in total; in two out of the 118 breasts operated on (1.6%). In G1 patients; postoperative bleeding; which required repeated hemostasis occurred on the day of the surgery; while in G2 there was skin necrosis which required excision and coverage with a dermoadipose flap from epigastrium in one case.
In the period of over 30 days from the surgery; reoperation was required in 3 patients (4 breasts). In G1; it concerned skin fistulae in three breasts; which had to be excised (3.8%) while in G2 one patient (2.5%) was operated on for an abscess in the course of radiation therapy (RT).
Another group of patients in which a repeated surgery was necessary; there were patients; which required re-resection of the surgical margin after R1 resection, That was necessary in case of 6 patients (6 breasts); 3 cases in each of the study groups - 3.8% and 7.5% in G1 and G2; respectively. In 5 patients; the nipple areola complex was removed. In one patient; it was possible to additionally cut out a part of the skin and subcutaneous tissue on the site of the original tumor location. As no neoplastic cells were found in the additionally resected tissues; the surgeries were deemed to be oncologically radical. Two patients (2 breasts - 1.6%); one in each group; underwent adjuvant radiation therapy (RT) due to R1 resection. In both of those cases; the surgical margin could not be broadened. Postoperative radiation therapy was administered to a total of 13 reconstructed breasts (11%) -10 breasts (12.8%) of G1 patients and 3 breasts (7.5%) of G2 patients. Summary of the reoperations and indications to radiation therapy is shown in [Table 6].
| Group1 | Group2 | p value | ||
|---|---|---|---|---|
| Reoperations due to complications | 4 (5.1 %) breasts | 2(5%)breasts | 1 | |
| Within 30 days from surgery (breasts) | 1 – haematoma (1.3%) | 1 – skin necrosis 2.5%) | NS | |
| Over 30 days from surgery (breasts) | 3 (3.8%) breasts | 1 (2.5%)breast | NS | |
| [2- skin fistula with infection (exchange of the implant with an expander) | [1 (2,5%) breast - abscess - removal of the implant] | NS | ||
| 1- skin fistula without infection (fistula excised)] | ||||
| Procedure in the case of R1 | N=4 | N=4 | ||
| Reoperations performed due to R1 | 3 (3.8%) | 3 (7.5%) | NS | |
| Adjuvant radiation therapy due to R1 | 1 (1.2%) | 1 (2.5%) | NS | |
| Implant removal (breasts) | 2 (2.5%) | 1 (2.5%) | NS |
Table 6: Reoperations and radiation therapy.
Axillary lymphadenectomies which were performed concurrently with mastectomy; with immediate breast reconstruction (only in G1); did not contribute to the development of complications while the radiation therapy undergone by one patient in G2 made implant removal necessary due to an abscess [17].
Discussion
The above analysis illustrates complications developing after mastectomy with immediate breast reconstruction; with the use of two types of meshes dedicated for reconstructive breast surgeries: Serasynth and SeragynBR.
The study group covered in total 118 breast reconstructions performed over the past three years; in the author’s department. The subpectoral method with the use of SeragynBR was gradually replaced with the prepectoral method of breast reconstruction; in which absorbable Serasynth meshes are used. Such a trend is noticeable all over the world [18].
In the opinion of the authors of this article; and others prepectoral operations are technically simpler and allow to obtain a better appearance of the breast already on the operation table Serasynth mesh is sufficiently soft and plastic to envelope the implant in a simple and fast way and subsequently place it and fix it in the skin pocket after mastectomy.
In case of reconstructions with the use of SeragynBR; and thus mainly retropectoral reconstruction; what constitutes an obvious difficulty is the need to cut off the inferior attachment of the pectoral major muscle and to suture the mesh along the whole cut off length; which can involve an increased risk of bleeding and intense pain after the surgery; which is also emphasized by other authors [19]. Yet; work with this mesh was as simple during the surgical procedure; as it was in the case of Serasynth meshes.
Both meshes are simple to use; plastic and do not require special preparation as it is; for instance; in the case of ADM mesh. They can be cut in any way; folded around the implant and are not palpable through the skin.
In Poland; the cost of a Serasynth mesh of the largest size; i.e. 28.5 x 17.5 cm; is approximately € 800. SeragynBR meshes are less expensive; the largest size mesh is approximately € 350. In comparison with other synthetic absorbable meshes or ADM; described products are less expensive.
The most common complication in the study groups was seroma collection; which was observed in 17.9% of the breasts reconstructed with the use of Serasynth mesh and in 25% of breasts reconstructed with the use of SeragynBR mesh. This is rather a high percentage of complications when compared with what is reported in other publications; but it may result from the fact that the authors adopted the concept of faster and more frequent puncture of the lymphocele instead of delaying this procedure. It is hard to define clearly; whether lymphoceles are a postoperative complication or a natural side effect of the surgical procedure with the use of breast implants and synthetic meshes. The percentage of lymphoceles reported in literature is assessed at a relatively high range of 3% to even 85% after mastectomies or surgeries in the region of the axillary fossa [20].
Seroma does not develop solely after operations with the use of meshes. It occurs commonly in the postoperative course after mastectomies and operations in the region of the axillary fossa. High BMI; use of electrocoagulation; low suction pressure; and early removal of drains or delayed physiotherapy are known to be factors affecting the quantity of lymph leaking. Numerous other factors responsible for the accumulation of lymph are still unconfirmed and lymph punctures remain the only effective form of treatment. The presence of lymphocele in three of the patients operated on accompanied skin ischemia and this can be seen as the cause of lymph accumulation in such cases.
Very similar findings with regards to the occurrence of seroma in patients in which Seragyn BR mesh was used for reconstruction were reported by Machleidt who analyzed 148 breasts operated on in 119 patients. The material describes seroma in 25% of cases and hematoma and infections in ca. 14% of the cases. In our material; these complications accounted for a significantly lower percentage in both analyzed groups. The percentage of reoperations and cases in which the mesh had to be removed; reported by Machleidt was also higher and amounted to 11.5%, in our material; in the Group2; two breasts (5%) required repeated surgery and in one of those cases the mesh and the implant were removed (2.5%). In Group1; three patients (4 breasts) required reoperation (5.1%) [21].
In both groups of patients; the mean duration of the drain insertion was ca. 13 days and was similar as reported in other publications. Yet; it was a rather long period; which may indicate that implant and mesh placements can constitute an important element which contributes to prolonging lymph accumulation.
Chattejee and Nahabedian in their meta-analysis covering 14 publications and assessing 654 operated breasts; show that the most common complications of prepectoral surgeries with the use of meshes include seroma collection (6.7%) and skin flaps necrosis (7.8%) followed by infections (4.2%); hematomas and wound dehiscence; 3.4% and 3.2%; respectively. In our material; the percentage of seromas in Group1 amounted to 17.9% while that of skin ischemia to 8.9%. In Group2; these complications were even more common - seromas - 25%; skin ischemia – 20%. In the opinion of the authors; complications such as ischemia and necrosis of skin flaps or wound margins are not linked to the type of mesh used for the reconstruction nor to the type of reconstruction but rather to the operation technique; the experience of the operator and individual anatomical conditions as well as the anatomy of the mammary gland.
In our clinic; the assessment of the blood supply and thickness of skin flaps in the described patients was made based on a clinical evaluation by a surgeon. In other studies; their authors also based their decision as to the selection of the subpectoral versus prepectoral method on the clinical evaluation of the flaps. The objective ways of the evaluation of skin perfusion include; for instance; cameras for the detection of indocyanine green. This way of assessing skin flaps perfusion is suggested by Jones and Anthonyband others. This is a very effective method of the evaluation of the normal perfusion of the skin and can have a significant influence on the choice of the reconstruction method as well as prevent potential complications.
Inflammation of the skin and subcutaneous tissue developed in 7.6% (6 breasts) in Group1 and in 17.5% (7 breasts) in Group2. The majority of them subsided spontaneously or after prolonged antibiotic therapy. In publications dealing with immediate reconstructions; the percentage of infections is similar or even higher as reported by Potter where infections developed in 26% of operations with synthetic meshes and 22% of cases with the use of biological meshes. In the article by Jeevan; this percentage amounted to 24.1% in the case of mastectomy with immediate breast reconstruction. A higher percentage of infections (12%) were also described by Casella in a two-stage mastectomy with a prepectoral breast reconstruction with an expander and Tiloop Bra meshes [22].
In the above analysis the removal of implants was necessary in two patients (3 breasts) which is 2.5% of operated breasts in both analyzed groups. In both cases; prolonged lymph accumulation was reported. This percentage is consistent with other studies and lower than that given by Jeevan. In the latter; the removal of the implant was necessary in 8.9% of patients. In another publication by Nealon; the percentage of lost implants was higher and amounted to 4.9% after subpectoral reconstructions and 3.5% after prepectoral reconstructions. In these patients; biological meshes were used. Similar percentages of implant losses (6.5%) after breast reconstructions with the use of ADM were reported by Cumo. In the prospective study by Potter; the mean percentage of implant losses in patients after immediate reconstructions was at the level of 10% for synthetic meshes and 8% for biological meshes. In turn; Hansson compared complications in patients after reconstructive breast surgeries with the use of biological mesh; on the one hand; and synthetic mesh; on the other. The percentage of implant losses after breast reconstructions with a synthetic mesh was similar to the findings presented by the authors of this study and amounted to 2%; in comparison to 8.5% of the 8.5% of implant losses after breast reconstruction with the use of ADM mesh; which is consistent with other publications. The frequency of seroma collection in this material was also higher after the use of ADM (38%); in comparison with breast reconstruction with synthetic meshes (3.8%).
What deserves attention; are a growing number of patients requiring neoadjuvant chemotherapy or adjuvant radiation therapy which results from changes in the qualification for treatment in recent years. In the analyzed groups; this concerned; respectively; 42.8% and 32.4% of patients who received neo-adjuvant chemotherapy. What is important in these groups of patients is careful qualification for surgical treatment and good communication with patients as regards possible influence of a surgery with reconstruction on potential delay of the oncological treatment with a potentially higher percentage of complications. This concerns both the qualification for adjuvant chemotherapy and adjuvant radiation therapy. This is also emphasized by other authors. In our material; one patient (1 breast - 2.4%) from Group2 had interrupted radiation therapy because of an abscess in the operated breast and the need to remove the implant.
Another important factor of significant influence on the development of complications is high BMI > 30. In our group of patients only one patient had a BMI of over 30 (BMI-32) and developed superficial skin ischemia and lymphorrhea. Seven patients with BMI of 27-30 developed superficial skin ischemia and three prolonged lymph accumulation. This can confirm the dependence of the occurrence of these complications on BMI but the number of patients with high BMI is too small to give objective results. The assessment of the aesthetic effects and quality of life was not the subject of this study [23].
Conclusions
The authors assessed complications; which occurred in case of patients operated with the use of two types of meshes - partly absorbable SeragynBR and fully absorbable Serasynth. The most common complication in both assessed groups was the occurrence of prolonged seroma collection; which however; did not contribute in any significant way to the development of serious complications such as removal of the implant or infection. Fewer complications were reported in the group of patients in whom fully-absorbable Serasynth mesh was used for the reconstruction and the percentage of complications in this group was twice lower than in patients after operations with SeragynBR. Other complications which developed after the placement of the two types of meshes were similar to those presented in other publications on mastectomy with immediate breast reconstruction with the use of synthetic meshes. The percentage of implant losses in the discussed group of patients was lower than that described in literature.
In spite of the complications; the development of which was not statistically significant; it can be concluded that both types of meshes are safe additions to reconstructive breast surgeries. The group of patients will remain under further observation so that late effects of the operations can be assessed over a longer period.
Data availability
The supporting data is available in the Department of Breast Cancer and Reconstruction at Maria Sklodowska – Curie Memorial Cancer Center and Institute of Oncology in Warsaw; Poland.
Conflict of Interest: The authors declare that they have no competing interests.
Funding statement
The research did not receive specific funding; but was performed as part of the employment of the authors at Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology in Warsaw; Poland.
Acknowledgement
We express special thanks to Prof. Ralf Ohlinger from the Clinic and Polyclinic for Obstetrics and Gynaecology; University Medicine Greifswald; Greifswald; Germany for the presentation of surgical techniques with the use of the meshes described and the possibility of having practical training.
References
- Safran T, Al-Halabi B, Viezel-Mathieu A (2020) Direct-to-Implant; Prepectoral Breast Reconstruction: A Single-Surgeon Experience with 201 Consecutive Patients. Plast Reconstr Surg 145:686-696.
- Louw R, Nahabedian M (2017) Prepectoral Breast Reconstruction. Plast Reconstr Surg 140:51-59.
- Gerber B, Marx M, Untch M (2015) Breast Reconstruction Following Cancer Treatment. Dtsch Arztebl Int 112:593-600.
- Alderman A, Gutowski KA, Ahuja A (2014) ASPS clinical practice guideline summary on breast reconstruction with expanders and implants. Plast Reconstr Surg 134:648-655.
- Srivastava V, Basu S, Shukla KV (2012) Seroma Formation after Breast Cancer Surgery: What We Have Learned in the Last Two Decades. J Breast Cancer 15:373-80.
- Sumanas J, Khavanin N, Kim JY (2016) Seroma in Prosthetic Breast Reconstruction. Plast Reconstr Surg 137:1104-16.
- Liliav B, Patel P, Jacobson A (2019) Prepectoral Breast Reconstruction: A Technical Algorithm. Plast Reconstr Surg Glob Open 7:21-27.
- Nealon K, Weitzman R, Sobti N (2020) Prepectoral Direct-to-Implant Breast Reconstruction: Safety Outcome Endpoints and Delineation of Risk Factors. Plast Reconstr Surg 145:898-908.
- Gardani M, Bertozzi N, Grieco MP (2017) Breast reconstruction with anatomical implants. Ann Med Surg 21:96-104.
- Becker H, Lind II J (2013) the use of synthetic mesh in reconstructive; revision; and cosmetic breast surgery. Aesth Plast Surg 37:914-21.
- Cuomo R (2020) Submuscular and Pre-pectoral ADM Assisted Immediate Breast Reconstruction: A Literature Review. Medicina 56:256.
- Casella D, Bernini M Orzalesi L (2014) TiLoop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg 37:599-604.
- Machleidt A, Schmidt-Feuerheerd N, Blohmer J (2018) Reconstructive breast surgery with partially absorbable bi-component Seragyn® BR soft mesh. Arch Gynecol Obstet 298:755-61.
- Srinivasa D, Holland M, Sbitany H (2019) Optimizing perioperative strategies to maximize success with prepectoral breast reconstruction. Gland Surg 8:19-26.
- Chatterjee A, Nahabedian MY, Gabriel A (2018) Early assessment of post-surgical outcomes with prepectoral breast reconstruction. J Surg Oncol 117:1119-30.
- Jones J; Antony AK (2019) direct to implant pre-pectoral breast reconstruction. Gland surg 8:53-60.
- Sinnott J, Persing S, Pronovost M (2018) Impact of Post mastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 25:2899-908.
- Potter S, Conroy EJ, Cutress RI (2019) Short-term safety outcomes of mastectomy and immediate implant-based breast reconstruction with and without mesh (iBRA). Lancet Oncol 20:254-66.
- Jeevan R, Cromwell DA, Browne JP (2014) Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. Plast Reconstr Aesthet Surg 67:1333-44.
- Casella D, Calabrese C, Bianchi S (2015) Subcutaneous Tissue Expander Placement with Synthetic Titanium-Coated Mesh in Breast Reconstruction. Plast Recontr Surg Glob Open 3:577.
- Vidya R, Masila J, Cawthorn S (2017) Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 23:670-6.
- Hansson E, Edvinsson Ach, Elander A (2021) First-year complications after immediate breast reconstruction with a biological and a synthetic mesh in the same patient. J Surg Oncol 123:80-8.
- Thorarinson A, Frojd V, Kolby L (2017) Patient determinants as independent risk factors for postoperative complications of breast reconstruction. Gland Surg 6:355-67.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: Grous A, Mazur S, Winter P, Kozak K, Jagiello-Gruszfeld A, et al. (2022) Immediate Breast Reconstructions after Mastectomy due to Breast Cancers with the Use of Serasynth and Seragynbr Synthetic Meshes. Single-Oncological Center Experience, Analysis of Complications. J Med Imp Surg 7: 150.
Copyright: © 2022 Grous A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Usage
- Total views: 1383
- [From(publication date): 0-2022 - Dec 25, 2024]
- Breakdown by view type
- HTML page views: 1193
- PDF downloads: 190