Research Article Open Access
Identification of Volatile and Protein Profiles in the Sting and Mandibular Glands of the Worker Honey Bee (Apis cerana indica)
| G. Lakshmi Priya1, P. Rameshkumar1,2, P. Ponmanickam1, R. Eswaran3, D.N.P. Sudarmani1 and T. Rajagopal1* | |
| 1Post Graduate Department of Biotechnology, Ayya Nadar Janaki Ammal College (Autonomous), Sivakasi-626 124, Tamil Nadu, India | |
| 2Centre for Pheromone Technology, Department of Animal Science, School of Life Sciences, Bharathidasan University, Tiruchirappalli-620 024, Tamil Nadu, India | |
| 3Department of Zoology, Madura College (Autonomous), Madurai-625 011, Tamil Nadu, India | |
| *Corresponding Author : | T Rajagopal Post Graduate Department of Biotechnology Ayya Nadar Janaki Ammal College (Autonomous) Sivakasi-626 124 Tamil Nadu, India Tel: 91-04562-254100 Fax: +91-04562-254970 E-mail: deer_raj@yahoo.co.in |
| Received January 08, 2013; Accepted February 04, 2013; Published February 07, 2013 | |
| Citation: Priya GL, Rameshkumar P, Ponmanickam P, Eswaran R, Sudarmani DNP et al. (2013) Identification of Volatile and Protein Profiles in the Sting and Mandibular Glands of the Worker Honey Bee (Apis cerana indica). Biochem Physiol 2:108. doi:10.4172/2168-9652.1000108 | |
| Copyright: © 2013 Priya GL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
In social insect, the perception of volatile molecules, odorants and releasing pheromones, is mediated via specialized olfactory organs is generally thought to be accomplished by odorant-binding proteins and pheromonecarrying proteins. These proteins play an important role in the solubilization, transport and deactivation of pheromones. In honeybee, the sting and mandibular glands are one of the major pheromonal sources and it is believed to contain volatile compounds and proteins (pheromone carrying or binding) for chemical communication. Therefore, the present investigation was carried out to identify the pheromones and proteins in sting and mandibular glands in adult worker bee, Apis cerana indica GC-MS and SDS-PAGE. Nearly twenty eight volatile compounds were identified in the sting and mandibular glands of the worker bee. Amongst, the 19 compounds were identified in the mandibular gland and 16 compounds in the sting. Amongst certain compounds peak appeared specific or common to particular glands. The present results suggest that glandular specific volatiles which may evoke the behaviourally important chemical signals in the Apis cerana indica. The present study revealed that the total content of protein was higher in the mandibular than sting gland. In fact, the protein profiles like 21.1, 25, 26, 29, 43, 61, 97.9 and 110 kDa have noted in the sting and mandibular glands. Among these fractions, the 43 and 26 kDa polypeptides appeared prominently in the sting and mandibular glands, respectively. Further, electophoretic profile showed 43 kDa mass protein intensity was relatively high in sting gland, whereas 26 kDa mass protein intensity was relatively higher in the mandibular gland. Based on the volatile identification and proteins in the sting and mandibular glands of worker bees supported by literature, it is concluded that the sting and mandibular gland-specific volatile compounds which may convey the specific information regarding the alarm or repellent forage-marking scent.
| Keywords |
| Apis cerana indica; Mandibular gland; Sting; Pheromonebinding protein; Chemical signal |
| Introduction |
| Insects have a good sense of olfaction. Their olfactory organs containing over a thousand Olfactory Receptor Neurons (ORNs) which have an olfactory ability to sense odour chemicals with remarkable sensitivity and specificity and to code both chemical exposure and other external physical stimuli [1]. When a chemical message is exchanged between members of the same or different species, the chemicals involved in these interactions are called “semiochemicals”. By contrast, the chemical signal used in communication among members of the same species is called a “pheromone”, a term first coined by Peter Karlson and Adolf Butenandt, in 1959 as a substitute for the older, selfcontradictory ectohormone [2-4]. Pheromones may be classified as olfactory or oral according to the site of their perception. Also, their various actions can be distinguished as releaser effects, comprising the classical stimulus-response mediated by the nervous system or primer effects, in which endocrine and reproductive systems are altered physiologically [5]. |
| The origin of pheromones is situated in exocrine glands and the perception of volatile molecules, odorants and pheromones are mediated via specialized olfactory organs, the sensilla, located mainly on the antennae [6-9]. In insects, the transport of airborne, hydrophobic odorants and pheromones through the sensillum lymph is generally thought to be accomplished by odorant-binding proteins [10]. The volatile pheromones are transported efficiently in aqueous body secretion for which the need to be bound to proteins known as lipocalins [11]. In insects, OBP (Odorant Binding Protein) were first described in the antennae of male Lepidoptera and named Pheromone-Binding Proteins (PBP) after their ability to bind pheromonal components [12,13]. They are observed in large amounts in males (10 mM in Lepidoptera), occurring as several acidic proteins of 15-20 kDa or isoforms in the same species [14,15]. Although these proteins were generally found to be specifically associated with olfactory sensilla, recent data report a possible role of related proteins in contact chemoreception, as illustrated in flies [16,17]. However, the acidic proteins involved in the long-term stability of volatiles in the environment (persistence) and slowly release the volatiles for long durational effect [18,19]. |
| In general, the honey bees are eusocial insect and the female honey bees develop into two castes, queens or workers depending on the environment at the larval stages [20,21]. It is reported that the coordination of the different tasks i.e., care of brood, growth and maintenance of colony by workers are partly mediated by chemical signals [21-23]. Behavioral evidence for division of reproduction and labour in the colony indicates the importance of pheromones in both queen-worker and worker-worker interactions, including mediating the regulation of task allocation [23]. For instance, the honey bee Apis mellifera, sex’s express different odor repertoires: there is a wide spectrum of general odors together with different pheromones for workers, which are sterile females, and a more restricted specificity for queen pheromone as a sex attractant for the male drones [24-26]. It is reported that Dufour gland secretes esters and eicosenol pheromones by Apis mellifera and A. cerana, which are utilized in defense by worker bees or reproduction in queen bees [27]. |
| There have been few reports on pheromone carrying proteins and odorant binding proteins in the Apis cerana indica [28]. In the literature consulted, there is no knowledge about the characterization of pheromone and its carrying proteins of the sting and mandibular gland of worker honey bee of Apis cerana indica. In the pursuit of all these considerations, present work offers a very simple technique for identification of volatile and protein profiles in the sting and madibular glands of worker honey bee of Apis cerana indica. |
| Materials and Methods |
| Sample collection |
| Worker bees, Apis cerana indica was collected from the natural hive in the village of N. Pudupatti near Srivilliputhur, Tamil Nadu and were sealed in plastic bags. Samples were immediately stored at -20°C until further analysis. The species (Apis cerana indica) identification and confirmation was done by using various field guides and other available literature in our institute [29]. The specimens (honey bee) were collected with the help of honey hunters as per the standard guidelines of Institute of Animal Ethical Committee. |
| Fifty five worker honey bees were anesthetized on ice and the sting and mandibular glands were carefully excised from the abdomen and head with sterile blade. After collection, the samples were placed in glass vial with Teflon-lined stoppers and stored at -20°C for further analysis. |
| Preparation of extracts |
| The crude extract from the sting and mandibular glands were prepared by homogenization with Phosphate Buffer Saline (pH 7.2) in well-sterilized homogenizer under ice-cold condition, followed by centrifugation at 10,000 rpm for 15 minutes. The clear supernatant was used for volatile compound analysis. Further, the supernatant was collected and dialyzed (Spectra/Por molecular porous membrane 12-14 MWCO, Thomas Scientific, USA) against pre-cooled Millipore water (MilliQ Water) with several water changes. Alternatively, extract was concentrated to a few milliliters by N2 pressure dialysis with YM10 filters (Amicon membrane-8010), of 10 kDa cutoff size. The total protein was determined according to Bradford [30] using BSA (Bovine Serum Albumin) as a standard. |
| Identification of volatile compounds by GC-MS |
| Dichloromethane (DCM) was used as a solvent in GC-MS analysis. From each sample 1 ml extract was taken separately and mixed with 1 ml DCM (1:1 ratio) and filtered through a silica gel column (60-120 meshes) and concentrated under vacuum (temperature<30°C) for fractionation and further processed for chemical identification. |
| The sample was fractionated and chemical compounds were identified by Gas Chromatography-linked Mass Spectrometry (GCMS; QP- 5050, Schimadzu, Japan) [31]. 1 μl of the extract was injected into the GC-MS system on a 30 m glass capillary column with a film thickness of 0.25 μm (30 m×0.2 mm) is coated with UCON HB 2000) using the following temperature programme; initial oven temperature of 40°C for 4 min increasing to 250°C at 15°C/min; and then held at 250°C for 10 min. The gas chromatography (Schimadzu GC 15A) was equipped with FID detector connected to an integrator. The relative amount of each component was reported as the percent of the ion current. The GC-MS was under the computer control at 70 eV using Helium as reagent gas at 95 eV performed chemical ionization. Identification of unknown compounds was made by probability-based matching using the computer library built within the NICT 12 system. |
| Sodium dodecyl sulphate polyacrlamide gel electrophoresis (SDS PAGE) |
| To determine the protein profiles of sting and mandibular gland extracts on the basis of its molecular weight, the separating gel (12%) and stacking gel (5%) was carried out following the method of Laemmli [32]. 50 μg of proteins were loaded onto gel for comparison. For determination of the molecular weight, 4 μl of protein standard (Protein Molecular Weight Marker-Medium range, Genei, Bangalore, India) was applied on the gel. |
| Protein detection by Coomassie Brilliant Blue (CBB) staining |
| After electrophoresis, the gel were rinsed with distilled water and mixed with 0.5% CBB R-250 in a solution of 40% methanol and 10% acetic acid at room temperature for 2 hours and then de-stained using the same solution until an appropriate background was obtained. Finally, the gel was washed with distilled water. The molecular weight and quantification of the polypeptides were performed by a gel documentation system and analysis soft-ware (Quantity One, Bio Rad, CA, USA). The band area was measured in pixel. |
| Results |
| Chemical profiles |
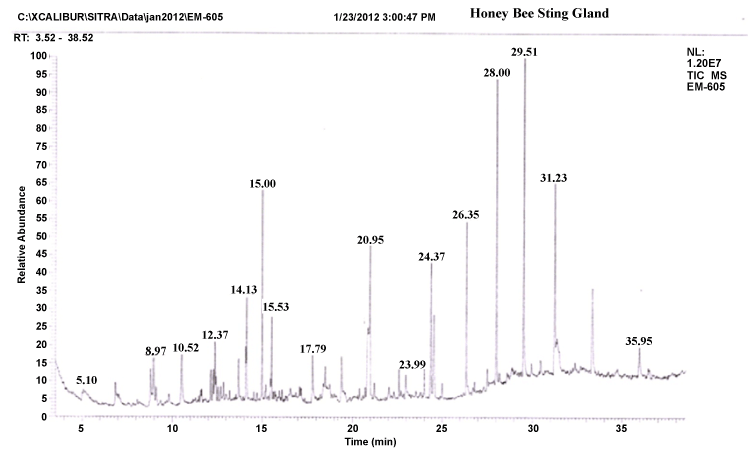
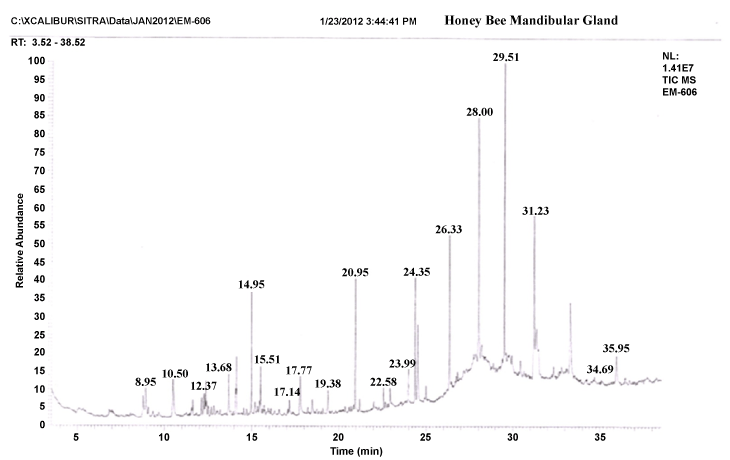
| The gas chromatograms of figures 1 and 2 shows the volatile profiles obtained from sting and mandibular glands of worker bee. In each gland, the sample showed 16–19 detectable peaks (Table 1). Nearly 28 detectable peaks were noted in the sting and mandibular glands of the worker bee. The constituents identified in the sting and mandibular gland sample were alkanes, alcohols, ketones, carboxylic acid, aldehyde, pyron, furan and expoxide. Among the different constituents, alkanes (16 volatiles) were predominantly present in the both gland samples compared to other constituents. Visual examination of all the chromatograms showed that there was a consistent qualitative difference in the chemical profiles in regard to sting and mandibular glands of worker bee. Among 28 volatile compounds, 19 compounds were identified in the mandibular gland and 16 compounds in the sting. |
| Comparison of the identified compounds sting and mandibular gland revealed that certain compounds were appeared specific or common to particular glands. For instance, among the 16 compounds present in the sting gland of worker bee, 9 compounds viz. 2,2-bis(1,1-dimethylethyl)-6-methyl- 2H-pyran, heptadecane, undecane, hexatriacontane, 2-methyl-10-butanol, pentadecane, hexadecanoic acid, docosane and heptacosane seemed to be specific to sting gland sample. Indeed, certain compounds were present in almost all gland samples. For example, Heptacosane, 3,5-bis(dimethoxymethyl)-1H- 1,2,4-triazole, 2-Benzylidene-3-oxo-4-(octylsulfanyl)-2,3-dihydrothiophene- 1-dioxide, 2-nonanol, tertratetracontane, dotriacontane and hexacosane were present in sting and mandibular glands. Further, the compounds like Nonadecane, octadecane, octacosane, isopropyl dodecanoate, docosane, 2,5-dimethoxy-3,4-dipentoxytetrahydrofuran, 3-methyl-2,2- dimethoxycyclopentan-1-one, hexadecane, pentadecane, 2-heptanone, pentatriacontane and 3-methoxy-5-methylene-2(5H)-furanone appeared only in the mandibular gland extract. |
| Protein profiles of sting and mandibular glands |
| The present investigation showed the protein concentration was found to vary in the sting and mandibular glands of worker bee. The mean protein concentration was effectively recorded of the mandibular gland (88.75 ± 0.15 mg/g) than sting (12.40 ± 2.35 mg/g). |
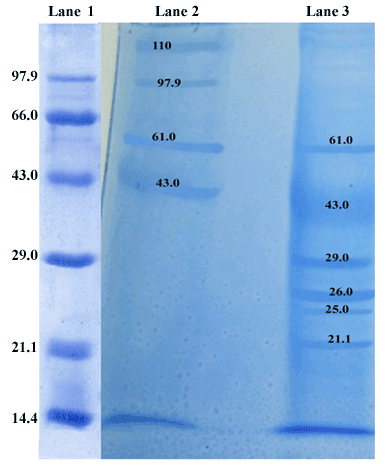
| The protein profiles of sting glands were compared to that of mandibular glands of worker bee. SDS-PAGE protein profiles of sting and mandibular glands expressed several proteins, in which the different molecular mass of proteins was found a distributed over a wide range like 21.1, 25, 26, 29, 43, 61, 97.9 and 110 kDa, respectively (Figure 3). The staining intensity of all major polypeptides was found to be varying in sting and mandibular glands of worker bee. Among these fractions, the 43 and 26 kDa polypeptides appeared prominently in the sting and mandibular glands respectively. The intensity of 43 kDa mass protein was relatively higher (p<0.05) in sting gland, whereas 26 kDa mass protein intensity was relatively higher (p<0.05) in the mandibular gland (Table 1). By contrast, 61 kDa polypeptide intensity was high in the sting as compared to mandibular gland. These observations were confirmed by the band areas determined by densitometric scanning. Furthermore, 110 and 97.9 kDa polypeptides were unique presence in the sting gland. The mandibular gland sample contained 29, 26, 25 and 21.1 kDa polypeptides, which were absent in the sting gland. |
| Discussion |
| Many activities of social insects are regulated by bouquets of semiochemicals. Odours are composed of complex mixtures of organic volatile compounds. While certain specific components of those mixtures referred to as “pheromones” undoubtedly exist, less specific mixture components may also have importance for the overall chemical message perceived by the insect olfactory organs. In the present study, twenty eight volatiles compounds were identified in the sting and mandibular glands worker bee. The volatiles identified in the sting and mandibular glands belong to the alkanes, alcohols, ketones, carboxylic acid, aldehyde, pyron, furan and expoxide classes of compounds. These classes of compounds have already been reported from the mandibular, sting, frontal, postpharyngeal glands, Dufour’s gland and venom glandular secretion of different social insects like ants, bees, wasps and termites [8,33]. |
| In the present study, more number of alkane compounds was identified in the both sting and mandibular glands extract of the worker bee. Achiraman and Archunan [31] described the alkane compounds are probably common metabolic end products in vertebrates and invertebrates. For example, an alkane, 1-iodoundecane identified in estrus bovine urine is reported to play a significant role in the attraction of bulls [34], and another volatile, 1,5-diemethyl-6-8-dioxobicyclo (3.2,1) octane is found to act as a pheromone during musth in elephants [35]. Similarly in insects, alkane acts as a bitrophic herbivore plant interactions as well as attractants for oviposition or attraction for feeding [36]. In addition, the mixture of seven monomethylalkane (i.e., 9-methylheptacosane, 11-methylheptacosane, 9-methylnonacosane, 11-methylnonacosane, 13-methylheptacosane, 13-methylnonacosane and 15-methylnonacosane) compounds at natural concentrations to elicit the male mating behavior in Gastrophysa atrocyanea [37]. Therefore, the alkane compounds may be the important candidature for pheromone as it expresses the general chemical communication between drone-workers or queen-workers or workers-brood bees. |
| It is well established that sting gland is one of the main source of the alarm substances in a wide variety of ants and bees [38]. It is important to note that the peak area and peak height of 2-methyl-10-butanol and 2-nonanol compounds are significantly higher in the GC profile. These compounds identified specifically in the sting gland, it may be considered as behavioural important chemical signals in the worker bee for communication between worker-drones or worker-queen or worker-brooders. The similar compound 2-methyl-10-butanol is reported in the sting gland of Apis mellifera [39]. It is remarkable to note that the compound 2-methyl-10-butanol is detected in other insects with slight variation in structure. For example, 2-methyl-3- butane-2-ol was identified as a component of the alarm pheromone of Vespula crabro [40]. Wager and Breed reported that 2-nonanol is a primary orientation role of alarm pheromone is to alert bees and attract them to moving objects and that it is probably inaccurate to assert a primary role for localization through chemotaxis for alarm pheromone [38,39]. The present findings demonstrate the presence of these specific volatiles which are pheromones in unrelated species may also evoke the behaviourally important (i.e., alarm signals) chemical signals in Apis cerana indica. |
| In insects, the most elaborate chemical communication systems have evolved in the context of mating and social behavior [41]. In honey bees, these communication systems are closely linked by mandibular gland secretions to attract drons or alarm signals [26]. 12 mandibular glandular volatiles (Nonadecane, octadecane, octacosane, isopropyl dodecanoate, docosane, 2,5-dimethoxy-3,4-dipentoxytetrahydrofuran, 3-methyl-2,2-dimethoxycyclopentan-1-one, hexadecane, pentadecane, 2-heptanone, pentatriacontane and 3-methoxy-5-methylene- 2(5H)-furanone) present in the worker bee, the concentration of 2-heptanone compound (peak height and area) were relatively higher in the mandibular gland of worker bee. Similar result was reported by Pankiw [42], the mandibular gland of worker bees also produce the alarm substance like 2-haptonone (2HPT). With increasing age of workers bees, the size of the mandibular gland and amount of 2HPT progressively increases. Stout and Goulson [43] found that 2HPT it may act as a repellent forage-marking scent, i.e., to avoid the probing of flowers that have recently been depleted of nectar or pollen. Therefore, the present findings suggest that mandibular gland specific odours (volatiles) may rely on repellent forage-marking scent. |
| In the present study, total protein content in the mandibular gland was significantly higher than sting gland of the worker bee. Booth and White [44] reported that the high concentration of proteins in the mandibular gland of worker bee reflects a high rate of intracellular synthesis of these proteins. The SDS-PAGE profiles of sting and mandibular gland revealed the presence of different molecular mass proteins like 21.1, 25, 26, 29, 43, 61, 97.9 and 110 kDa, respectively. Among these fractions, the 43 and 26 kDa proteins appeared prominently in the sting and mandibular glands respectively. The present findings are consistent with the earlier reports on Pheromone Carrier Proteins (PCPs) typically around 17-30 kDa that have a high level homology and include Odorant Binding Proteins (OBPs) produced by invertebrate chemosensory epithelia [18]. In addition, the N-terminal sequence (LEDAPRVKTPGAIGYYKI) for the 60 kDa protein was determined in Apis mellifera L. and it was reported as which involved in the antennal functions characteristic of drons [21]. In vertebrates, the pheromone carrying protein, α2u-globulin (18-21 kDa) was reported in the preputial gland of both adult [45,46] and pup [47], and urine of house rat [48] while the intensity of this protein was higher in the preputial gland of adult than the prepubertal rat. Moreover, another protein, aphrodisin (17 kDa) secreted in hamster vaginal fluid by vaginal tissue and Bartholin gland was also reported to be a pheromone carrier and facilitated the male copulatory behaviour [49]. |
| For efficient transport in aqueous body secretions, pheromones needed to bind proteins (pheromone-carrying proteins), belonging to a large family of lipocalins [11,50,51]. Proteins are present at high concentration in biological fluid, for both the delivery (pheromonecarrying proteins, or PCPs) and perception (odorant-binding proteins, or OBPs) of chemicals involved in pheromone communication [14,52,53]. For instance, Bmor Pheromone-Binding Protein (Bmor PBP), the structure easily accommodates the pheromonal compound (ligand) like (E10, Z12)-hexadecadienol responsible for male attraction [54,55]. Moreover, OBP of ASP1(24.5 kDa -Antennal-specific proteins 1) has been identified in the antenna of drons honey bee (Apis mellifera L.) and bound with two volatile compounds such as 9-keto- 2(E)-decenoic acid (9-ODA) and 9-hydroxy-2(E)-decemoic acid (9- HDA). These two pheromones are produced by queen mandibular gland, which act as a sex attractant for drons [56,57]. In Drosophila melanogaster, Os-D (13-kDa) protein that contains a putative nuclear import sequence, and an acid-rich region. The OBP from ApolPBP has (0.64 Kd) its pheromone (E,Z)-6,11-hexadecadienyl-1-acetate and (21 Kd) for binding with the homolog (E,Z)-4,9-tetradecadienyl-1- acetate in the silk moth (Antheraea polyphemus) [58]. Another class of chemosensory proteins (10–20 kDa, CSPs) has been described in some insect species such as Drosophila melanogaster [16,59], desert locus Schistocerca gregaria [60] and Locusta migratoria [61,62]. Another group of proteins, called general odorant-binding proteins (18 kDa, GOBPs) [15] have also been identified in moth antennae. They showed the substantial sequence similarity to the PBPs and have been proposed to carry odorants, as opposed to pheromones [15]. |
| Interestingly, there are some gland-specific proteins are appeared in the sting (110-, 97.9-KDa) and mandibular (29-, 25-, 21.1-KDa) glands. These proteins may carry the volatile in Apis cerana indica secretory gland. Ponmanickam et al. [63] reported that α2u-globulin (18 kDa, pheromone carrying protein) protein specially appeared in the preputial gland of intact male rat as compared to castrated male. Thus, the present findings suggest that these gland–specific proteins may involve in the long-term stability of volatiles in the environment (persistence) and slowly release the volatiles for long durational effect. |
| It is suspected that identified specific volatile compounds may bind with 43 and 26 kDa proteins of sting and mandibular glands respectively. Hence, these proteins identified in the present study may consider being the carrier for sting and mandibular glandular pheromones of Apis carana indica. Based on the volatile compounds and proteins (i.e., 43 and 26 kDa) in the sting and mandibular glands of worker bees supported by literature, it is concluded that these volatile compounds which may convey information regarding the alarm or repellent forage-marking scent. |
| Acknowledgements |
| The authors thank the Principal and the management of Ayya Nadar Janaki Ammal College, Sivakasi for facilities and encouragement. We thank Dr. G. Archunan, Director, Center for Pheromone Technology, Professor of Animal Science, School of Life Sciences, Bharathidasan University, Tiruchirappalli for encouragement and suggestions. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14516
- [From(publication date):
May-2013 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 10073
- PDF downloads : 4443
