Research Article Open Access
Identification of Serum Anti-GADD34 Antibody as a Common Marker of Diabetes Mellitus and Parkinson Disease
Kazuo S1,2,†, Tomiyoshi G2,3,†, Masahiro M1, Satoshi K1, Shigeki H1, Setsu S1, Minako B1,4, Mayumi M1, Akiyuki U1, Kenichiro K5, Minoru T6, Akiko H6, Masashi Y6, Kazuki K6, Harukiyo K6, Ryoichi I6, Koutaro Y6, Seiichiro M7,8,9, Toshio M9, Eiichi K7, Yoichi Y7, Tomoo M7, Yasuo I7, Yoshio K10, Rika N2,3, Natsuko S2,3, Hideyuki K3, Hao W2,11, Xiao-Meng Z2 and Takaki H2*1Department of Neurology, Graduate School of Medicine, Chiba University, Chiba 260-8670, Japan
2Department of Biochemistry and Genetics, Graduate School of Medicine, Chiba University, Chiba 260-8670, Japan
3Medical Project Division, Research Development Center, Fujikura Kasei Co., Saitama 340-0203, Japan
4Department of Molecular Diagnosis, Graduate School of Medicine, Chiba University, Chiba 260-8670, Japan
5Department of Internal Medicine 3, University of Yamanashi School of Medicine, Yamanashi 409-3898, Japan
6Department of Clinical Cell Biology and Medicine, Graduate School of Medicine, Chiba University, Chiba 260-8670, Japan
7Department of Neurological Surgery, Graduate School of Medicine, Chiba University, Chiba 260-8670, Japan
8Department of Neurological Surgery, Chiba Prefectural Sawara Hospital, Chiba 287-0003, Japan
9Department of Neurological Surgery, Chiba Cerebral and Cardiovascular Center, Chiba 290-0512, Japan
10Department of Cardiovascular Medicine, Graduate School of Medicine, Chiba University, Chiba 260-8670, Japan
11Department of Anesthesia, The First Affiliated Hospital, Jinan University, Guangzhou 510630, P. R. China
- *Corresponding Author:
- Takaki H
Department of Biochemistry and Genetics
Chiba University, Graduate School of Medicine, Japan
Tel: 81432262541
E-mail: hiwasa_takaki@faculty.chiba-u.jp
Received date: July 14, 2017; Accepted date: July 27, 2017; Published date: August 03, 2017
Citation: Kazuo S, Tomiyoshi G, Masahiro M, Satoshi K, Shigeki H, et al. (2017) Identification of Serum Anti-GADD34 Antibody as a Common Marker of Diabetes Mellitus and Parkinson Disease. J Alzheimers Dis Parkinsonism 7:358. doi:10.4172/2161-0460.1000358
Copyright: © 2017 Kazuo S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Background: Growth arrest and DNA-damage-inducible gene 34 (GADD34) has been identified as an antigen by serological identification of antigens by cDNA expression cloning (SEREX) using the sera of patients with atherosclerosis. It is possible that GADD34 is associated with atherosclerosis-related diseases such as diabetes mellitus (DM), acute-phase cerebral infarction (aCI), cardiovascular disease (CVD), and chronic kidney disease (CKD) as well as endoplasmic reticulum stress-related Parkinson disease (PD). Methods: GADD34 protein was bacterially expressed and purified. Amplified luminescent proximity homogeneous assay (AlphaLISA) was used to evaluate serum antibody levels against GADD34 protein in serum samples. Results: AlphaLISA revealed significantly higher serum antibody levels against GADD34 protein in patients with DM, aCI and CVD than those in healthy donors (HDs). The difference in levels between DM and HD was more prominent than that between aCI or CVD and HDs. The anti-GADD34 antibody levels were also elevated in the sera of diabetic CKD patients; thus, the anti-GADD34 antibodies appeared to be the most associated with DM, which is also a risk factor of PD. Anti-GADD34 antibody levels were also higher in patients with PD but not in those with amyotrophic lateral sclerosis as compared with those in HDs. Conclusion: Anti-GADD34 antibody may be a useful diagnostic tool for DM and PD. GADD34 may account for the pathophysiological relationship between DM and PD.
Keywords
GADD34; PPP1R15A; Parkinson disease; Diabetes mellitus; Serum antibody biomarker
Abbreviations
ABI: Ankle-Branchial Index; ACI: Acute- Phase Cerebral Infarction; ALB: Albumin; AlphaLISA: Amplified Luminescence Proximity Homogeneous Assay-Linked Immunosorbent Assay; ALS: Amyotrophic Lateral Sclerosis; AST: Aspartate Amino Transferase; ATF4: Activating Transcription Factor 4; Atg12: Autophagy 12; ATP2B4: ATPase Ca++ transporting plasma membrane 4; AUC: Area Under the ROC Curve; BMI: Body Mass Index; BMP- 1: Bone Morphogenetic Protein 1; CAVI: Cardio-Ankle Vascular Index; CHOP: C/EBP Homologous Protein; CI: Confidence Interval; CKD: Chronic Kidney Disease; CRE: Creatinine; CVD: Cardiovascular Disease; DHPS, Deoxyhypusine Synthase; DM: Diabetes Mellitus; ECSA: Esophageal Carcinoma SEREX Antigen; ER: Endoplasmic Reticulum; E. coli: Escherichia coli; GAD65: 65kDa form of Glutamic Acid Decarboxylase; GADD3: Growth Arrest and DNA-Damage- Inducible Gene 34; GST: Glutathione-S-Transferase; Hb: Hemoglobin; HbA1c: Glycated Hemoglobin; HD: Healthy Donor; HDL-C, High- Density Lipoprotein Cholesterol; Hsp: Heat Shock Protein; IA-2: Insulinoma Antigen 2; LDL-C: Low-Density Lipoprotein Cholesterol; LRRK2: Leucine-Rich Repeat Kinase 2; MAX IMT: Maximum Intima- Media Thickness; PD: Parkinson Disease; PERK: Protein Kinase RNA-like Endoplasmic Reticulum Kinase; PINK1: PTEN-Induced Putative Kinase 1; PRKN: Parkin RBR E3 Ubiquitin Protein Ligase; ROC: Receiver Operating Curve; RPA2: Replication Protein A2; SD: Standard Deviation; SEREX: Serological Identification of Antigens by Recombinant cDNA Expression Cloning; SH3BP5: SH3 Domain- Binding Protein 5; SH3GL1: SH3-Domain GRB2-like 1; s-GADD34- Abs: Serum Anti-GADD34 Antibodies; SNCA: α-Synuclein; SOSTDC1: Sclerostin Domain-Containing Protein 1; TACSTD2, Tumor- Associated Calcium Signal Transducer 2; TRIM21: Tripartite Motif- Containing 21; TUBB2C: Tubulin Beta-2C; ZnT8: Zinc Transporter 8.
Introduction
Parkinson disease (PD) is a common neurodegenerative disease with core pathological features including progressive dopaminergic neuron loss in the substantia nigra and the accumulation of intraplasmic α-synuclein (SNCA), which forms Lewy bodies [1]. Main motor symptoms of PD are tremor, slowness of movement, rigidity and poor balance, in addition to the non-motor symptoms represented by neuropsychiatric conditions (dementia, depression), sleep-related disorder), autonomic dysfunction (constipation, urinary disturbance, etc.) and sensory symptoms including reduced olfactory function [2]. The accurate diagnosis in early sage PD is still difficult, mainly due to the lack of disease-specific biomarkers. Therefore, fluid diagnostic biomarkers of sporadic PD is still of need.
Genetic and environmental factors are considered as a pivotal role in the pathogenesis of PD. There are two major protein degradation pathways in eukaryotic cells: the ubiquitin-proteasome system and the autophagy-lysosome pathway (ALP) [3]. Protein synthesis and degradation are maintained in in equilibrium, which preserves the intracellular environment. Lately, studying the causal relationship between neuronal autophagy and PD has become a topic of interest. Additionally, genetic mutations in PD including SNCA, LRRK2, PINK1 and PRKN are involved in the dysfunction in ALP system [4-6]. An autophagosome formation and altered degradation of intracellular proteins may be the major pathogenetic pathway of PD. On the other hand, biochemical analysis of the brains of patients with PD indicates that endoplasmic reticulum (ER) stress is also one of the main drivers of dopaminergic neuronal loss [7]. ER stress is normally involved in the steady state regulation of the protein quality control pathway, and is typically evoked when there is an increase in the false folding and aggregation of proteins [8]. In sum, dysfunction of protein quality control is underlying pathophysiological basis in PD [9].
In this study, we identified growth arrest and DNA-damageinducible gene 34 (GADD34; protein phosphatase 1, regulatory subunit 15A/PPP1R15A), as an antigen recognized by serum IgG in patients with ischemic stroke [10]. On the unfolded protein response, translation factor eIF2α is phosphorylated by activated PERK, and the phosphorylated eIF2α induces global repression of protein synthesis except transcription factor ATF4, which then transactivates autophagyinducing Atg12, apoptosis/autophagy-inducing CHOP and GADD34 [11]. Increased GADD34 can dephosphorylate eIF2α to resume protein synthesis including insulin [12,13]. Phosphorylated PERK and phosphorylated eIF2α were co-localized with SNCA in Lewy bodies of dopaminergic neurons of patients with PD [14]. GADD34 may have a suppressive role in the induction of autophagy. We examined anti-GADD34 antibody levels in patients with PD as well as with other atherosclerosis-related diseases such as diabetes mellitus (DM), acute-phase cerebral infarction (aCI), cardiovascular disease (CVD) and chronic kidney disease (CKD).
Materials and Methods
Patient and healthy donor (HD) sera
This study was approved by the Local Ethical Review Boards of the Chiba University Graduate School of Medicine (Chiba, Japan), the Kumamoto University Graduate School of Medicine (Kumamoto, Japan) and the Chiba Prefectural Sawara Hospital (Chiba, Japan). Sera were collected from patients who had provided written informed consent. Each serum sample was centrifuged at 3,000x g for 10 min and the supernatant was stored at −80°C until use. Repeated thawing and freezing of samples were avoided.
Serum samples from HDs and patients with DM, CVD, PD and amyotrophic lateral sclerosis (ALS) were obtained from Chiba University Hospital. Samples from patients with aCI were obtained from Chiba Prefectural Sawara Hospital. Samples from patients with CKD were from the Kumamoto cohort [15,16].
Expression and purification of GADD34 protein
Glutathione S-transferase (GST)-tagged GADD34 protein and control GST protein were expressed in Escherichia coli (E. coli) and were purified by glutathione-Sepharose (GE Healthcare Life Sciencess, Pittsburgh, PA) column chromatography as described previously [10,17,18].
Amplified luminescence proximity homogeneous assay (AlphaLISA)
AlphaLISA was performed in 384-well microtiter plates (white opaque OptiPlate™, PerkinElmer, Waltham, MA) containing either 2.5 μL of 1:100-diluted serum with 2.5 μL of GST or GST-GADD34 protein (10 μg/mL) in AlphaLISA buffer (25 mM HEPES, pH 7.4, 0.1% casein, 0.5% Triton X-100, 1 mg/mL dextran-500, and 0.05% Proclin-300). The reaction mixture was incubated at room temperature for 7-10 h. Anti-human IgG-conjugated acceptor beads (2.5 μL at 40 μg/mL) and glutathione-conjugated donor beads (2.5 μL at 40 μg/mL) were then added and incubated at room temperature in the dark for 7 days. Chemical emissions were read on an EnSpire Alpha microplate reader (PerkinElmer), as previously described [19-24]. Specific reactions were calculated by subtracting the Alpha counts of the GST control from the counts of GST-GADD34 fusion proteins.
Statistical Analyses
The Mann-Whitney U test and student’s t test were used to determine the significance of differences between the two groups. Correlations were calculated using Spearman’s rank-order correlation analysis. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). The predictive values of putative disease markers were assessed via receiver operating characteristic (ROC) curve analysis, and cutoff values were set to maximize the sums of sensitivity and specificity. All the tests were two-tailed and P values of <0.05 were considered statistically significant.
Results
Elevation of serum antibody levels against GADD34 proteins in patients with DM
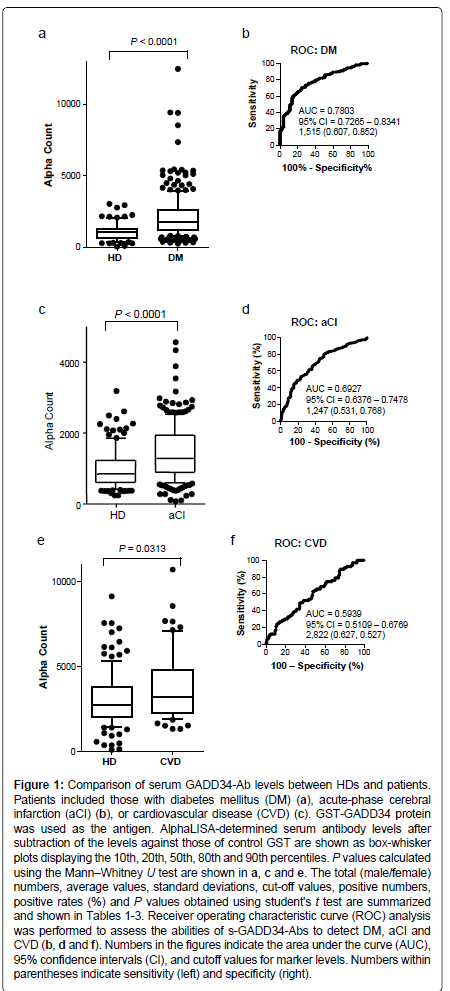
We examined the levels of antibodies against GADD34 protein in the sera of HDs and patients with DM or CVD, using the highly sensitive and stable AlphaLISA method [19-24]. Levels of serum antibodies against GADD34 protein (s-GADD34-Abs) were significantly higher in samples from patients with DM than in those from HDs (P<0.0001; Mann-Whitney U test) (Figure 1). At a cutoff value equivalent to the average plus two SDs of the HD specimen values, the s-GADD34-Ab positive rates in HDs and patients with DM were 3.7% and 32.0%, respectively (Table 1). ROC analysis was performed to evaluate the abilities of these antibody markers to detect DM. The area under the ROC curve (AUC) for s-GADD34-Abs was 0.7803 (95% confidence interval (CI): 0.7265-0.8341), yielding sensitivity and specificity values of 60.7% and 85.2%, respectively, for the diagnosis of DM.
| Sample information | HD | DM | |
|---|---|---|---|
| Total sample number | 81 | 275 | |
| Male/Female | 46/35 | 158/117 | |
| Type 1 DM/Type 2 DM | 0/0 | 26/216 | |
| Age (Average ± SD) | 45.20 ± 10.95 | 63.12 ± 12.04 | |
| Alpha analysis (antibody level) | GADD34-Ab | ||
| HD | Average | 1,077 | |
| SD | 616 | ||
| Cutoff value | 2,308 | ||
| Positive number | 3 | ||
| Positive rate (%) | 3.7% | ||
| DM | Average | 2,200 | |
| SD | 1,855 | ||
| Positive number | 88 | ||
| Positive rate (%) | 32.0% | ||
| P value (vs HD) | 3.47E-16 | ||
Table 1: Comparison of the serum anti-GADD34 antibody levels between healthy donors (HD) and patients with diabetes mellitus (DM). The upper panel includes sample information, such as total sample numbers, numbers of male and female specimens, numbers of type 1 and type 2 DM and average age ± standard deviation (SD) of the subjects. The lower panel summarizes the summary of serum antibody levels examined by AlphaLISA. Purified GST-GADD34 and control GST proteins were used as antigens. The average and SD antibody levels (Alpha counts), cut-off values, positive numbers, and positive rates (%) are shown in the table. Cutoff values were determined as the average HD values plus two SDs. Positive samples for which the Alpha counts exceeded the cutoff value were scored. P values versus HD were calculated using the students' t test. P values <0.05 and positive rates >10% are marked in bold. Box-whisker plots of the same results are shown in Figure 1a.
Figure 1: Comparison of serum GADD34-Ab levels between HDs and patients. Patients included those with diabetes mellitus (DM) (a), acute-phase cerebral infarction (aCI) (b), or cardiovascular disease (CVD) (c). GST-GADD34 protein was used as the antigen. AlphaLISA-determined serum antibody levels after subtraction of the levels against those of control GST are shown as box-whisker plots displaying the 10th, 20th, 50th, 80th and 90th percentiles. P values calculated using the MannâÂ?Â?Whitney U test are shown in a, c and e. The total (male/female) numbers, average values, standard deviations, cut-off values, positive numbers, positive rates (%) and P values obtained using student's t test are summarized and shown in Tables 1-3. Receiver operating characteristic curve (ROC) analysis was performed to assess the abilities of s-GADD34-Abs to detect DM, aCI and CVD (b, d and f). Numbers in the figures indicate the area under the curve (AUC), 95% confidence intervals (CI), and cutoff values for marker levels. Numbers within parentheses indicate sensitivity (left) and specificity (right).
Elevation of serum antibody levels against GADD34 proteins in patients with aCI and CVD
Levels of s-GADD34-Abs were also significantly higher in patients with aCI or CVD than in HDs but they were less prominent. s-GADD34-Ab positivity rates in patients with aCI or CVD were 18.0% and 10.4%, respectively (Tables 2 and 3). ROC analysis revealed that AUCs of s-GADD34-Abs were 0.6927 (95% CI: 0.6376-0.7478) for aCI and 0.5939 (95% CI: 0.5109-0.6769) for CVD, which were lower than that for DM. The sensitivity and specificity were 53.1% and 76.8%, respectively, for aCI and 62.7% and 52.7%, respectively, for CVD. The P value of s-GADD34-Ab levels of patients with aCI or CVD versus HDs calculated using the Mann-Whitney U test or the student’s t test (Tables 2 and 3), were higher than those of patients with DM versus HDs.
| Sample information | HD | aCI | |
|---|---|---|---|
| Total sample number | 138 | 228 | |
| Male/Female | 87/51 | 129/99 | |
| Age (Average ± SD) | 51.63 ± 12.70 | 77.04 ± 11.08 | |
| Alpha analysis (antibody level) | GADD34-Ab | ||
| HD | Average | 992 | |
| SD | 552 | ||
| Cut-off value | 2,095 | ||
| Positive number | 9 | ||
| Positive rate (%) | 6.5% | ||
| aCI | Average | 1,444 | |
| SD | 764 | ||
| Positive number | 41 | ||
| Positive rate (%) | 18.0% | ||
| P value (vs. HD) | 1.96E-10 | ||
Table 2: Comparison of serum antibody levels against GADD34 between HDs and patients with acute-phase cerebral infarction (aCI) examined by AlphaLISA. The antigens used were purified GST-GADD34 and control GST proteins. Numbers are as described in Table 1. Box-whisker plots of the same results are shown in Figure 1c.
| Sample information | HD | CVD | |
|---|---|---|---|
| Total sample number | 128 | 67 | |
| Male/Female | 35/23 | 22/12 | |
| Age (Average ± SD) | 43.10 ± 12.93 | 62.18 ± 12.80 | |
| Alpha analysis (antibody level) | GADD34-Ab | ||
| HD | Average | 3,045 | |
| SD | 1,609 | ||
| Cutoff value | 6,263 | ||
| Positive number | 6 | ||
| Positive rate (%) | 4.7% | ||
| CVD | Average | 3,733 | |
| SD | 1,937 | ||
| Positive number | 7 | ||
| Positive rate (%) | 10.4% | ||
| P (vs HD) | 1.42E-02 | ||
Table 3: Comparison of serum antibody levels against GADD34 between HDs and patients with cardiovascular disease (CVD) examined by AlphaLISA. The antigens used were purified GST-GADD34 and control GST proteins. Numbers are as described in Table 1. Box-whisker plots of the same results are shown in Figure 1e.
| Sample information | HD | Type 1-CKD | Type 2-CKD | Type 3-CKD | |
|---|---|---|---|---|---|
| Total sample number | 82 | 145 | 32 | 123 | |
| Male/Female | 44/38 | 106/39 | 21/11 | 70/53 | |
| Age (Average ± SD) | 44.10 ± 11.19 | 66.04 ± 10.38 | 76.03 ± 9.78 | 61.98 ± 11.69 | |
| Alpha analysis (antibody level) | GADD34-Ab | ||||
| HD | Average | 923 | |||
| SD | 723 | ||||
| Cutoff value | 2,369 | ||||
| Positive number | 6 | ||||
| Positive rate (%) | 7.3% | ||||
| Type 1-CKD | Average | 2,006 | |||
| SD | 2,085 | ||||
| Positive number | 40 | ||||
| Positive rate (%) | 27.6% | ||||
| P (vs. HD) | 4.85E-08 | ||||
| Type 2-CKD | Average | 1,635 | |||
| SD | 850 | ||||
| Positive number | 6 | ||||
| Positive rate (%) | 18.8% | ||||
| P (vs. HD) | 1.17E-04 | ||||
| Type 3-CKD | Average | 1,438 | |||
| SD | 1,158 | ||||
| Positive number | 20 | ||||
| Positive rate (%) | 16.3% | ||||
| P (vs. HD) | 1.21E-04 | ||||
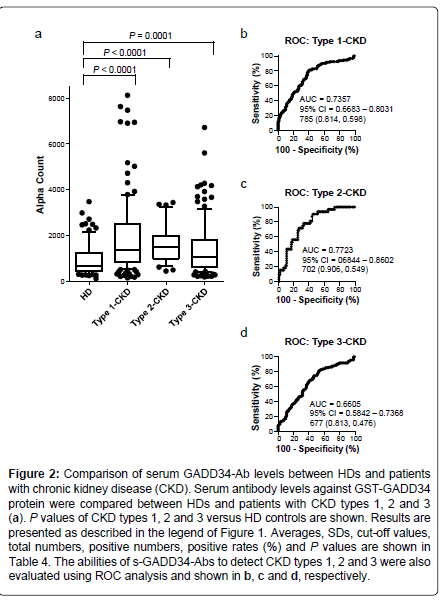
Table 4: Comparison of serum antibody levels against GADD34 between HDs and patients with chronic kidney disease (CKD) examined by AlphaLISA. CKD types -1, -2 and -3 are diabetic kidney disease, nephrosclerosis, and glomerulonephritis, respectively. The antigens used were purified GST-GADD34 and control GST proteins. Shown numbers are as described in Table 1. Box-whisker plots of the same results are shown in Figure 2a.
Elevation of levels of s-GADD34-Abs in patients with CKD
We then examined antibody levels in the sera of patients with CKD, which is also closely related to atherosclerosis. CKD was divided into three groups as follows: type 1, diabetic kidney disease; type 2, nephrosclerosis; and type 3, glomerulonephritis. Patients from all three groups of CKD had significantly higher serum levels of s-GADD34-Abs than the HDs (Figure 2). The s-GADD34-Ab positivity rates in HDs and patients with CKD type 1, type 2 and type 3 were 7.3%, 27.6%, 18.8% and 16.3% (Table 4). P value of CKD type 1 versus HD was much lower than those of CKD types 2 and 3. ROC analysis revealed that AUCs of s-GADD34-Abs were 0.7357 (95% CI: 0.6683-0.8031) for CKD type 1, 0.7723 (95% CI: 0.6844-0.8602) for CKD type 2 and 0.6605 (95% CI: 0.5842-0.7368) for CKD type 3. The relatively high AUC values of CKD type 2 may have been affected by low sample numbers (n=32).
Figure 2: Comparison of serum GADD34-Ab levels between HDs and patients with chronic kidney disease (CKD). Serum antibody levels against GST-GADD34 protein were compared between HDs and patients with CKD types 1, 2 and 3 (a). P values of CKD types 1, 2 and 3 versus HD controls are shown. Results are presented as described in the legend of Figure 1 . Averages, SDs, cut-off values, total numbers, positive numbers, positive rates (%) and P values are shown in Table 4. The abilities of s-GADD34-Abs to detect CKD types 1, 2 and 3 were also evaluated using ROC analysis and shown in b, c and d, respectively.
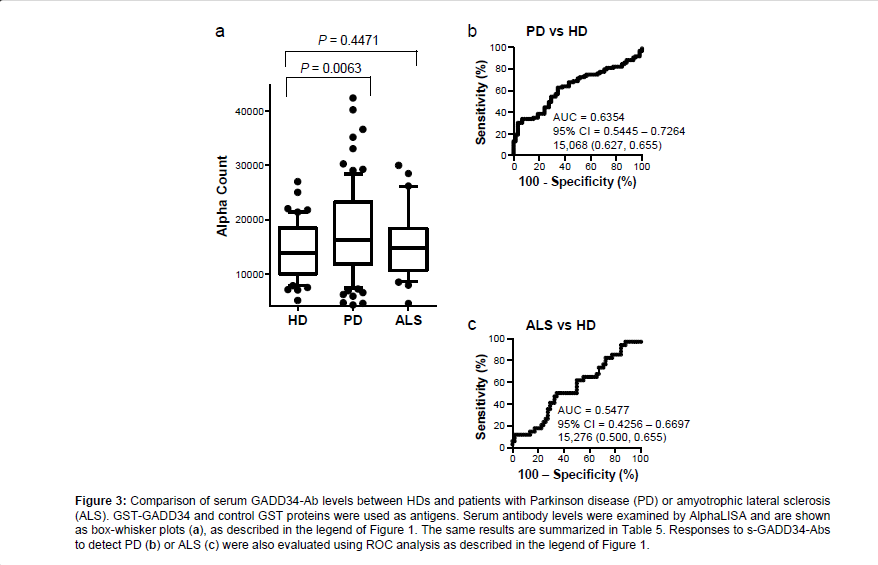
Elevation of levels of s-GADD34-Abs in patients with PD
We then examined the levels of s-GADD34-Abs in the sera of patients with PD or ALS. All serum specimens of HDs and patients with PD or ALS were obtained from Chiba University Hospital. The AlphaLISA results demonstrated that levels of s-GADD34-Ab were significantly higher in patients with PD than in HDs, but not in patients with ALS compared with the HDs (Figure 3). Using the cutoff values described in the previous section, s-GADD34-Ab positivity rates in HDs, patients with PD and those with ALS were 3.4%, 20.5%, and 11.8% (Table 5). ROC analysis revealed that the AUCs of s-GADD34- Abs were 0.6354 (95% CI: 0.5445-0.7264) for PD and 0.5477 (95% CI: 0.4256-0.6697) for ALS. Serum GADD34 antibodies were found to be useful as a diagnostic marker of PD, but not of ALS.
| Sample information | HD | PD | ALS | |
|---|---|---|---|---|
| Total sample number | 58 | 83 | 34 | |
| Male/Female | 35/23 | 37/46 | 22/12 | |
| Age (Average ± SD) | 43.10 ± 12.93 | 67.40 ± 11.40 | 62.18 ± 12.80 | |
| Alpha analysis (antibody level) | GADD34-Ab | |||
| HD | Average | 14,245 | ||
| SD | 5,058 | |||
| Cutoff value | 24,361 | |||
| Positive number | 2 | |||
| Positive rate (%) | 3.4% | |||
| PD | Average | 17,909 | ||
| SD | 8,334 | |||
| Positive number | 17 | |||
| Positive rate (%) | 20.5% | |||
| P (vs. HD) | 0.0015 | |||
| ALS | Average | 15,374 | ||
| SD | 5,959 | |||
| Positive number | 4 | |||
| Positive rate (%) | 11.8% | |||
| P (vs. HD) | 0.358 | |||
Table 5: Comparison of serum anti-GADD34 antibody levels between HDs and patients with Parkinson disease (PD) or amyotrophic lateral sclerosis (ALS) examined by AlphaLISA. Numbers are as described in Table 1. Box-whisker plots of the same results are shown in Figure 3a.
Figure 3: Comparison of serum GADD34-Ab levels between HDs and patients with Parkinson disease (PD) or amyotrophic lateral sclerosis (ALS). GST-GADD34 and control GST proteins were used as antigens. Serum antibody levels were examined by AlphaLISA and are shown as box-whisker plots (a), as described in the legend of Figure 1. The same results are summarized in Table 5. Responses to s-GADD34-Abs to detect PD (b) or ALS (c) were also evaluated using ROC analysis as described in the legend of Figure 1.
Correlation analysis
Spearman’s correlation analysis was performed to determine the correlation between s-GADD34-Ab levels and subject parameters, which included general information, such as age, body height, body weight and body mass index (BMI); degree of artery stenosis, including the maximum intima-media thickness (max IMT), plaque score, cardio-ankle vascular index (CAVI, right and left) and anklebranchial index (ABI, right and left); and smoking habit duration (years). The following blood test data were also included; low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol, alanine aminotransferase, aspartate aminotransferase (AST), alkaline phosphatase, lactate dehydrogenase, γ-glutamyl transpeptidase, total protein, albumin (ALB), creatinine (CRE), hemoglobin A1c (HbA1c), triglyceride, blood urea nitrogen, total bilirubin, ferritin, iron, sodium, chlorine, potassium, calcium, phosphate, magnesium, hemoglobin (Hb), hematocrit, red blood cell count, white blood cell count and platelet cell count [19-21,23,24]. The s-GADD34-Ab level was found to significantly correlate with artery stenosis including max IMT, plaque score, and CAVI (right and left), but not with ABI, suggesting that s-GADD34-Ab distinguishes atherosclerosis in upper body (Table 6). The results also indicated a significant association of antibody levels with age, AST and ferritin but they indicated an inverse correlation with HDL-C, ALB, CRE, Hb and platelet number.
| Spearman | ||||
|---|---|---|---|---|
| Subjects' information | Abbreviation | r value | P value | |
| General | Age | 0.179 | 0.0019 | |
| Body height | Height | -0.053 | 0.3653 | |
| Body weight | Weight | -0.088 | 0.1302 | |
| Body mass index | BMI | -0.070 | 0.2308 | |
| Artery stenosis | Maximum intima-media thickness | max IMT | 0.151 | 0.0091 |
| Plaque Score | 0.193 | 0.0009 | ||
| Cardio ankle vascular index (right) | CAVI (R) | 0.221 | 0.0002 | |
| Cardio ankle vascular index (left) | CAVI (L) | 0.178 | 0.0027 | |
| Ankle-branchial index (right) | ABI (R) | -0.060 | 0.3040 | |
| Ankle-branchial index (left) | ABI (L) | -0.104 | 0.0759 | |
| Life style | Smoking habit period | 0.054 | 0.3573 | |
| Blood test | LDL-cholesterol | LDL-C | -0.037 | 0.5193 |
| HDL-cholesterol | HDL-C | -0.132 | 0.0222 | |
| Total cholesterol | T-CHO | -0.076 | 0.1906 | |
| Alanine aminotransferase | ALT (GPT) | 0.057 | 0.3240 | |
| Aspartate aminotransferase | AST (GOT) | 0.216 | 0.0002 | |
| Alkaline phosphatase | ALP | 0.075 | 0.1970 | |
| Lactate dehydrogenase | LDH | 0.083 | 0.1501 | |
| Gamma-glutamyl transpeptidase | g-GTP | 0.030 | 0.6004 | |
| Total Protein | TP | -0.047 | 0.4165 | |
| Albumin | ALB | -0.189 | 0.0010 | |
| Creatinin | CRE | -0.173 | 0.0027 | |
| Hemoglobin A1c | HbA1c | -0.145 | 0.0795 | |
| Triglyceride | TG | -0.020 | 0.7238 | |
| Blood urea nitrogen | BUN | -0.100 | 0.0851 | |
| Total bilirubin | tBil | 0.071 | 0.2179 | |
| Ferritin | 0.205 | 0.0003 | ||
| Iron | Fe | -0.110 | 0.0580 | |
| Sodium | Na | -0.005 | 0.9317 | |
| Chlorine | Cl | 0.014 | 0.8061 | |
| Potassium | K | -0.051 | 0.3775 | |
| Calcium | Ca | -0.106 | 0.0663 | |
| Phosphate | P | 0.038 | 0.5091 | |
| Magnesium | Mg | 0.016 | 0.7786 | |
| Hemoglobin | Hb | -0.142 | 0.0138 | |
| Hamatocrit | Hc | -0.104 | 0.0714 | |
| Red blood cell number | RBC | -0.101 | 0.0811 | |
| White blood cell number | WBC | -0.088 | 0.1278 | |
| Platelet number | PLT | -0.241 | <0.0001 |
Table 6: Correlation analysis of s-GADD34-Ab levels with data on subjects in the Kumamoto cohort. Correlation coefficients (r values) and P values were obtained through Spearman's rank-order correlation analysis, as shown. Widely used abbreviations are also shown. Significant correlations (P<0.05) are marked in bold text.
Discussion
Currently, an increasing number of humoral antibody markers for diseases have been reported, including oxidized low-density lipoprotein and heat shock protein (Hsps) in CVD, Hsp60 in stroke, insulin, GAD65, IA-2 and ZnT8 in type-1 DM and p53 in cancer [25- 33]. Such antibody markers are useful not only as diagnostic tools but also as probes to investigate pathomechanisms, as exemplified by insulin, GAD65, IA-2 and AcT8 in type-1 DM [34]. We also identified the following autoantibody-recognized antigens by recombinant cDNA expression cloning (SEREX) method or protein array method: TACSTD2, TRIM21, makorin1 and ECSA in esophageal squamous cell carcinoma, SH3GL1 and filamin C in low-grade glioma, talin-1 in multiple sclerosis, RPA2 and SOSTDC1 in ischemic stroke, TUBB2C and adiponectin in DM, and ATP2B4, BMP-1, DHPS, and SH3BP5 in arteriosclerosis-related diseases [10,17-39]. The most prominent features of antibody markers are the large variation of the antibody levels induced by repeated exposure of small amounts of antigens and an easy measurement of stable immunoglobulins.
GADD34 was identified as an antigen recognized by IgG antibody in the sera of patients with ischemic stroke [10]. Further analysis of s-GADD34-Ab levels in DM, aCI, CVD and CKD, which are atherosclerosis-related diseases in this study, revealed a particularly large difference between HDs and patients with DM or diabetic CKD (Figures 1 and 2). Further Spearman’s rank-order correlation analysis revealed that s-GADD34-Ab levels were correlated with CAVI and plaque score, which reflect the progression of atherosclerosis (Table 6). This is consistent with our finding that s-GADD34-Ab values in type-2 CKD/nephrosclerosis showed similarly high AUC values as type-1 diabetic CKD (Figures 1b, 2b and 2c). While our analysis was limited due to the small sample size of type-2 CKD, it is plausible that the major cause of the development of s-GADD34-Abs may be DM, which frequently induces atherosclerosis leading to the onset of aCI and CVD.
There is epidemiologic evidence to suggest the relationship between DM and PD. Hu et al. at the National Institute of Public Health in Finland followed a total of 51,000 men and women aged 25-74 years who had no medical history of PD for 18 years [40]. As a result, the risk of PD onset in patients with type-2 DM was 83% higher than that in those without DM. Additional factors such as BMI, alcohol use, coffee and tea consumption, smoking and physical activity did not alter this risk. Sun et al. examined a study group in Taiwan composed of 600,314 diabetic patients and 472,188 non-diabetic patients and evaluated the risk of developing PD for 9 years [41]. The number of PD onset per 10,000 people was 3.59 in the DM group per year and 2.15 in the non-DM group, representing a covariate adjusted hazard ratio of 1.61. This ratio was reducing to 1.37 after treatment; therefore, the study team concluded that DM is associated with an increased risk of PD onset. Brauer et al. performed a retrospective cohort study in which patients with DM were followed from 1999 to 2013, and found that PD incidence was reduced by 28% in 44,597 anti-diabetic glitazoneexposed individuals [42]. This result was compared to a matched 120,373 other anti-diabetic medication users, which resulted in the finding that DM may have a causal role in developing PD.
It has been well documented that ER stress followed by autophagy was frequently observed in neurodegenerative diseases including PD, Alzheimer’s disease, and ALS. During ER stress, the unfolded protein response activates PERK, which then phosphorylates eIF2α. The phosphorylated eIF2α induces the transient shutdown of protein translation, but allows the translation of ATF4, which then induces gene expression of CHOP, GADD34 and pro-autophagic genes [11]. Consistently, the expression of GADD34 was increased in oligodendrocytes in Alzheimer disease [43]. Persistent shutoff of protein synthesis results in autophagic cell death, yet induced GADD34 may have an anti-autophagic role by dephosphorylating eIF2α to resume normal protein synthesis [44]. GADD34-/- mouse embryonic fibroblasts show delayed recovery from the shutoff of protein synthesis by ER stresses [45]. Mice defective in the elF2α phosphorylation develop DM, and active eIF2α is indispensable for insulin synthesis after ER stress [13,46]. Therefore, GADD34 and its substrate eIF2α may account for the common pathway in ER stress leading to the development of DM and PD. The development of autoantibodies against GADD34 in DM and PD may be the result of overexpression of GADD34 protein induced by ER stress. Alternatively, if GADD34 proteins can be adsorbed or disturbed by their autoantibodies, such autoantibodies may have causal effects on the development of DM and PD. Further analysis is required to reveal the precise mechanism.
Conclusion
We believe that the serum anti-GADD34 antibody is a common marker for DM and PD and that GADD34 may account for the relationship between DM and PD.
Acknowledgement
This work was supported, in part, by research grants from the Japan Agency for Medical Research and Development (AMED) (Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus), Japan Science and Technology Agency (JST) and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan.
References
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, et al. (2017) Parkinson disease. Nat Rev Dis Primers 3: 17013.
- Nat Rev Dis Primers
- Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson's disease. J Neurochem 139: 318-324
- Yorimitsu T, Klionsky DJ (2005) Autophagy: Molecular machinery for self-eating. Cell Death Differ 12: 1542-1552
- Chan SL, Tan EK (2017) Targeting LRRK2 in Parkinson's disease: An update on recent developments. Expert Opin Ther Targets 21: 601-610
- Xilouri M, Stefanis L (2011) Autophagic pathways in Parkinson disease and related disorders. Expert Rev Mol Med 13: e8
- Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C (2010) Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7 and LRRK2 genes: A mutation update. Hum Mutat 31: 763-780
- Mercado G, Castillo V, Soto P, Sidhu A (2016) ER stress and Parkinson's disease: Pathological inputs that converge into the secretory pathway. Brain Res 1648: 626-632
- Walden H, Muqit MM (2017) Ubiquitin and Parkinson's disease through the looking glass of genetics. Biochem J 474: 1439-1451
- Kinghorn KJ, Asghari AM, Castillo-Quan JI (2017) The emerging role of autophagic-lysosomal dysfunction in Gaucher disease and Parkinson's disease. Neural Regen Res 12: 380-384
- Machida T, Kubota M, Kobayashi E, Iwadate Y, Saeki N, et al. (2015) Identification of stroke-associated-antigens via screening of recombinant proteins from the human expression cDNA library (SEREX). J Translat Med 13: 71
- Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta 1833: 3460-3470
- Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2a. J Cell Biol 153: 1011-1021
- Akai R, Hosoda A, Yoshino M, Iwawaki T. (2015) Constitutive role of GADD34 and CReP in cancellation of phospho-eIF2a-dependent translational attenuation and insulin biosynthesis in pancreatic ÃÂ? cells. Genes Cells 20: 871-886
- Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, et al. (2008) Serine 129 phosphorylation of a-synuclein induces unfolded protein response-mediated cell death. J Biol Chem 283: 23179-23188
- Nishiura R, Fujimoto S, Sato Y, Yamada K, Hisanaga S, et al. (2009) Elevated osteoprotegerin levels predict cardiovascular events in new hemodialysis patients. Am J Nephrol 29: 257-263
- Komatsu H, Fujimoto S, Hara S, Fukuda A, Fukudome K, et al. (2009) Recent therapeutic strategies improve renal outcome in patients with IgA nephropathy. Am J Nephrol 30: 19-25
- Nakashima K, Shimada H, Ochiai T, Kuboshima M, Kuroiwa N, et al. (2004) Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer 112: 1029-1035
- Kagaya A, Shimada H, Shiratori T, Kuboshima M, Nakashima-Fujita K, et al. (2011) Identification of a novel SEREX antigen family, ECSA, in esophageal squamous cell carcinoma. Proteome Sci 9: Article ID: 31
- Goto K, Sugiyama T, Matsumura R, Zhang XM, Kimura R, et al. (2015) Identification of cerebral infarction-specific antibody markers from autoantibodies detected in patients with systemic lupus erythematosus. J Mol Biomark Diagnos 6: 2
- Hiwasa T, Machida T, Zhang XM, Kimura R, Wang H, et al. (2015) Elevated levels of autoantibodies against ATP2B4 and BMP-1 in sera of patients with atherosclerosis-related diseases. Immunome Res 11: 097
- Hiwasa T, Zhan XM, Kimura R, Machida T, Kitamura K, et al. (2015) Association of serum antibody levels against TUBB2C with diabetes and cerebral infarction. Integ Biomed Sci 1: 49-63
- Hiwasa T, Zhang XM, Kimura R, Ohno M, Chen PM, et al. (2016) Elevated adiponectin antibody levels in sera of patients with atherosclerosis-related coronary artery disease, cerebral infarction and diabetes mellitus. J Circ Biomark 5: 8
- Nakamura R, Tomiyoshi G, Shinmen N, Kuroda H, Kudo T, et al. (2017) An anti-deoxyhypusine synthase antibody as a marker of atherosclerosis-related cerebral infarction, myocardial infarction, diabetes mellitus and chronic kidney disease. SM Atheroscler J 1: 1001
- Hiwasa T, Tomiyoshi G, Nakamura R, Shinmen N, Kuroda H, et al. (2017) Serum SH3BP5-specific antibody level is a biomarker of atherosclerosis. Immunome Res 13: 2
- Satta N, Vuilleumier N (2015) Auto-antibodies as possible markers and mediators of ischemic, dilated and rhythmic cardiopathies. Curr Drug Targets 16: 342-360
- Fesmire J, Wolfson-Reichlin M, Reichlin M (2010) Effects of autoimmune antibodies anti-lipoprotein lipase, anti-low density lipoprotein and anti-oxidized low density lipoprotein on lipid metabolism and atherosclerosis in systemic lupus erythematosus. Rev Bras Reumatol 50: 539-551
- Kramer J, Harcos P, Prohászka Z, Horváth L, Karádi I, et al. (2000) Frequencies of certain complement protein alleles and serum levels of anti-heat-shock protein antibodies in cerebrovascular diseases. Stroke 31: 2648-2652.
- Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F (2013) Evidence on the pathogenic role of auto-antibodies in acute cardiovascular diseases. Thromb Haemost 109: 854-868
- Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, et al. (1983) Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222: 1337-1339
- Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E (1995) Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K auto-antigen and a target of islet-cell antibodies. J Immunol 155: 5419-5426.
- Baekkeskov S, Aanstoot H, Christgau S, Reetz A, Solimena MS, et al. (1990) Identification of the 64K auto-antigen in insulin dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347: 151-156
- Shimada H, Yajima S, Oshima Y, Hiwasa T, Tagawa M, et al. (2012) Impact of serum biomarkers on esophageal squamous cell carcinoma. Esophagus 9: 131-140.
- Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, et al. (2006) In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119: 4199-4206
- Wenzlau JM, Hutton JC (2013) Novel diabetes autoantibodies and prediction of type 1 diabetes. Curr Diab Rep 13: 5
- Kuboshima M, Shimada H, Liu TL, Nomura F, Takiguchi M, et al. (2006) Presence of serum tripartite motif-containing 21 antibodies in patients with esophageal squamous cell carcinoma. Cancer Sci 97: 380-386
- Shimada H, Shiratori T, Yasuraoka M, Kagaya A, Kuboshima M, et al. (2009) Identification of Makorin 1 as a novel SEREX antigen of esophageal squamous cell carcinoma. BMC Cancer 9: 232
- Matsutani T, Hiwasa T, Takiguchi M, Oide T, Kunimatsu M, et al. (2012) Autologous antibody to src-homology 3-domain GRB2-like 1 specifically increases in the sera of patients with low-grade gliomas. J Exp Clin Cancer Res 31: 85
- Adachi-Hayama M, Adachi A, Shinozaki N, Matsutani T, Hiwasa T, et al. (2014) Circulating anti-filamin C antibody as a potential serum biomarker for low-grade gliomas. BMC Cancer 14: 452
- Muto M, Mori M, Hiwasa T, Takiguchi M, Iwadate Y, et al. (2015) Novel serum autoantibodies against talin1 in multiple sclerosis: Possible pathogenetic roles of the antibodies. J Neuroimmunol 284: 30-36
- Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J (2007) Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 30: 842-847
- Sun Y, Chang YH, Chen HF, Su YH, Su HF, et ak, (2012) Risk of Parkinson disease onset in patients with diabetes: 9 year population-based cohort study with age and sex stratifications. Diabetes Care 35: 1047-1049
- Brauer R, Bhaskaran K, Chaturvedi N, Dexter DT, Smeeth L, et al. (2015) Glitazone treatment and incidence of Parkinson's disease among people with diabetes: A retrospective cohort study. PLoS Med 12: e1001854
- Honjo Y, Ayaki T, Tomiyama T, Horibe T, Ito H, et al. (2015) Increased GADD34 in oligodendrocytes in Alzheimer's disease. Neurosci Lett 602: 50-55
- Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, et al. (2009) Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2a) dephosphorylation in mammalian development. Proc Natl Acad Sci USA 106: 1832-1837
- Kojima E, Takeuchi A, Haneda M, Yagi A, Hasegawa T, et al. (2003) The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J 17: 1573-1575
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 3818
- [From(publication date):
August-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 2955
- PDF downloads : 863