Research Article Open Access
Identification of Preanalytical Errors in the Clinical Laboratory of North Indian Tertiary Care Hospital
| Monika Gupta1*, Dharamveer Yadav2, Sandhya Mishra2 and Praveen Sharma3 | |
| 1Department of Biochemistry, Dr. S. N. Medical College, Jodhpur, India | |
| 2Department of Biochemistry, SMS Medical College, Jaipur, India | |
| 3Department of Biochemistry, All India Institute of Medical Sciences, Jodhpur, India | |
| Corresponding Author : | Monika Gupta Department of Biochemistry Dr. S. N. Medical College, Jodhpur, India Tel: +91-9413841965 E-mail: maturemona@gmail.com |
| Received: August 19, 2015 Accepted: September 08, 2015 Published: September 15, 2015 | |
| Citation: Gupta M, Yadav D, Mishra S, Sharma P (2015) Identification of Preanalytical Errors in the Clinical Laboratory of North Indian Tertiary Care Hospital. Biochem Physiol 4:181. doi: 10.4172/2168-9652.1000181 | |
| Copyright: © 2015 Gupta M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Introduction: In an era where mechanization of laboratory automation has drastically reduced the errors due to the analytical phase of testing, errors due to the preanalytical phase are largely responsible for the decrease in quality of clinical laboratory results.
Materials and methods: The present study was conducted at one of the tertiary care hospital at State Capital to find out the incidence of preanalytical errors between inpatients and outpatients samples collected into vacuated (Serum and EDTA) tubes and without evacuated (Serum and EDTA) tubes.
Results: The frequency of total errors observed was 25.80% and 27.96% for inpatients samples collected into BD Vacutainer® tubes and without evacuated tubes respectively while for outpatients it was 20.75% and 29.27%. The total errors (30.24%) irrespective of the blood container used were 34.92% and 25.55% for inpatients and outpatients, respectively.
Conclusions: Preanalytical variables can produce unpredictable and unfavorable impacts on the wellbeing of patients because of preanalytical variables which could affect more than 30% of laboratory results.
| Keywords |
| Preanalytical variables; Vacuated tubes; Without evacuated tubes; Inpatients; Outpatients |
| Introduction |
| As laboratory results play a key role in the diagnostic procedure, a high validity of the laboratory result is an important precondition for the efficacy in clinical medicine. Clinical laboratories have witnessed major changes due to technological progress and economic demands and further remarkable advances in instrument technology, automation and computer science have greatly simplified many aspects of previously tedious tasks in laboratory diagnostics, creating a greater volume of routine work, and significantly improving the quality of results of laboratory testing [1]. Following the development and successful implementation of high-quality analytical standards, analytical errors are no longer the main factor influencing the reliability and clinical utilization of laboratory diagnostics. Errors occurring within the extraanalytical phases are still the prevailing source of concern [2]. |
| The process of laboratory medicine is typically divided into three main phases (pre-analytical, analytical and post-analytical), with each of them variably affected by uncertainties and errors [3]. Thus, the wide range of variables affecting a clinical laboratory result in the “pre-analytic phase” (the period prior to the actual analysis of a specimen) are grouped together as the “pre-analytic variables” and may be broadly categorized into: (i) Patient/individual related and (ii) Processing-related variables [4]. |
| Under the broad umbrella of the preanalytical phase specimen collection, handling and processing variables, physiological variables such as the effect of lifestyle, age, gender, pregnancy and menstruation and endogenous variables such as drugs and circulating antibodies can be included [5]. Some of the preanalytical variables such as specimen variables can be controlled, while acknowledge of uncontrollable variables need to be well understood in order to be able to separate their effects from disease related changes affecting laboratory results [6]. |
| There has been increasing recognition that the situation is less favorable in the preanalytical phase of the testing process such that there is now general acceptance of the need to focus on improvements in this area. Because up to 60% of the testing process is centered on the preanalytical phase and preanalytical errors have been reported to account for more than two thirds of all laboratory errors [7]. Most preanalytical errors result from system flaws and insufficient audit with operators involved in specimen collection/handling responsibilities [8]. |
| Since preanalytical variability exerts a strong influence on laboratory organization, healthcare expenditures and patient outcome, governance of this crucial phase of the total testing process by the reduction of uncertainty offers the greatest potential for improving total quality and enhances stakeholders’ satisfaction [9]. |
| In view of the significance of preanalytical variables, the present study has been conducted to find out the incidence of preanalytical errors in inpatients and outpatients samples collected either in BD Vacutainer® (Serum and EDTA) tubes or without evacuated (Serum and EDTA) tubes. |
| Materials and Methods |
| This study was performed to compare the incidence of preanalytical errors in inpatients and outpatients samples collected into vacuated (Serum and EDTA) tubes and without evacuated (Serum and EDTA) tubes. Vacuated tubes were provided by Becton Dickinson (India) Pvt. Ltd. This study has covered only routine blood specimens for inpatients and outpatients received in Central Lab of SMS Hospital, Jaipur, India for biochemical and hematological analysis and therefore did not involve collection of any additional blood specimens. |
| In Total, 10,000 patient samples (Inpatients-4000, Outpatients-6000) were monitored for various preanalytical specimen quality checks. Outpatient’s samples were collected by phlebotomists from the patients that had been referred to the laboratories for the analysis of various laboratory parameters. Sample collection for inpatients was performed by nursing staff and specimens were transported manually by ward staff or relatives of patients from the individual wards to the laboratory for analysis. Open Collection mode of specimen collection was used for both inpatients and outpatients. |
| Open venous blood collection mode |
| All blood specimens under Open Collection Mode were collected using normal hypodermic needle and disposable syringes (5 ml or 10 ml) procured from normal hospital purchase process. Open Collection was carried out by choosing one of the following methods using different specimens containers. |
| (a) Open Collection using BD Vacutainer® Tube: Venous blood was collected using needle and syringe (as mentioned above). After sufficient amount of blood was drawn from patient in syringe, needle was mutilated using needle burner. The mutilated needles were then re-capped and removed from the syringe. The venous blood was transferred to either BD Vacutainer® Serum Tube (red top, 5 ml) or BD Vacutainer® EDTA Tube (purple top, 5 ml) after the Hemogard™ cap of the tube was removed. Precautions were taken to minimize froth formation while transferring the specimen. The syringe and the recapped, mutilated needle were discarded in hypochlorite solution. BD Vacutainer® Serum Tubes samples were left at room temperature for 60 min before centrifugation at 1500 x g for 10 minutes using REMI Centrifuge machine. |
| (b) Open Collection Using without evacuated Tubes: Specimen collected using needle syringe (as mentioned above) were transferred to either Plain Serum Tube or Plain EDTA Tube. All care was taken to reduce any froth formation during the transfer of specimens. Samples were transferred at room temperature to the laboratory in a sample tray in upright position. After minimum 60 minutes of collection, Plain Serum Tubes samples were centrifuged at 1500 x g for 10 min using REMI Centrifuge machine. |
| Open Collections were carried out using manual data management. At the time of collection of all specimens, the phlebotomists/ Nurses were required to fill up a Form. This Form traveled along with the specimen to the laboratory. The Form was divided into two sections, one filled at the time of collection, while the other was filled once the specimen entered into the laboratory (site of analysis). All the preanalytical specimen quality checks were carried out in the laboratory. |
| All the Specimens were checked for preanalytical quality using the method/criteria given in the Table 1. |
| The Forms for both the study groups (open venous blood collection using either BD Vacutainer® Tubes or without evacuated Tubes) were coded and transferred manually into Microsoft Excel® Format. The sums of errors in inpatients and outpatients were calculated. The relative frequencies of Pre-analytical errors in total specimens collected from inpatients and outpatients were also calculated and presented as percentage. |
| Results and Discussion |
| Major revolution has been observed in the field of biochemical laboratory testing in the last few decades. The lab medicine plays a pivotal role in the provision of healthcare to the masses and hence there is an ever increasing demand for reliability and accuracy of the lab tests. There are whole gamuts of factors that contribute to accurate test results in the biochemistry laboratories. These factors can be classified into three phases: pre-analytical, analytical and post-analytical. The advances in the technology like automation and computerization of the tests have led to reduction in the errors during analytical phase but there still remains a high level of inconsistency in the total testing process. The major contributors being the pre-analytical errors are complex as they involve numerous steps and various levels of professionals [10]. |
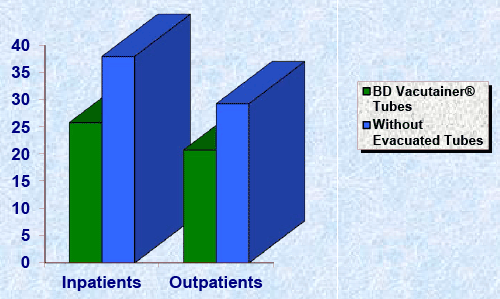
| The present study is an attempt to find out the frequency of various preanlytical errors in Inpatients and Outpatients of Open Venous Sample Collection. In this study, we have analyzed total 10,000 specimens collected into BD Vacutainer® Tubes (3000) and without evacuated tubes (7000) to compare the incidence of preanalytical errors in Inpatients (4000) and Outpatients (6000) (Table 2). The observations were made for the quality check of samples especially for various preanalytical errors have been presented in the Tables (Table 3-6) and Figure 1. All the preanalytical specimen quality checks were carried out in the laboratory. |
| Preanalytical errors in Inpatients and Outpatients were calculated by the relative frequencies to the total specimens and presented as percentage. All the specimens of inpatients and outpatients were confirmed for following preanalytical errors: |
| Specimen identification and tracking related errors |
| We have examined samples of Inpatients and outpatients collected into BD Vacutainer® Tubes and without evacuated tubes for various Specimen Identification and Tracking related errors which includes Sample lost/Not received, Sample ID/Order form mismatch, One or Mandatory ID Missing, Test order form missing and Incorrect tube collected (Table 3-4). |
| In our study, sample identification related errors occurred more frequently in inpatients and plain tube open collection as compared to outpatients and BD Vacutainer® open collection (Table 5). Samples in Inpatients were collected by nursing staff and deposited into laboratory by ward staff/patient’s relative and in outpatients, sample collection and deposition was done by phlebotomists in laboratory itself so the incidence for these errors in inpatients was more as compared to outpatients. Problems such as incorrect sample identification or handling might occur beyond the blood drawing process, although their prevalence is reportedly much lower [11]. Misidentification of the specimen can occur outside the laboratory when it is collected or prepared for shipment or inside the laboratory when it is aliquoted for multiple tests [12]. |
| Specimen insufficient/leakage/spillage |
| We have identified samples of Inpatients and outpatients collected into BD Vacutainer® Tubes and without evacuated tubes for various errors like insufficient sample to perform test, Sample Leakage and Tube broken/Spillage (Table 3 and 4). |
| These errors were found more in inpatients due to the transportation of specimens from wards to laboratory. Without evacuated tubes are more susceptible for leakage/spillage because more prone to breakage or due to loosely attached rubber caps than BD Vacutainer® tubes (Table 5). In open venous blood collection, volume of blood delivered to the containers is subject to human error. With the fixed volume, syringes are used; there may be possibility of variation in the volume delivered into the open containers when smaller than needed volumes of collected specimen were coupled with the inclination of the phlebotomists/ nursing staff to avoid second venipuncture. In case of insufficient specimen, redraw of samples from the patients are required. |
| Sample clotted/volume related errors |
| We have analyzed samples of Inpatients and outpatients collected into BD Vacutainer® EDTA Tubes and without evacuated EDTA Tubes (1000) for sample clotted/volume related errors (Table 3 and 4). |
| When the samples are required in EDTA tubes and collected in Serum tubes or not properly mixed with anticoagulant, it became clotted hence not suitable for hematology testing. Underfilled/overfilled EDTA tubes could result in introduction of preanalytical errors due to changed sample to additive ratios and therefore potentially impact quality of results for certain parameters [13,14]. In hematology, a clotted specimen is the most frequent reason for rejection and the container type with the highest frequency of rejection is a pediatric tube [15]. |
| Hemolysis/fibrin clot |
| We have examined samples of Inpatients and outpatients collected into BD Vacutainer® Serum Tubes and without evacuated Serum Tubes for hemolysis/fibrin clot (Table 3 and 4). |
| These errors are two times higher in without evacuated tube open collection than BD Vacutainer® open collection (Table 5). Hemolysis occurs whenever there is trauma to relatively fragile red blood cells, either during collection or after collection. If blood is drawn with a syringe, drawing the plunger back forcefully or injecting blood into container using pressure could cause hemolysis [16]. Hemolysis could cause chemical, biological, immunological interference with reaction mechanism of several assays [17]. In vitro hemolysis, reflecting a more generalized process of blood and vascular cell damage that occurs during phlebotomy, is the most frequent reason for specimen rejection, five-fold more frequent than the next reason (insufficient specimen quantity), as indicated by the College of American Pathologists (CAP) Chemistry Specimen Acceptance Q-Probes study [11]. Therefore higher incidence of hemolysis in open collections could potentially pose one of the largest risks for specimen result not correlating with patient condition. Presence of latent fibrin in serum poses a risk of blocking analyzer probe resulting in no/reduced sample aspiration/ system breakdown. Post-centrifugation fibrin clot can be formed due to insufficient time given for specimen to clot before centrifugation. This can also happen if the patient is on dialysis or on anticoagulant therapy [16]. |
| Frequency of total errors in inpatients whose samples are collected into BD Vacutainer® tubes and without evacuated tubes are 25.80% and 27.96% and in outpatients whose samples are collected into BD Vacutainer® tubes and without evacuated tubes are 20.75% and 29.27%. Total errors in inpatients and outpatients are 34.92% and 25.55% (Table 6, Figure 1). In view of the above results, it is evident that the occurrence of preanalytical errors is more in inpatients and without evacuated tubes open collection than outpatients and BD Vacutainer® tubes open collection so we can reduce the incidence of preanalytical errors by using evacuated tubes for the sample collection. |
| In a similar study it was reported that evacuated closed blood collection resulted in an approximate 100-fold reduction in the incidence of hemolysis in samples and 200-fold reduction in incidence of insufficient specimen quantity. It was also found that incidence of specimen contamination, improper volume of sample collected, and specimen spillage was also lower when the evacuated collection system was used [18]. |
| Conclusion |
| In the age of evidence-based medicine, results of laboratory testing are integral to the clinical decision making, to assist diagnosis, guide or monitor therapy and predict health outcomes. While laboratory results are objective, they should not be used in isolation; they supplement and do not supplant clinical decision. While the majority of attention has been focused on the analytical process, consideration should also be applied to the preanalytical phase as well, as this process affects the reliability of test results, consuming valuable healthcare resources and possibly compromising treatment outcomes. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15091
- [From(publication date):
December-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10443
- PDF downloads : 4648
