Identification of Microflora Associated with Groundnut (Arachis Hypogaea L.) Seeds and their Impact on Physical Impairment and Germination Percentages of Groundnut Seeds
Received: 02-Jun-2024 / Manuscript No. acst-24-140965 / Editor assigned: 05-Jun-2024 / PreQC No. acst-24-140965 / Reviewed: 19-Jun-2024 / QC No. acst-24-140965 / Revised: 23-Jun-2024 / Manuscript No. acst-24-140965 / Published Date: 30-Jun-2024
Abstract
This study was to investigate the incidence of seed-borne fungi on the groundnut seeds isolate and identify the seedborne fungi associated with some of groundnut seeds and obtain information on the pathological effect of these fungi on the peanut seed germination. The results of these investigations revealed the existence of four notable seed-borne fungal pathogens, specifically A. niger, A. flavus, A. tamre, and one Aspergillus pp., in the designated research area. A. Niger was identified as the predominant fungus affecting groundnut seeds among these pathogens. This particular finding suggests that the presence of A. Niger might inhibit the growth of other fungi through competitive interactions in the environment. Previous research by Oladipupo (2011) and Bahattcharya and Raha (2002) have pointed out that these species can lower seed germination rates and cause damage during storage. The findings are consistent with the results of my study, which demonstrate that these fungi play a role in causing pathological effects on groundnut seeds, resulting in issues such as seed shriveling, discoloration, and decreased germination rates due to seed-borne fungal infections. The conducted study emphasizes the importance of treating seeds purchased from markets with fungicides prior to planting to prevent seedborne diseases like seed rot, decay, and other related pathologies. Furthermore, it is very important to take steps to reduce the spread of seed-borne pathogens and the production of mycotoxins in groundnut seeds by improving storage conditions, which include maintaining low temperatures, humidity levels, and moisture content. Additionally, preventive measures should be implemented to avoid damage during post-harvest processes at storage and also during the sold at the market.
Keywords
Fungicides; Groudnut; Microflora; Seed damage germination
Introduction
Groundnut, (Arachis hypogaea L.,) is an annual plant that belongs to the leguminosae family. It is grown in 108 countries across approximately 22.2 million hectares of land. Specifically, 13.69 million hectares are in Asia (with India accounting for 8 million ha and China for 3.84 million ha), 7.39 million hectares in Sub-Saharan Africa, and 0.7 million hectares in Central and South America (FAO, 1995-2001; CGIAR Research, 2000). This crop ranks as the 13th most important food crop for edible oil and the 3rd most significant source of vegetable protein. Groundnut seeds are rich in high-quality edible oil (about 50%), easily digestible protein (25%), and carbohydrates (20%) (Al-Amod, 2015) [1]. The cultivation of groundnuts is primarily for its seeds, used for oil extraction, direct human consumption for protein and vitamins A and B. The by-product obtained after oil extraction from the seeds is valuable for poultry and animal feed. The shells can be utilized as fuel for making coarse boards and as a substitute for cork. The kernels can be consumed raw, roasted, or sweetened. Furthermore, the oil has various applications such as soap making, cosmetics, and lubricants (Ibiam and Egwu, 2011) [2].
Groundnut, also known as peanut, is one of the five main oil crops grown in the arid regions of Ethiopia (Wijnands et al., 2009). Italian explorers brought it from Eritrea to Hararghe in the early 1920s (Daniel, 2009). Key areas for groundnut production in Ethiopia include Babile, Gursum, Beles, Didessa, Gambella, and Pawe. Additionally, regions like Gamu Gofa, Illubabor, Gojam, Wello, and Wellega have been identified as having potential for production [3]. In the 2013/2014 cropping season, Ethiopia had an estimated groundnut production area of 79,947.03 hectares, yielding 112,088.72 tons (CSA, 2014). Despite its significant role in exports and the economy, various challenges exist that hinder the production and use of groundnut. Among these challenges, toxigenic fungal diseases are highlighted as the most critical, impacting seed quality due to spoilage and mycotoxin contamination.The cultivated groundnut (Arachis hypogaea L.) is an ancient crop of the New World, which originated in South America (southern Bolivia/north west Argentina region) where it was cultivated as early as 1000 B.C. Dissemination of the crop to Africa, Asia, Europe and the Pacific Islands occurred presumably in the sixteenth and seventeenth centuries with the discovery voyages of the Spanish, Portuguese, British and Dutch [4]. The term Arachis is derived from the Greek word "arachos", meaning a weed, and hypogaea, meaning underground chamber, i.e. in botanical terms, a weed with fruits produced below the soil surface). There are two most common names used for this crop i.e. groundnut or peanut. The term groundnut is used in most countries of Asia, Africa, Europe and Australia, while in North and South America it is commonly referred to as peanut [5]. There are two most common names used for this crop i.e. groundnut or peanut. The term groundnut is used in most countries of Asia, Africa, Europe and Australia, while in North and South America it is commonly referred to as peanut. The term groundnut refers to the pods with seeds that mature underground; the connotation of peanut is because this crop belongs to the leguminous family which includes also other (FAO, 1995-2001) [6].
Groundnut is grown in several agro-ecological systems and under numerous socioeconomic environments. Its cultivation is mostly confined to the tropical, subtropical, and warm temperate (zones) countries between 40°N and 40°S latitude. It is currently grown on 25.2 million hectares worldwide with a total production of 35.9 million metric tons and productivity of 1.425 tons per hectare, with developing countries in Asia (66%) and Africa (25%) as the major producers (FAO, 2006). In 2009, China, India and the United States were the three largest producers of groundnut (USDA-FAS, 2010). The crop is now grown in about 108 countries of the world. Asia with 63.4% land mass produces 71.72% of world groundnut production followed by Africa with 18.6% production and North-Central America with 7.5% (Crop gallery: Groundnut, 2008). It grows best in light, sandy loam soil. It requires five months of warm weather and annual rainfall of 500 to 1000mm.the pod ripen 120to150 days after the seed are planted. Temperature between 250 c to 300c is optimum for plant development (weiss, 200). Globally, 50% groundnut produced is used for oil extraction, 37% for confectionery and 12% for seed purpose. Groundnut haulms provide excellent hay for feed livestock. Groundnut cake is a high protein livestock feed. It is also manufacture of margarine and soap. High quality oil is used in the pharmaceutical industry. The ground nut husk can be burned for energy generation or used in manufacture of particle board (Crop gallery: Groundnut, 2008). Fungi growing on stored seeds such as groundnut, can reduce the germination rate along with loss in the quantum of carbohydrate, protein and total oil content, induces increased moisture content, free fatty acid content and enhancing other biochemical changes. The tropical climate with high temperature and high relative humidity along with unscientific storage conditions adversely affect the preservation of cereal grains, oilseeds, etc., which lead to the total loss of seed quality (Ameer Junaithal Begum, 2013).
Fungi can be rendered as the most harmful microorganism and so far, 46 fungal diseases were recorded on groundnut and 67 fungi were associated with various symptoms type (Wikipedia, 2012). Groundnut is attacked by a number of pathogenic fungi of economic importance. Aspergillus niger, Fusarium, Penicilium and Cladosporium are the predominant fungal genera associated with grains in storage, and aflatoxins of all mycotoxins is of utmost concern (CAST, 2003). This is due to their carcinogens and immunosuppressive effects in both humans and domestic animals (Turner et al., 2015). Janardhan et al. (2011) found that Aspergillus is a common mould in tropical and sub-tropical countries and causes aflatoxin contamination as a result of moulding of badly stored commodities, such as groundnut, cereal and cotton seeds [7]. Chavan and Kakde (2008), reported that groundnut seeds are highly susceptible to diseases, as they serve as a source of stored nutrients for fungi causes discoloration, rotting, shrinking, seed necrosis, loss in germination capacity and toxification to oil seeds. Aflatoxin contamination of groundnut could occur before harvest while the crop is maturing in the field, during harvest, and post-harvest in storage. It prevents groundnut producers from accessing bigger western markets, increases dependency on foreign food aid, stifles economic opportunities, and adversely affects consumer health. According to FAO (2002) developing countries account for approximately 95% of world groundnut production, but are unable to sell large quantities of groundnut on the international market because of aflatoxin contamination. For instance, a food processing company in Ethiopia imported groundnuts from India, while groundnut producers in Gursum and Bablie could not find market to sell their crop (Guchi et al, 2014). In Ethiopia, an earlier report showed mean levels of aflatoxin B1 of 34.7 and 105 µg/kg in a sample of groundnut and peanut butter respectively (Bisrat and Gebre, 1981). Amare et al (1995) reported aflatoxin levels of 5-250 µg/kg in groundnut seed from eastern Ethiopia. Recently, Alemayehu (2012) reported that total aflatoxin levels in Aspergillus flavus positive samples of groundnut seed varied between 15 and 11865 µg/kg.
There for the objectives of this study was to investigate the incidence of seed-borne fungi on the groundnut seeds isolate and identify the seed-borne fungi associated with some of groundnut seeds and obtain information on the pathological effect of these fungi on the peanut seed germination.
Material and Method
Collection of seed sample
Groundnut seeds were collected from four different sellers all within Jimma central market. These seed samples were investigated at Jimma University College of agriculture and veterinary medicine plant pathology laboratory.
Detection agar plate method (APM)
Peanut seed samples were analyzed for their association of seed mycoflora by using standard Agar plate method (ISTA, 1985). From each sample seed a working sample of 400seeds, 70 seeds were taken at random. at the time of isolation, the samples were surface sterilized with 5% Chlorox aqueous solution for approximately one minute. These seeds were rinsed in sterilized distilled water then blot dried. Five seeds were plated in each petridish. The plated seeds were incubated at 25 ± 20C for 7 days and then pure cultured [8].
Identification of fungal isolates
Petri plates having diseased specimens were observed to identify the seed borne fungi on the basis of colony color. Further confirmation was made by preparing the slides and observed under microscope with low as well as high magnifications (4, 10, 40, and 100X). A drop of water was placed in the center of slide, the small portion of fungi culture was cut out with the aid of sterilized inoculating needle. The cut piece was put directly in the water droplet and tease out, a cover slip was then covered over the teased portion, it was mounted on the microscopic stage and viewed. The viewing was first done with lower magnification (x4) and later with higher magnification (x40). The nature of mycelium, the types of fruiting body and spore structure served as criteria for the identification of isolate. The isolates were identified and confirmed with mycological atlas as described by fisher 1988.
Data collected
Fungal species found growing on the surface of seeds, Type and frequency of occurrence of identified fungal species was recorded. Percentage frequency (PF) of occurrence of fungal was calculated by using the following formula: PF= (No. of seeds on which fungus appears / Total number of seeds) X 100. Percent of germination (PG) of seed varieties are determined as proportion of germinated seed over the total number of seed and computed by using the following formula: PG= No. of seeds germination/ Total number of seeds) X 100 [9].
Results
Occurrence of seed borne fungi on groundnut seeds
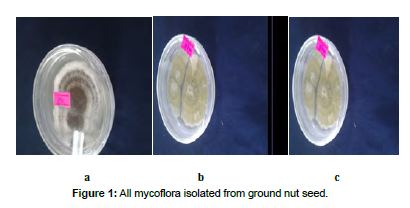
The agar plate method technique has proven more suitable for the detection of Aspergillus Niger, Aspergillus flavus and another Aspergillus spp. Among the fungal species that were isolated, Aspergillus Niger show the highest mean frequency flowed by Aspergillus tamrri Aspergillus flavus and other Aspergillus spp. across the hole seller in Jimma central market. The highest average frequency of fungal pathogen was absorbed the sample taken from kullober2, then followed by Kullober1, Bishisha1 and 2 respectively (Figure 1&2) (Table 1).
| Market name | Aspergillus niger | Aspergillus tamrii | Aspergillus flavus | other Aspergillus pp | Mean |
|---|---|---|---|---|---|
| Kulober1 | 52.68 | 46.6 | 36.05 | 15.2 | 35.98 |
| Kulober2 | 42.78 | 33.42 | 45.6 | 32.93 | 36.27 |
| Bishishe1 | 34.91 | 26.7 | 25.1 | 22.47 | 27.30 |
| Bishishe2 | 32.46 | 31.33 | 25.24 | 14.19 | 25.81 |
| Mean | 40.68 | 34.26 | 29.2 | 21.2 |
Table 1: Seed physical damage and germination %
As different authors wrote about seed born fungi associated with the groundnut seed that affected the germination percentage and seed imrgence, these results also showed that, the germination percentage across the whole seller in Gimma central market were not more than 50%. The highest and lowest germination percentage was observed the sample taken from Bishishe1 and Kullober2 respectively. As many reporters also wrote the physical damage (seed shriveling and discoloration) of seed also good indicator for the presence of mycoflora associated with the seed. In these results the highest and lowest physical seed damage was absorbed from the sample taken from Kullobere2 and Bishishe1 respectively (Figure 3, 4&5).
Discussion
Results of these investigation revealed that at least four important seed-borne fungal pathogens namely A. Niger, A. flavus, A.tamre and one Aspergillus pp.in the study area. Out of all those pathogen, A. Niger was the predominate fungi on groundnut seed. This observation suggested that, A. Niger inhibit the growth of other fungi due to the competition for in the site. The findings of the present investigation agree with the findings of other investigators such as Oladipupo (2011) and Chavan (2011) found association of similar fungi with peanut seeds. Rasheed et al. (2004) found A. Niger and A. flavus were predominant in groundnut and seed coat was greatly infected by fungi followed by cotyledon and axis. Such similar reports have been made by Rasheed et al., (2004) on groundnut seed. It was also reported that A. flavus was the important mycotoxins producer and produce aflatoxin B1, B2, G1 and G2 which are hepatocarcinogenic (Syed et al., 2013). As Oladipupo, (2011) Bahattcharya and Raha, (2002) reported these species have been to reduce the germination of seed and damage the seeds in the storage. these studies agree with my study that showed those fungi were associated with the pathological effect on groundnut seed, such seed shriveling, seed coloration and low percentage germination was as a result of infection of seed-borne fungi on those seeds [10].
Conclusion and Recommendation
The experiment has helped to show that seed collected from open markets for purpose of planting must be treated with fungicide to avoid seeding disease such as seed rote, seed decay, and other pathological effect. There is also need to reduce the growth seed born pathogen and mycotoxin production in ground nut seed by improving the storage condition, that is low temperature, low humidity and low moisture content. The damage done during post harvest operation should also be avoided.
References
- Alemayehu C, Abdi M, Amare A, Helge S (2012) Natural occurrence of aflatoxins in groundnut.
- Amare A, Dawit A, Mengistu H (1995) Mycoflora, aflatoxins and resistance of groundnut cultivars’ from Eastern Ethiopia. SINET: Eth J Sci 18: 117-131.
- Alemayehu C, Abdi M, Amare A, Helge S (2012) Natural occurrence of aflatoxins in groundnut (Arachis hypogaea L.) from eastern Ethiopia. Food Cont 30: 602-605.
- Bhattacharya K, Raha S (2002) Deteriorative changes of maize, groundnut andsoybean seeds by fungi storage. Mycopathologia 155: 135-141.
- Guchi E, Ayalew A, Dejene M, Ketema M, Asalf B, et al. (2014) Occurrence of Aspergillus Species in Groundnut (Arachis hypogaea L.) along the Value Chain in Different Agro-Ecological Zones of Eastern Ethiopia. Journal of Applied & Environmental Microbiology 2: 309-317.
- Hell K, Cardwell KF, Setamou M, Poehling HM (2000) The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, West Africa. J Stored Products Res 36: 365-382.
- Kaaya AN, Kyamuhangire W (2006) The effect of storage time and agroecological zone on mould incidence and aflatoxin contamination of maize from traders in Uganda. Int J Food Microbiol 110: 217-223.
- Payne GA (1998) Process of contamination by aflatoxin-producing fungi and their impact on crops. 279-300.
- Peraica M, Radic B, Lucic A, Pavlovic M (1999) Diseases caused by molds in humans. Bulletins of the World Health Organization.
- Schatzki TF, and Haddon WF (2002) Rapid, non-destructive selection of peanuts for high aflatoxin content by soaking and tandem mass spectrometry. J Agri Food Chem 50: 3062-3069.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Gebisa LA (2024) Identification of Microflora Associated with Groundnut(Arachis Hypogaea L.) Seeds and their Impact on Physical Impairment andGermination Percentages of Groundnut Seeds. Adv Crop Sci Tech 12: 712.
Copyright: © 2024 Gebisa LA. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 690
- [From(publication date): 0-2024 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 512
- PDF downloads: 178