Research Article Open Access
Identification of High-Temperature Tolerant and Agronomically Viable Tomato (S. lycopersicum) Genotypes from a Diverse Germplasm Collection
Muhammed Alsamir1,2, Nabil M Ahmad1*, Claudia Keitel3, Tariq Mahmood1 and Richard Trethowan1
1Plant Breeding Institute, University of Sydney, Cobbitty, NSW 2570, Australia
2The Date Palm Research Center, University of Basrah, Basrah, Iraq
3Centre for Carbon Water and Food, University of Sydney, Cobbitty, NSW 2570, Australia
- *Corresponding Author:
- Nabil M Ahmad
Plant Breeding Institute, University of Sydney
Cobbitty, NSW 2570, Australia
Tel: +61 2 9351 8829
E-mail: nabil.ahmad@sydney.edu.au
Received Date: July 26, 2017 Accepted Date: August 04, 2017 Published Date: August 11, 2017
Citation: Alsamir M, Ahmad NM, Keitel C, Mahmood T, Trethowan R (2017) Identification of High-Temperature Tolerant and Agronomically Viable Tomato (S. lycopersicum) Genotypes from a Diverse Germplasm Collection. Adv Crop Sci Tech 5: 299. doi: 10.4172/2329-8863.1000299
Copyright: © 2017 Alsamir M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Advances in Crop Science and Technology
Abstract
The aim of most crop heat stress tolerance improvement programs is to increase productivity, not just survival, under high-temperature. Two cycles of experiments were conducted on forty four diverse tomato (S. lycopersicum) lines collected from UC Davis. These included one genotype each of the wild species S. pimpinellifolium (LA0373), S. Pennellii (LA0716) and S. chillense (LA1930). Experiments were conducted in both a growth-chamber and a polytunnel house. Three physiological parameters; stomatal conductance, electrolyte leakage (EL) and chlorophyll fluorescence were used to evaluate the first cycle materials in a growth chamber. Two-month old tomato plants were exposed to heat stress of 44°C for four hours and traits were assessed prior to and following heat shock treatment. Electrolyte leakage differentiated the materials best and was therefore used in subsequent evaluations.
In the second cycle of evaluation, the agronomic superiority of the more heat tolerant materials was assessed using an Agronomic Superiority Index (Ag Index) calculated using Euclidean distance for fruit set %, fruit yield, plant dry weight and EL. Plants were grown hydroponically in cocopeat bags in a tunnel house for two crop seasons. The temperature in the tunnel house was maintained using natural sunlight and controlled ventilation. The Ag Index successfully identified heat tolerant and agronomically superior genotypes and fruit yield and the Ag Index were strongly correlated (R2=0.96). Two lines, LA4284 and LA3847, were classified as superior for heat tolerance and agronomic performance. The EL response in the growth chamber and the tunnel house was significantly correlated (R2=0.30), thus validating the use of EL for screening. Histological studies on the selected lines confirmed that pollen development was significantly impaired by heat stress.
Keywords
Agronomic superiority index; Chlorophyll fluorescence; Electrolyte leakage; Heat stress; Stomatal conductance; Tomato germplasm
Introduction
The tomato (Solanum lycopersicum L.) is an important vegetable in most regions of the world for both field and greenhouse production. The tomato crop is currently the second largest of the major vegetable commodities in Australia [1] however, the size of the harvest fluctuates annually. The national production in 2013-14 was 326,189 tonnes or 28% less than the previous season. This was largely due to a reduction in the production area (18%) and unfavorable conditions in parts of New South Wales, Victoria and Queensland [1]. Tomatoes were ranked 16th in Australian agricultural production for quantity and value in 2010. However, they ranked 20th in 2011 for value and did not rank within the top 20 producing countries in that year [2]. China, India and the USA are the largest producers of tomatoes with China surpassing the USA in 1995 and maintaining this position ever since. Countries such as Italy, Egypt, Iran and Turkey produce substantial quantities and there is a gradual trend of increasing production globally [2].
The optimum temperature for tomato cultivation is 25°C during the day and 20°C at night. However, tomato cultivation is constrained by the dual challenges of global warming and increasing world population and production must increase in the tropics and sub-tropics, where high temperature often disturbs plant establishment, to meet future demand. Improved knowledge of the physiological processes that limit plant productivity under hot conditions will enable the development of more heat tolerant cultivars.
In most production regions, tomato plants are often exposed to extreme environmental conditions including high temperatures and drought. The frequency of extreme events is likely to increase with predicted climate change [3]. An increase in the Earth’s surface temperature of between 1.5 and 11°C, projected by 2100, will pose serious constraints on plant growth and reproduction [4,5]. When day/ night temperatures exceed 26/20°C, fruit set is interrupted and tomato yield subsequently reduced [6-9].
Several factors were reported to contribute to reduced fruit set under high temperatures in tomato. These include: reduced flower production and ovule and pollen viability as well as increased pollen dehiscence [3]. Additionally, stigma and stylar exsertion in response to high temperatures and high assimilate translocation rates adversely affect fruit set [10].
However, the major factor responsible for the failure of tomato fruit set under suboptimal temperature is the high sensitivity of flower development to temperature changes. The anthers are more vulnerable than the female organs to these changes [11,12]. Alterations in tomato anthers, including failure of adequate dehiscence and tapetum development, occur under heat stress during the early phases of pollen development; 7-15 d before anthesis [12]. However, heat stress also affects late pollen development and can cause impairment of pollen and normal anther development [11,12].
High temperature during reproductive development has been associated with several alterations in the morphology of tomato flower structures [13,14] and can cause significant flower drop [15] which consequently decreases fruit yield. Other reports suggest that high temperature affects stigma tube elongation and cone splitting, resulting in poor pollen germination and poor pollen tube growth [16]. However, the impact of high temperature is not limited to lowering and fruit set. Others report that high-temperatures affect the development and maturity of the fruit thus reducing yield and quality, largely due to decreased lycopene content [17,18].
The effect of heat stress on pollen viability was also associated with alterations in carbohydrate metabolism in various parts of the anther during development [19]. Bhadula [20] reported that reduced carbohydrate metabolism in the tomato anther leads to abnormal pollen development.
High temperature also affects photosynthesis [21], alters membrane fluidity and may disrupt the overall balance of metabolic processes, leading to over-production of reactive oxygen species (ROS) and oxidative stress-induced damage [22]. Physiological imbalances in stress-protective metabolites, such as carbohydrates, polyamines and proline have also been reported [13,19,23].
Plants are often exposed to abiotic stresses during the life cycle and they have developed mechanisms to overcome such stresses including the accumulation of compatible solutes [24,25], elevated transpiration rates that promote leaf cooling and more efficient photosynthesis. These solutes are low molecular-weight metabolites that are soluble in water and non-toxic at high concentrations. Representative compatible solutes, which differ among species, may include certain polyols, sugars, amino acids, betaines and some other compounds [26]. Stomatal conductance [27] can be used to assess transpiration rates and therefore estimate evaporative cooling. Impacts of hightemperature stress on photosynthesis can be measured using chlorophyll fluorescence as this trait assesses electron flow through photosystem II in heat damaged tissue.
The sensitivity of three relatively fast screening methods used previously to detect heat tolerant genotypes was evaluated in the current study. The three methods; stomatal conductance, chlorophyll fluorescence and EL, were combined with morphological data to: i) identify tomato genotypes with high-temperature tolerance, ii) distinguish agronomically superior genotypes under heat stress and iii) assess changes in floral characteristics under heat stress.
Materials and Methods
All plant material was grown at The University of Sydney’s Plant Breeding Institute, Cobbitty (34.02°S, 150.67°E, 87 masl). An initial screen of the material was conducted in a controlled environment facility at the Centre for Carbon, Water and Food (Environment I); the agronomic superiority of the heat-tolerant material identified in the initial screen was then evaluated in a poly-tunnel house at the Plant Breeding Institute (Environment II).
Environment I
Seeds of 44 domesticated tomato genotypes (Solanum lycopersicum ) including one genotype each of the wild species S. pimpinellifolium (LA0373), S. Pennellii (LA0716) and S. chillense (LA1930) were sourced from the tomato genetic resources center (TGRC), University of California, Davis (Table 1). These materials were subsequently sown in culture trays containing peat moss and placed in a germination room maintained at 26/20°C day/night temperature. Seedlings were transplanted 25 days after planting into 20 cm diameter pots filled with a 0-8 mm Composted Pine Bark: 0-3 mm Composted Pine Bark: Prop Sand mixture (8:1:1 in volume) and supplemented @ 0.4 kg/m3 with all trace elements, 1 kg/m3 Gypsum, 1 kg/m3 Superphosphate, 0.25 kg/m3 KNO3 (13% N), 0.25 kg/m3 Nitroform (38% N), 1.5 kg/m3 Magrilime.
| List ID | Genotype ID | List ID | Genotype ID | List ID | Genotype ID |
|---|---|---|---|---|---|
| 1 | LA0373 | 16 | LA3875 | 31 | LA4234 |
| 2 | LA0716 | 17 | LA3876 | 32 | LA4235 |
| 3 | LA1930 | 18 | LA3878 | 33 | LA4236 |
| 4 | LA2375 | 19 | LA3879 | 34 | LA4237 |
| 5 | LA2661 | 20 | LA3882 | 35 | LA4247 |
| 6 | LA3320 | 21 | LA3883 | 36 | LA4248 |
| 7 | LA3344 | 22 | LA3886 | 37 | LA4249 |
| 8 | LA3345 | 23 | LA3889 | 38 | LA4252 |
| 9 | LA3847 | 24 | LA3892 | 39 | LA4256 |
| 10 | LA3866 | 25 | LA3893 | 40 | LA4257 |
| 11 | LA3867 | 26 | LA3906 | 41 | LA4272 |
| 12 | LA3869 | 27 | LA4230 | 42 | LA4273 |
| 13 | LA3870 | 28 | LA4231 | 43 | LA4283 |
| 14 | LA3871 | 29 | LA4232 | 44 | LA4284 |
| 15 | LA3874 | 30 | LA4233 | 45 | Roma |
| 46 | Rio Grande |
Table 1: List of tomato genotypes evaluated in the experiments.
In the preliminary screening experiment, two growth chambers were set and maintained at two temperature levels; the control at 26/20°C day/night and heat stress treatment at 42/26°C day/night temperature. Humidity was not controlled but was not significantly different between the two chambers.
Physiological parameters
Chlorophyll fluorescence and stomatal conductance: Chlorophyll fluorescence, defined as the ratio of variable to maximal fluorescence in both dark-adapted (Fv/Fm) and light adapted leaves (Fv’/Fm’), was measured using a Licor 6400XT infra-red gas analyser (IRGA, Li-Cor Inc., Lincoln, NE, USA) fitted with a fluorescence chamber. As stomatal conductance (gs, mmol m-2 s-1) measured using an IRGA takes considerable time (generally c. 5 to 10 min per leaf), gs was determined on both leaf surfaces and added to calculate total conductance using a porometer (SC-1, Decagon Devices, Pullman, WA, USA), where measurements only take c. 30 sec. Chlorophyll fluorescence and stomatal conductance were assessed on the top-most mature leaf of each plant.
Electrolyte leakage: Electrolyte leakage was determined using the conductivity method according to Lafuente [28]. Six leaf segments of uniform maturity from each plant were cut into discs and washed three times with de-ionized water to eliminate external surface residues. Discs were placed in 50 ml Greiner centrifuge tubes (Sigma Aldrich, Australia) with 20 ml of de-ionized water and shaken at 80 rpm for 20 hours. The conductivity of the solution was subsequently read with a conductivity meter (Edge, Hanna Instruments Inc. HI11310 single ceramic, double junction, refillable pH electrode with temperature sensor). The samples were then autoclaved at 121°C and 15 psi pressure for 15 min to kill the tissue and burst all cells and conductivity was recorded again. The percentage of electrolytes originally diffused was calculated as follows: Electrolyte (%)=C1/C2 × 100, where C1 and C2 are the conductivities of the solution before and after autoclaving, respectively.
Environment II
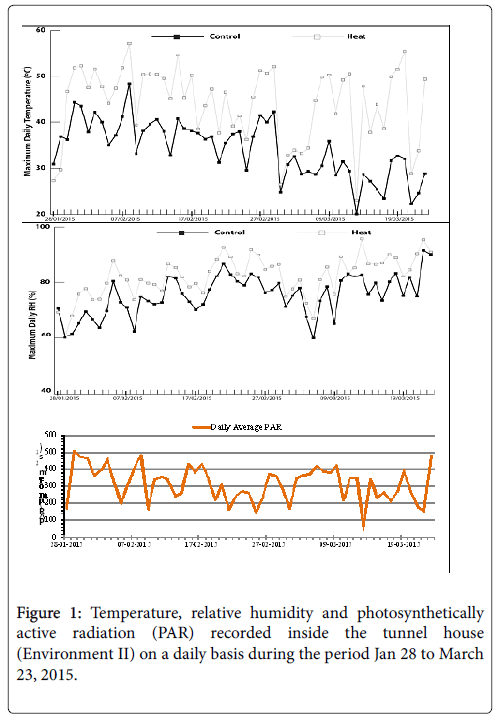
The same set of materials as Environment I and two standard checks were grown hydroponically in a poly-tunnel house in cocopeat bags. The experiment was conducted using two planting dates; mid-season and late-season. Each cocopeat bag contained one plant and two replications were maintained. The plants were fertigated with a nutrient solution formulated for growing commercial tomatoes. Two temperature treatments were created in two separate sections of the tunnel-house. Management protocols in both sections were identical with the exception of the temperature treatment. The high temperature section accumulated heat from the sun and temperatures at midday reached 50°C and above on some days (measured at c. 2 m height and c. 50 cm above the canopy). The temperature in the hot section was maintained using controlled ventilation. On hot days, the ventilation was more closely monitored to avoid heat damage to the plants. The temperature, relative humidity and photosynthetically active radiation (PAR) in the tunnel-house was recorded (Figure 1) using a CR200X data logger (Campbell Scientific Australia, Townsville, Qld, Australia). The data were recorded at maturity.
Morphological parameters
Floral structure and fertility: Data captured in each tunnel-house section for each genotype included: number of days from sowing to the first flower appearance, floral bud and flower production per truss for the first four trusses, number of flowers with exserted stigmas, antheridial cone splitting and other floral anomalies, seeded and seedless (parthenocarpic) fruits and undeveloped flowers and/or aborted flowers. Fruits and flowers still attached to the peduncle at harvest but not large enough for visual determination of seed content were recorded as undeveloped flowers. Aborting flowers, yellowing or partial separation in the abscission layer and peduncles without fruits or flowers were recorded as aborted flowers [29]. Pollen viability, pollen production and anther dehiscence were assessed following established protocols [12,14]. Pollen was collected from freshly-opened flowers. Before incubation in vitro , pollen grains were hydrated for 30 min over a moist filter paper in a Petri dish at 22.5°C. An appropriate amount of pollen grains were then spread onto a 30 μl liquid germination medium and placed on a microscope slide. The slide was placed on a piece of moist filter paper in a plastic dish. The dish was sealed tightly and incubated at 25 ± 1°C in the dark. A pollen grain was considered germinated when the pollen tube was equal to or larger than the grain diameter (25-30 μm).
Light microscopy: Floral buds and flowers were examined under a binocular microscope (Zeiss Stemi 2000-C with KL 1500 LCD light source, Carl Zeiss Oberkochen, Germany) and photographed with a Canon D500 SLR camera (Tokyo, Japan). Histological observations and analyses were performed on floral structures at various stages of development following fixation in formalin acetic alcohol (FAA; 5 parts formalin: 5 parts glacial acetic acid: 90 parts 50% ethanol (v/v/v)) and stored in 70% ethanol. The tissues were dehydrated through an ethanol series and then embedded in paraffin with a 58-60°C melting point for microtoming. Serial sections were cut using a rotary microtome (Spencer 820: American Optical Co, Buffalo, NY) at 5 μm in thickness, stained with Safranin-O and Fast Green FCF [30] and subsequently dehydrated through an alcohol series to absolute ethanol and mounted in DPX (BDH, Poole, UK). The samples were examined using normal bright-field optics on a Leica DM 2500 M light microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany) and photographed with a Leica DFC500 12-megapixel digital colour camera mounted on the same microscope using Leica Application Suite Software Version 4.0.0.
Data collection/analysis: The following parameters were recorded:
Electrolyte leakage (EL): Electrolyte leakage was recorded at the start of flowering following the protocol described under Environment I above. The EL score presented is the mean of four independent measurements taken on different plants in each treatment.
Number of flowers and fruits: All flowers and fruit were counted per plant throughout the growing season and the flower/fruit set ratio subsequently calculated for each genotype.
Fruit yield/plant and shoot biomass: All fruit was harvested at maturity from each plant and weighed to determine fruit yield/plant (fresh weight). After the completion of the experiment, the shoots were harvested, dried in an oven at 65°C for 48 h and weighed to determine shoot biomass (dry weight).
Agronomic superiority index (Ag Index): The materials were also assessed for their agronomic performance. An agronomic superiority index (Ag Index) or Euclidean Distance (ED) was calculated based on flower-fruit set ratio, fruit weight, plant dry weight and electrolyte leakage to estimate the agronomic superiority of a genotype [31]. ED measured the distance of a genotype from an ideal phenotypic expression in the materials evaluated; the shorter the distance the closer the genotype to the ideal phenotypic expression and vice versa. The Ag index based on EL and agronomic data was then calculated. The square of the difference between ideal and actual genotypic responses was summed for each trait and the square root calculated. The Ag Index compared a certain genotypic response with the ideal response. The materials were ranked both on the basis of their electrolyte leakage and the ED score. ED was calculated as follows:
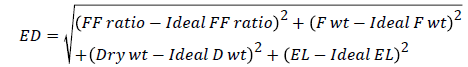
Where FF ratio is flower fruit set ratio, Fwt is the fruit weight, Dry wt is the plant dry weight, and EL is the electrolyte leakage.
Statistical analysis: Data were statistically analysed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). EL and chlorophyll fluorescence data were analysed using a one-way ANOVA procedure. Genotype and environment were considered fixed effects and replication as random effects. The differences between means were determined according to Fisher’s Least Significant Difference (LSD) test at P ≤ 0.05. The significance of correlation coefficients was evaluated using Pearson’s correlation coefficient at P ≤ 0.05.
Results
Environment 1
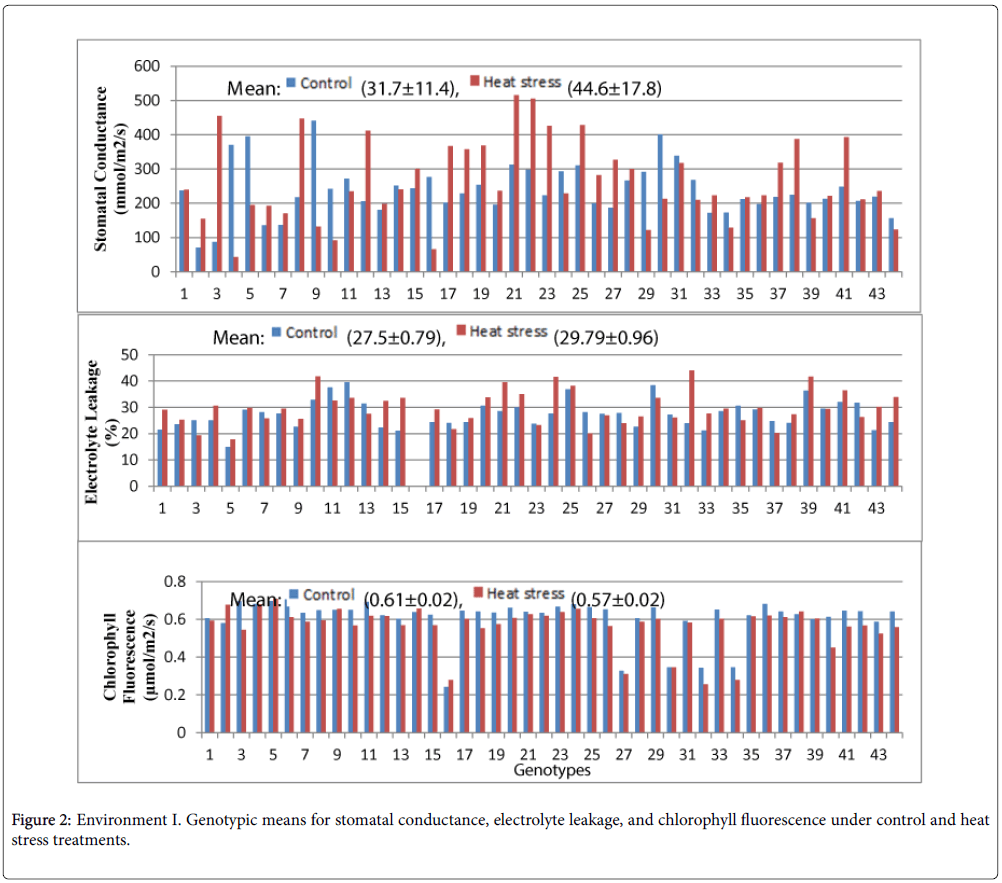
The preliminary screening of stomatal conductance, chlorophyll fluorescence and electrolyte leakage in Environment 1 is presented in Figure 2. The highest stomatal conductance in the control treatment was observed in the genotype LA3847 (440.5 mmol m-2 s-1), wheras the wild tomato LA0716 (70.95 mmol m-2 s-1) produced the lowest. The mean stomatal conductance in the control treatment was 31.7 ± 11.4 (mmol m-2 s-1). However, the highest stomatal conductance under heat stress was obseved in LA3883 (532.95 mmol m-2 s-1) and the lowest in LA2375 (42.8 mmol m-2 s-1). The mean stomatal conductance under heat stress was 44.6 ± 17.8 (mmol m-2 s-1). A relatively high standard error was recorded for stomatal conductance in both treatments.
The lowest electrolyte leakage in both the control and heat stress treatments was observed in LA2661 (14.91 and 17.78 μmhos cm-1). The mean of the control and heat stress treatments was 27.5 ± 0.79 and 29.79 ± 0.96 μmhos cm-1, respectively. The genotype with the highest electrolyte leakage in the control was LA3869 (39.52 μmhos cm-1) and under heat stress LA4235 (44.10 μmhos cm-1). The genotypic differences were well defined and the standard error was relatively low.
The highest chlorophyll fluorescence in the control was observed in LA3320 (0.706 μmol m-2s-1) and the lowest in LA3875 (0.24 μmol m-2s-1). The mean value of chlorophyll fluorescence under the control and heat stress treatment was 0.61 ± 0.02 and 0.57 ± 0.02 μmol m-2s-1, respectively (Figure 3). The highest chlorophyll fluorescence under heat stress was observed in LA4252 (0.641 μmol m-2s-1) and the lowest in LA4235 (0.257 μmol m-2 s-1). The standard errors were relatively low, although the genotypic differences were generally less significant than observed for EL (Appendix 1).
EL was used to evaluate materials in Environment II as this trait differentiated the germplasm better than the other traits assessed.
Therefore, stomatal conductance and chlorophyll fluorescence were discontinued.
Environment II
Signifcant differences among genotypes were found for flower-fruit set ratio, fruit weight, plant dry weight and electrolyte leakage (Table 2). The genotype-gnvironment (G × E) interaction was also significant for all traits except EL. The means for each trait are presented in Table 3.
| Trait | SOV | DF | MS | Prob |
|---|---|---|---|---|
| Fruit set | Entry | 23 | 20.08 | <.001 |
| Entry.Env | 6 | 12.32 | <.001 | |
| Fruit weight | Entry | 23 | 7822.6 | <.001 |
| Entry.Env | 6 | 2702.7 | <.001 | |
| Pl dry weight | Entry | 45 | 9498.28 | <.001 |
| Entry.Env | 43 | 985.06 | <.001 | |
| EL | Entry | 45 | 285.78 | < .001 |
| Entry.Env | 43 | 4.58 | 0.59 |
Table 2: Mean squares from analysis of variance for various traits in Environment II.
| Line ID | Fruit set (%)* | Fruit weight (g) | Pl dry weight (g) | EL (%) |
|---|---|---|---|---|
| LA0373 | 2.3 | 2.87 | 185.9 | 31.24 |
| LA0716 | 120.2 | 23.2 | ||
| LA1930 | 204.1 | 27.66 | ||
| LA2375 | 88.4 | 29.08 | ||
| LA2661 | 4.06 | 35 | 92.4 | 58.68 |
| LA3320 | 4.24 | 70.12 | 161.5 | 39.77 |
| LA3344 | 6.23 | 45.52 | 91.2 | 27.31 |
| LA3345 | 6.81 | 23.3 | 149.9 | 38.22 |
| LA3847 | 7.35 | 82.77 | 153.2 | 28.62 |
| LA3866 | 146.3 | 37.41 | ||
| LA3867 | 5.21 | 15.25 | 155.4 | 33.95 |
| LA3869 | 88.5 | 32.79 | ||
| LA3870 | 63.8 | 38.75 | ||
| LA3871 | 111.4 | 32.57 | ||
| LA3874 | 9.64 | 7.2 | 161.7 | 42.03 |
| LA3875 | 0.35 | 4 | 144.9 | 52.23 |
| LA3876 | 150.5 | 30.39 | ||
| LA3878 | 57 | 26.37 | ||
| LA3879 | 116 | 33.24 | ||
| LA3882 | 105.7 | 33.65 | ||
| LA3883 | 0.97 | 23.62 | 241.4 | 51.26 |
| LA3886 | 1.73 | 14.75 | 160.3 | 43.63 |
| LA3889 | 1.67 | 2 | 133.8 | 29.3 |
| LA3892 | 0.78 | 65 | 220 | 46.68 |
| LA3893 | 277.5 | 40.98 | ||
| LA3906 | 2.21 | 2.57 | 110.7 | 23.75 |
| LA4230 | 0.9 | 34.75 | 179.7 | 29.99 |
| LA4231 | 2.03 | 10.25 | 131.5 | 22.75 |
| LA4232 | 4.67 | 4.05 | 76.9 | 24.66 |
| LA4233 | 72 | 28.19 | ||
| LA4234 | 36.7 | 31.67 | ||
| LA4235 | 1.41 | 6.87 | 135.9 | 51.39 |
| LA4236 | 189.2 | 33.91 | ||
| LA4237 | 251.7 | 30.4 | ||
| LA4247 | 178.3 | 28 | ||
| LA4248 | 173.2 | 30.95 | ||
| LA4249 | 118.6 | 30.36 | ||
| LA4252 | 1.11 | 5.9 | 133 | 29.79 |
| LA4256 | 3.44 | 91 | 176.9 | 48.92 |
| LA4257 | 2.5 | 20.72 | 158 | 31.57 |
| LA4272 | 0.38 | 8.62 | 122.7 | 41 |
| LA4273 | 1.01 | 12.25 | 201.2 | 29.97 |
| LA4283 | 171.7 | 37.72 | ||
| LA4284 | 5.14 | 160.25 | 156.9 | 31.8 |
| Roma | 218.7 | 23.47 | ||
| R Grande | 91.8 | 29.64 | ||
| Mean LSD | 3.17 ± 0.50 0.95 | 31.19 ± 7.64 10.55 | 144.45 ± 7.73 1.85 | 34.50 ± 1.25 0.66 |
Table 3: Means of various traits under Environment II. *Percentage fruit set was calculated by dividing the number of fruit set by the total number of flowers.
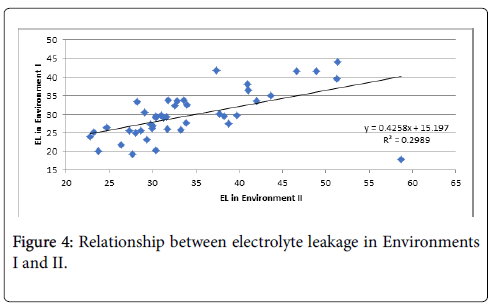
The lowest EL was found in LA4231 and the highest in LA2661. EL assessed in the growth chamber and tunnel-house were significantly correlated, although some rank changes were observed between the two environments (Figure 4).
Of the 46 genotypes exposed to high-temperature stress only 24 set fruit. The highest fruit yield of 160 g was recorded for the genotype LA4284 and the highest fruit set ratio of 9.64% for LA3874. LA3893 produced the highest above ground dry weight and LA4234 the lowest.
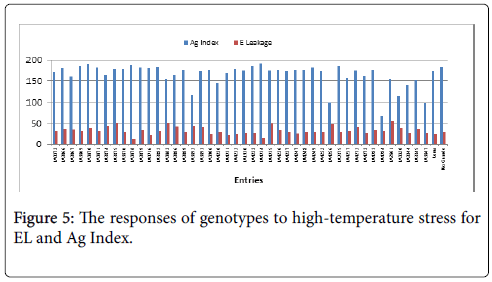
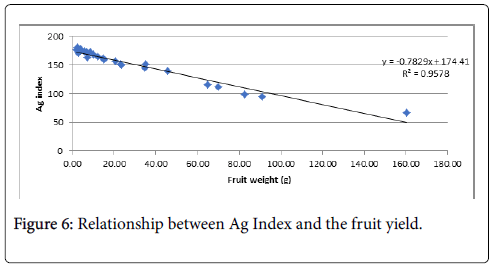
The Ag Index was then used to rank the genotypes (EL and Ag Index are presented in Figure 5). Genotypes with lower Ag Indices were agronomically superior. The lowest Ag Index was predicted for LA3878 and the highest index for LA2661. The Ag Index was very strongly correlated with fruit yield (Figure 6).
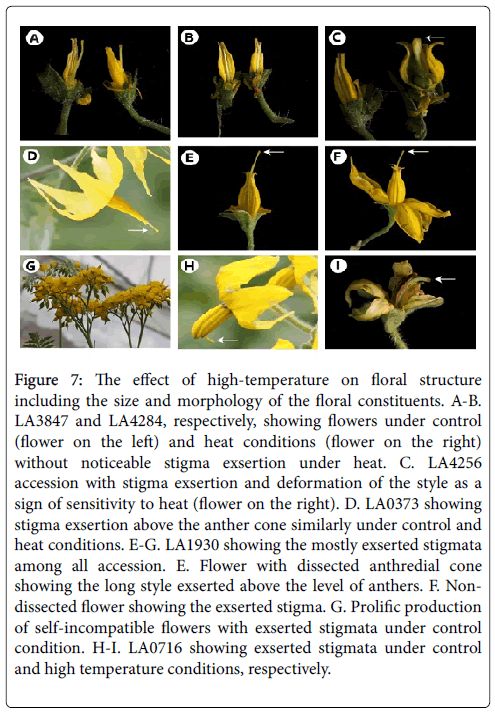
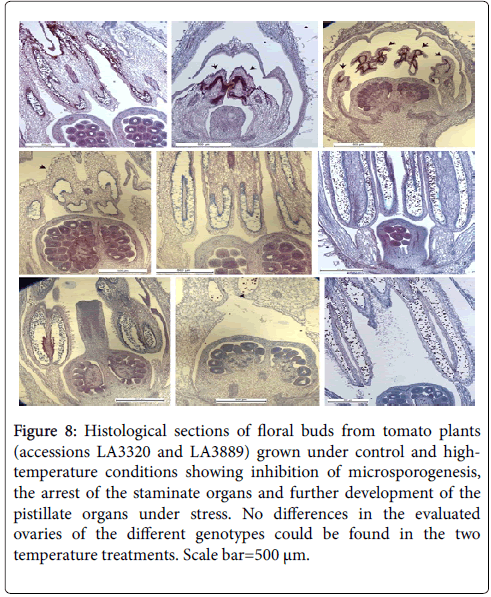
The critical heat sensitive stage was the period from meiosis to the tetrad breakup in anthers (Figure 7). The histological analysis (Figure 8) showed that heat stress induced adverse effects on male gametophyte development resulting in a reduction in the number of available pollen grains falling on the stigma. Therefore, poor pollination at flowering is likely the primary cause of sterility in tomato following exposure to high-temperature.
Figure 7: The effect of high-temperature on floral structure including the size and morphology of the floral constituents. A-B. LA3847 and LA4284, respectively, showing flowers under control (flower on the left) and heat conditions (flower on the right) without noticeable stigma exsertion under heat. C. LA4256 accession with stigma exsertion and deformation of the style as a sign of sensitivity to heat (flower on the right). D. LA0373 showing stigma exsertion above the anther cone similarly under control and heat conditions. E-G. LA1930 showing the mostly exserted stigmata among all accession. E. Flower with dissected anthredial cone showing the long style exserted above the level of anthers. F. Nondissected flower showing the exserted stigma. G. Prolific production of self-incompatible flowers with exserted stigmata under control condition. H-I. LA0716 showing exserted stigmata under control and high temperature conditions, respectively.
Figure 8: Histological sections of floral buds from tomato plants (accessions LA3320 and LA3889) grown under control and hightemperature conditions showing inhibition of microsporogenesis, the arrest of the staminate organs and further development of the pistillate organs under stress. No differences in the evaluated ovaries of the different genotypes could be found in the two temperature treatments. Scale bar=500 μm.
Discussion
Sufficient genetic diversity for EL, fruit set %, fruit yield and total plant dry weight was observed to justify tomato improvement for hightemperature stress tolerance.
In vivo chlorophyll fluorescence was reported to be a reliable method for determining temperature-induced changes in the photosynthetic apparatus of plants [15,32-34]. However, in the current study where a greater number of genotypes were assessed, EL better discriminated the materials both under control and heat stress conditions.
The current study aimed to identify materials with both hightemperature tolerance and good agronomic type. Heat tolerance potential was measured in terms of electrolyte leakage from leaf tissues in response to heat stress. However, while plants may survive heat stress, they may not be agronomically suitable for commercial production. For practical purposes the target genotype should be both high-temperature tolerant and agronomically superior.
Cell membrane function was altered by heat stress in the current study and electrolyte efflux significantly increased in the sensitive genotypes (Figure 2). Alsadon [35] classified the heat stress tolerance of some tomato cultivars in vitro into three groups: heat tolerant, moderately heat tolerant and heat sensitive. Similar groupings were observed in the current study. EL under heat stress was useful for classifying genotypes and was first suggested as a selection crierion for increasing heat stress tolerance by Blum [36].
An increase in electrolyte leakage was observed in stress sensitive accessions indicating increased permeability of cell membranes and reduced ability to retain solutes and water. Similar changes to membrane function under stress were observed in a range of plant species [37,38].
The EL score in environments I and II was correlated (R2=0.30) showing that the method is effective and stable across phenotyping conditions. Selection under controlled conditions in the growth room can be used in plant breeding to develop high-temperature tolerant genotypes for the poly-house and possibly the field. The inactivation of photosynthesis by high temperature is a function of membrane damage. Blum and Ebercon [39] reported that membrane disintegration is the primary symptom of heat injury and thermostability of the plasmalemma was later proposed as an effective indicator of thermo-tolerance in plants [40,41]. This can be selected indirectly using EL.
Earlier reports suggested that selection for slow leaf EL under heat stress could assist the development of heat resistant plants [36,42]. Thiaw and Hall [43] reported that a strong association between ability to set fruit under high temperature and the maintenance of membrane function may indicate pleiotropy. However, this hypothesis has never been confirmed and reduced fruit set is likely a physiological consequence of membrane damage.
Fruit and/or seed set is particularly sensitive to high temperature in several other warm season crops including cotton, sorghum, rice and common bean [44-47]. The current study confirmed that this stage of development is equally sensitive in tomato.
The very strong correlation (R2=0.96) observed between fruit yield and the Ag Index indicates that the Ag Index was an effective method for selecting genotypes with productivity under heat stress. Two genotypes, LA4284 and LA3847, identified as highly desirable based on the Ag Index had relatively low EL values and were therefore classified as suitable for future genetic improvement.
The effect of heat stress on the productivity of the tomato, including the wild species, was greatest at pollen development. Heat stress caused reduced pollen release and impaired pollen function; an observation similar to earlier. Successful pollination requires pollen hydration, germination, tube penetration, stigma tube elongation through the style, tube entry into the ovule and the release of two sperm nuclei into the embryo sac, resulting in double fertilization. This process was clearly disrupted in the current study.
This study also confirmed that certain developmental stages within the flower were more sensitive to high temperature. Pollen viability can be used as an indicator of fertility in tomato plants exposed to hightemperature as viability reduced significantly in the heat treatment.
Stigma exsertion also negatively impacted fruit setting, especially in heat sensitive accessions where stigma exsertion was observed to be more common (Figure 7). Excessive elongation of the styles within most flowers of heat sensitive genotypes minimized pollen access to the stigmas and reduced fertilization and normal fruit setting.
Wild relatives and landraces are potential sources of new genetic variation for new and known traits. This variation could be vital for maintaining long-term genetic gains in tomato. While the mechanisms controlling heat stress response in tomato are complex, inheritance studies suggest that major genes confer thermo-tolerance during reproductive development. If this is confirmed then the high temperature tolerance of tomato can be improved in the short to medium term.
Nevertheless, additional studies are required to precisely identify mechanisms of heat tolerance during reproductive development and source/sink interactions. Increasing climate variability necessitates the development of more efficient methods of germplasm screening and selection for high temperature stress tolerance.
Conclusions
Genetic variability exists in the tomato germplam for heat tolerance. Combining heat tolerance and agronomic vigour is possible as no negative correlation between stress tolerance and agronomic type was observed. Three types of genetic materials were identified; i) those that possess heat tolerance but lack agronomic vigour, ii) those that possess agronomic vigour but lack heat tolerance and iii) those that possess heat tolerance and agronomic vigour. All three types have utility in breeding depending upon the circumstances and the traits required.
EL was a reliable trait for differentiating heat tolerant and sensitive genotypes. Flower-fruit set ratio, fruit yield and plant dry matter were key traits that can be used in conjunction with EL to select genotypes suitable for heat stressed environments.
Results confirmed earlier findings that the reproductive phase in tomato is more sensitive to high temperature than vegetative growth and that the adverse effects of extreme temperatures on fruit set primarily impact the male gametophyte (microsporogenesis). Stigma exsertion in heat sensitive genotypes also contributed to low fruit setting.
Acknowledgments
Authors acknowledge the contributions made by the staff and students of the Plant Breeding Institute, University of Sydney for their help during these studies. Authors also thankfully acknowledge the contributions made by the Histopathology Laboratory, University of Sydney, for allowing the histological work to be done in the laboratory; TGRC, UC Davis for providing the tomato germplasm; and ACIAR vegetables project HORT/2012/002 for supporting the heat stress research on tomatoes.
Conflict of Interest
The authors declare no conflicts of interest.
References
- ABS (2014) Agricultural Commodities, Australian Bureau of Statistics, Canberra, Australia.
- FAOSTAT (2013) Production statistics. Food and Agriculture Organisation of the United Nations, Rome, Italy.
- Ahmadi ABE, Stevens MA (1979) Reproductive responses of heat-tolerant tomatoes to high temperature. Journal of the American Society for Horticultural Science 104: 686-691.
- Reddy KR, Kakani VG (2007) Screening Capsicum species of different origins for high temperature tolerance by in vitro pollen germination and pollen tube length. Scientia Horticulturae 112: 130-135.
- Stainforth DA, Aina T, Christensen C, Collins M, Faull N, et al. (2005) Uncertainty in predictions of the climate response to rising levels of greenhouse gases. Nature 433: 403-406.
- Bartsur A, Rudich J, Bravdo B (1985) High-temperature effects on CO2 gas-exchange in heat-tolerant and sensitive tomatoes. Journal of the American Society for Horticultural Science 110: 582-586.
- El Ahmadi AB, Stevens MA (1979) Reproductive responses of heat-tolerant tomatoes to high temperatures. Journal-American Society for Horticultural Science.
- Lohar D, Peat W (1998) Floral characteristics of heat-tolerant and heat-sensitive tomato (Lycopersicon esculentum Mill.) cultivars at high temperature. Scientia horticulturae 73: 53-60.
- Stevens M, Rudich J (1978) Genetic potential for overcoming physiological limitations on adaptability, yield, and quality in the tomato. Hort Science.
- Dinar M, Rudich J (1985) Effect of heat-stress on assimilate partitioning in tomato. Annals of Botany 56: 239-248.
- Peet M, Sato S, Gardner R (1998) Comparing heat stress effects on male‐fertile and male‐sterile tomatoes. Plant, Cell & Environment 21: 225-231.
- Sato S, Peet M, Thomas J (2000) Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant, Cell & Environment 23: 719-726.
- Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H (2006) Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Annals of Botany 97: 731-738.
- Sato S, Peet MM, Thomas JF (2002) Determining critical pre and post-anthesis periods and physiological processes in Lycopersicon esculentum Mill exposed to moderately elevated temperatures. Journal of Experimental Botany 53: 1187-1195.
- Camejo D, Rodriguez P, Morales A, Dell'Amico JM, Torrecillas A, et al. (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. Journal of Plant Physiology 162: 281-289.
- Abdul-Baki AA, Stommel JR (1995) Pollen Viability and Fruit Set of Tomato Genotypes under Optimumand High-temperature Regimes. Hort Science 30: 115-117.
- Al-Khatib K, Paulsen GM (1999) High-temperature effects on photosynthetic processes in temperate and tropical cereals. Crop Science 39: 119-125.
- Hall AE, Ziska LH (2000) Crop breeding strategies for the 21st century. Climate Change and Global Crop Productivity, pp: 407-423.
- Pressman E, Peet MM, Pharr DM (2002) The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Annals of Botany 90: 631-636.
- Bhadula SK, Sawhney VK (1989) Amylolytic Activity and Carbohydrate Levels During the Stamen Ontogeny of a Male Fertile and a ‘Gibberellin-Sensitive’ Male Sterile Mutant of Tomato (Lycopersicon esculentum). Journal of Experimental Botany 40: 789-794.
- Salvucci ME, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiology 134: 1460-1470.
- Larkindale J, Hall JD, Knight MR, Vierling E (2005) Heat stress phenotypes of arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology 138 882-897.
- Song JJ, Nada K, Tachibana S (2002) Suppression of S-adenosylmethionine decarboxylase activity is a major cause for high-temperature inhibition of pollen germination and tube growth in tomato (Lycopersicon esculentum Mill.). Plant and Cell Physiology 43: 619-627.
- Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. The plant cell 7: 1099-1111.
- Chen TH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current opinion in plant biology 5: 250-257.
- Rhodes D, Hanson A (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annual review of plant biology 44: 357-384.
- Acatrinei L (2010) Photosynthesis rate, transpiration and stomatal conductance of vegetable species in protected organic crops. Lucrări ùtiinĠifice, Seria Agronomi, Iasi 53: 32-35.
- Lafuente MT, Belver A, Guye MG, Saltveit ME (1991) Effect of temperature conditioning on chilling injury of cucumber cotyledons. Possible role of abscisic acid and heat shock proteins. Plant Physiology 95: 443-449.
- Sato S, Peet MM, Gardner RG (2004) Altered flower retention and developmental patterns in nine tomato cultivars under elevated temperature. Scientia Horticulturae 101: 95-101.
- Sass JE (1958) Botanical microtechnique. The Iowa State College Press, Iowa, USA, p: 228.
- Gomez-Pando R, Jimenez-Davalos J, Eguiluz-de la BA, Aguilar-Castellanos E, Falconí-Palomino J, et al. (2009) Field performance of new in vitro androgenesis-derived double haploids of barley. Euphytica 166: 269-276.
- Govindjee (1995) 63 years since Kautsky-chlorophyll-A fluorescence. Australian Journal of Plant Physiology 22: 131-160.
- Krause G, Weis E (1991) Chlorophyll fluorescence and photosynthesis: The bacics. Annu Rev Plant Physiol Plant Mol Biol 42: 313-349.
- Strasser BJ (1997) Donor side capacity of photosystem II probed by chlorophyll a fluorescence transients. Photosynthesis Research 52: 147-155.
- Alsadon A, Wahb-allah M, Khalil S (2006) In vitro evaluation of heat stress tolerance in some tomato cultivars. Journal of King Saud University 19: 13-24.
- Blum A (1988) Plant breeding for stress environments. CRC Press, Florida, USA, p: 223.
- Chengkun H, Suzhi G, Jiasen L (1996) Effect of drought stress on activated oxygen metabolism in tomato. J Fujian Agr Univ 25: 307-311.
- Karim Z, Chambrey R, Chalumeau C, Defontaine N, Warnock D, et al. (1999) Regulation by PKC isoforms of Na+/H+ exchanger in luminal membrane vesicles isolated from cortical tubules. American Journal of Physiology-Renal Physiology 277: F773-F778.
- Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Science 21: 43-47.
- Ristic Z, Williams G, Yang G, Martin B, Fullerton S (1996) Dehydration, damage to cellular membranes, and heat-shock proteins in maize hybrids from different climates. Journal of plant physiology 149: 424-432.
- Shanahan J, Edwards I, Quick J, Fenwick J (1990) Membrane thermostability and heat tolerance of spring wheat. Crop Science 30: 247-251.
- Blum A, Klueva N, Nguyen H (2001) Wheat cellular thermotolerance is related to yield under heat stress. Euphytica 117: 117-123.
- Thiaw S, Hall AE (2004) Comparison of selection for either leaf-electrolyte-leakage or pod set in enhancing heat tolerance and grain yield of cowpea. Field Crops Research 86: 239-253.
- Gipson J, Joham H (1968) Influence of night temperature on growth and development of cotton (Gossypium birsutum L.). I. Fruiting and boll development. Agronomy Journal 60: 292-295.
- Eastin J, Castleberry R, Gerik T, Hultquist J, Mahalakshmi V, et al. (1983) Physiological aspects of high temperature and water stress. Crop reactions to water and temperature stresses in humid temperate climates. Westview Press, Boulder.
- Mohammed AR, Tarpley L (2010) Effects of high night temperature and spikelet position on yield-related parameters of rice (Oryza sativa L.) plants. European Journal of Agronomy 33: 117-123.
- Konsens I, Ofir M, Kigel J (1991) The effect of temperature on the production and abscission of flowers and pods in snap bean (Phaseolus vulgaris L.). Annals of Botany 67: 391-399.
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 5837
- [From(publication date):
August-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 4832
- PDF downloads : 1005