Identification of Chemical Structures of Hypolipidemic Proanthocyanidins in Red Rice Enhancing Acyl CoA Oxidase Expression
Received: 09-Apr-2020 / Accepted Date: 24-Apr-2020 / Published Date: 05-May-2020 DOI: 10.4172/2375-4338.1000213
Abstract
Steamed red rice has been served in local religious ceremonies in Japan and is mainly consumed in Asian countries. However, only a few detailed chemical and biological studies have been performed on red rice to date. The present study aimed to clarify the chemical structure of red pigment and examined its effects on lipid metabolism, such as hepatic β-oxidation, in mice. The results obtained showed that red pigment up-regulated the mRNA expression of enzymes involved in lipid metabolism, such as acyl CoA oxidase 1 (ACOX-1), peroxisome proliferator-activated receptor β (PPAR β), and AMP-activated protein kinase (AMPK), which decompose fatty acids. Furthermore, the redcolored extract was fractionated by ACOX-1-enhancing activity to provide the proanthocyanidins involved in cytosolic β-oxidation. Spectroscopic data on proanthocyanidins revealed detailed characteristic structures, including 3- to 10- mer polymerization, a 3,4-cis configuration, more C4-C8 than C4-C6 linkages, and the existence of a B-ring bearing three hydroxy groups with/without a methyl moiety. In conclusion, red rice bran comprising proanthocyanidins has potential in the treatment of fatty liver and hyperlipidemia.
Keywords: Red rice bran; Proanthocyanidin; β -oxidation; ACOX-1
Introduction
Pigmented rice, such as purple and red rice, has been served in religious events from ancient times in Japan. Colored rice, called ancient rice, has been served as nutritious food for longevity or as celebration dishes in traditional ceremonies. In Asian countries, color rice is a popular ingredient in sticky rice. These colors are due to the presence of polyphenolic components[1-3]which exhibit a wide range of bioactivities[4-7]. Ice pigment exerts beneficial effects on health[8]and have been applied to dietary supplements and processed foods[9].Anthocyanins have been detected in black rice and identified as pigment constituents[10,11]. In contrast,color compounds in red rice have not yet been identified. A relationship was previously reported between color concentrations and phenolic polymerization[12].Furthermore, red rice extract has been shown to exhibit many biological activities, including glucosidase inhibitoryantiatherosclerotic, hepatoprotective, anti-inflammatory, and skin protective activitiesas well as anti-carcinogenic activities against breast cancer cells,18) hepatocarcinomaand lung adenocarcinoma.Among these activities, proanthocyanidins contribute to glucosidase inhibitory activity and anticarcinogenic activities[13-20].Therefore, the color source in red rice and its contribution to the biological activity of this rice are of interest. However, the effect of red rice proanthocyanidins on hepatic lipid metabolisnm has not been reported yet. To clarify these objects, we herein examined the hypolipidemic effects of red rice and its constituents and performed a structural analysis of the proanthocyanidins present in red rice.
Materials And Methods
Preparation of a red-colored extract from red rice
Dried red rice bran (500 g) obtained from Sichuan Xiangzhen Enterprise Co., Ltd. (China) was extracted with acetone (500 mL) at room temperature for 30 min three times. Acetone was removed from the extracts gathered under reduced pressure. The resultant residue was suspended in H2O (ca. 100 mL) and then washed with n-hexane (300 mL) three times. The H2O layer was charged on an ODS (300 g) column bed and then sequentially eluted with H2O (2 L) and acetonitrile (1.5 L). Elution by acetonitrile was concentrated under reduced pressure and dried, giving a red-colored extract (10.3 g).
Animals and cells
Animal experiments were performed in accordance with the Guidelines for Animal Experimentation (Japan Association for Laboratory Animal Science, 1987).All animal experiments were approved by the Ethics Committee of Oryza Oil & Fat Chemical Co., Ltd.. Ten-week-old male ddY mice were obtained from Japan SLC (Hamamatsu, Japan). Mice were housed in a plastic cage and fed the CE-2 (CLEA Japan Inc., Tokyo, Japan) diet and tap water. The HepG2 cell line was obtained from the JCRB Cell Bank (Tokyo, Japan).
High-fat diet-induced hypertriglycemia in mice
After acclimatization, mice were randomly divided into two groups: control (n = 6) and 200 mg/kg red-colored extract (n = 6). They were housed with free access to a high-fat diet (High Fat Diet 32, CLEA Japan, Inc.) and water. Each sample was suspended in 5% aqueous gum arabic and orally administered once a day for 12 days. After 12 hr of fasting, body weight was measured. Mice were sacrificed under anesthesia, and blood, liver, and abdominal fat were removed. Triglyceride (TG) and cholesterol levels in serum and the liver and blood glucose levels were measured using the Triglyceride E test Wako(FUJIFILM, Tokyo, Japan), Cholesterol E-test Wako, and GlucoseCII test Wako, respectively.
Real-time quantitative polymerase chain reaction (PCR) analysis of HepG2 liver cells
HepG2 cells were seeded on 24-well plates at a density of 4×104 cells/0.5 mL/well in Dulbecco’s Modified Eagle Medium (DMEM, FUJIFILM) supplemented with 10% fetal bovine serum (FBS, Invitrogen, USA). After 24 hr, the corresponding concentration of sample solution in dimethyl sulfoxide (DMSO) 50 μL (1% DMSO as the final conc.) was added, then incubated for another 20 hr. After washing cells with phosphate-buffered saline (PBS), total RNA was extracted using the RNAspin mini RNA isolation kit (GE Healthcare, USA) according to the manufacturer’s protocol. Regarding cDNA synthesis, total RNA solution (0.1 μg/20 μL) was treated with 10 mM dNTP mixture with a random hexamer (2 μL) at 65°C for 5 min and then cooled on ice. This was followed by the addition of 5× PrimeScript buffer (Takara Bio Inc., Japan) (6 μL), an RNAase inhibitor (Invitrogen) (0.5 μL), and PrimeScript (Takara Bio Inc.) (1 μL), and successive incubations at 25°C for 10 min, 42°C for 50 min, and 72 °C for 15 min provided cDNA. Quantitative PCR amplification was performed using SYBR Premix Ex Taq (Takara Bio Inc.) and the Thermal Cycler Dice Real Time System (Takara Bio Inc.). PCR reactions were performed for initial denaturation at 95°C for 10 sec, for 40 cycles at 95°C for 10 sec, and at 60°C for 30 sec, and for final dissociation at 95°C for 15 sec, at 60°C for 30 sec, and at 95°C for 15 sec. Data were analyzed using the comparative CT method with β-actin as the internal control. The sequences of specific primers were as follows:
acox1: forward 5’- actgcctatgccttccagtttgtgggcg -3’, reverse 5’-gagcttaactaccacatagtggcaatgt-3’
pparα: forward 5’- ccaacatggtgaaaccctgtctc -3’, reverse 5’- aagaacaccaccattcccacaga -3’
ampk: forward 5’- gagcccatagtaaccagccagcttggca -3’, reverse 5’- tggaaatgatgtgtggcctcagtccctg -3’
cpt1a: forward 5’- ggagaggagacagacaccatcca -3’, reverse 5’- caaaataggcctgacgacacctg -3’
β-actin: forward 5’- catcctcaccctgaagtaccccatcgag -3’, reverse 5’- acaggactccatgcccaggaaggaaggc -3’
Fractionation procedure from the red-colored extract from red rice
The red-colored extract from red rice (1g) was dissolved in MeOHH 2O (1:1, 10 mL) and then charged on a Toyopearl HW-40F (Tosoh Corporation) (70g) column bed. Sequential elutions by MeOH-H2O (1:1, 300 mL) and MeOH-H2O-acetone (1:1:1, 500 mL) gave four fractions (Fr. 1: 210 mg, Fr. 2: 350 mg, Fr. 3: 90 mg, and Fr. 4: 310 mg) that were separated by UV absorbance (λ=280 nm).Fr. 2 (30 mg) was subjected to normal phase HPLC (column: Cosmosil 5SL-II (10 mm i.d.×250 mm) (Nacalai Tesque), mobile phase: A; CH2Cl2-MeOH-H2Oformic acid (5:43:1:1), B; CH2Cl2-MeOH-H2O-formic acid (47:7:1:1), linear gradient A 10% (0 min)-40% (60 min)-100% (65 min)-100% (70 min), detection: UV (λ=280 nm), flow rate: 4 mL/min]to provide two fractions (Fr. 2-A: 9.7 mg, Fr. 2-B: 20.2 mg).
Spectroscopic analysis of Fr. 2-A
Nuclear magnetic resonance (NMR) spectra were measured by Varian Inova 600 (Agilent Technologies, Inc.) and samples were dissolved in aceteone-d6 with 0.05% tetramethylsilane as the internal standard. Matrixassisted laser desorption ionization (MALDI) - time of flight (TOF) - mass spectra (MS) were measured by REFLEX II (Bruker). Measurements were performed in the positive ion mode and reflector mode.
Statistical analysis
Results are expressed as the mean and S.E. The significance of differences was examined by a one-way ANOVA, followed by Dunnett’s test, and p<0.05 was considered to be significant.
Results and Discussion
Red-colored extract from red rice
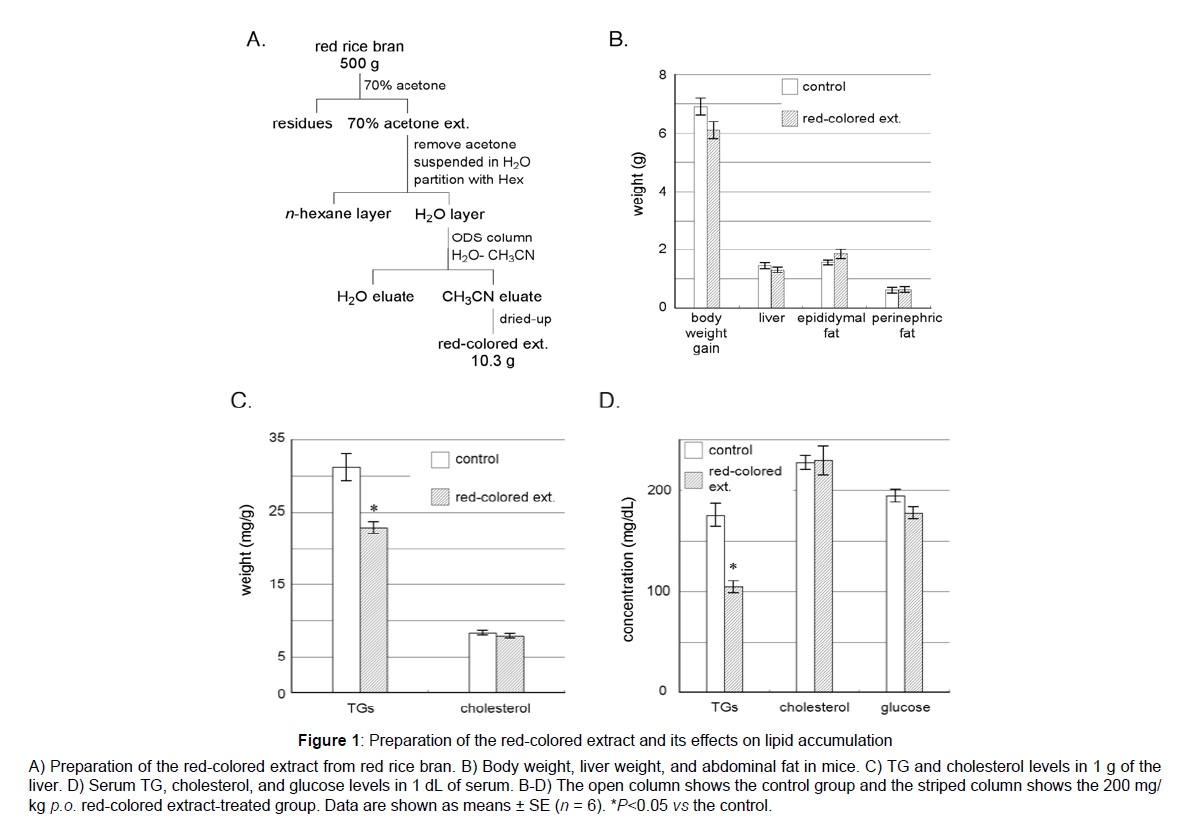
Red rice has frequently been used in traditional rites and festivals in Japan because its distinctive red color is suitable for celebrations. The source of the red color was previously proposed to be proanthocyanidins belonging to polyphenols[1],which have been suggested to suppress excessive fat accumulation leading to obesity, fatty liver, and hyperlipidemia[21-23].Thus, we attempted to elucidate the chemical structures of proanthocyanidins in red rice and their effects on lipid metabolism. Chinese red rice was extracted with 70% aqueous acetone, which has been used in the extraction of proanthocyanidins[24], at room temperature. After the removal of acetone from the extract under reduced pressure, the wet residue obtained was diluted with H2O and partitioned with n-hexane for de-fatting. The resultant H2O layer was applied to ODS column chromatography. After washing ODS with H2O, acetonitrile was added. Then acetonitrile eluent was completely dried under decompression to afford 10.3 g of a red-colored extract from 500 g of red riceFigure 1.
Figure 1: Preparation of the red-colored extract and its effects on lipid accumulation
A) Preparation of the red-colored extract from red rice bran. B) Body weight, liver weight, and abdominal fat in mice. C) TG and cholesterol levels in 1 g of the
liver. D) Serum TG, cholesterol, and glucose levels in 1 dL of serum. B-D) The open column shows the control group and the striped column shows the 200 mg/
kg p.o. red-colored extract-treated group. Data are shown as means ± SE (n = 6). *P<0.05 vs the control.
Effects of the red-colored extract on lipid metabolism parameters
The effects of the red-colored extract on lipid parameters in highfat diet-fed mice were examined. As shown in Fig. 1B, liver weight and the amount of fat around the epididymis and kidneys did not significantly differ between the control and red-colored extract-treated groups, whereas body weight was slightly lower in the red-colored extract-treated group (Fig. 1B). Thus the red-colored extract was found not to possessanti-obese effect which had been reported in apple proanthocyanidins[23].G levels in the liver were significantly decreased by the red-colored extract treatment, whereas cholesterol levels were not Figure 1C. Furthermore, TG levels in serum were markedly lower in the red-colored extract-treated group than in the control group, whereas no significant differences were observed in cholesterol or glucose levels between the two groups Figure 1C. The red pigment in red rice effectively decreased TG levels in serum and suppressed TG accumulation in the liver, butdid not reduce the amount of fat around the epididymis and kidney or decrease cholesterol levels in serum or the liver. From these results, the red colored extract was suggested to improve hepatic lipid metabolism in diet-induced hyperlipidemia in mice.Thus, the red-colored extract from red rice may be useful for preventing liver steatosis and fatty liver and attenuating hyperlipidemia.
Effects of the red-colored extract on the expression of enzymes for lipid metabolism
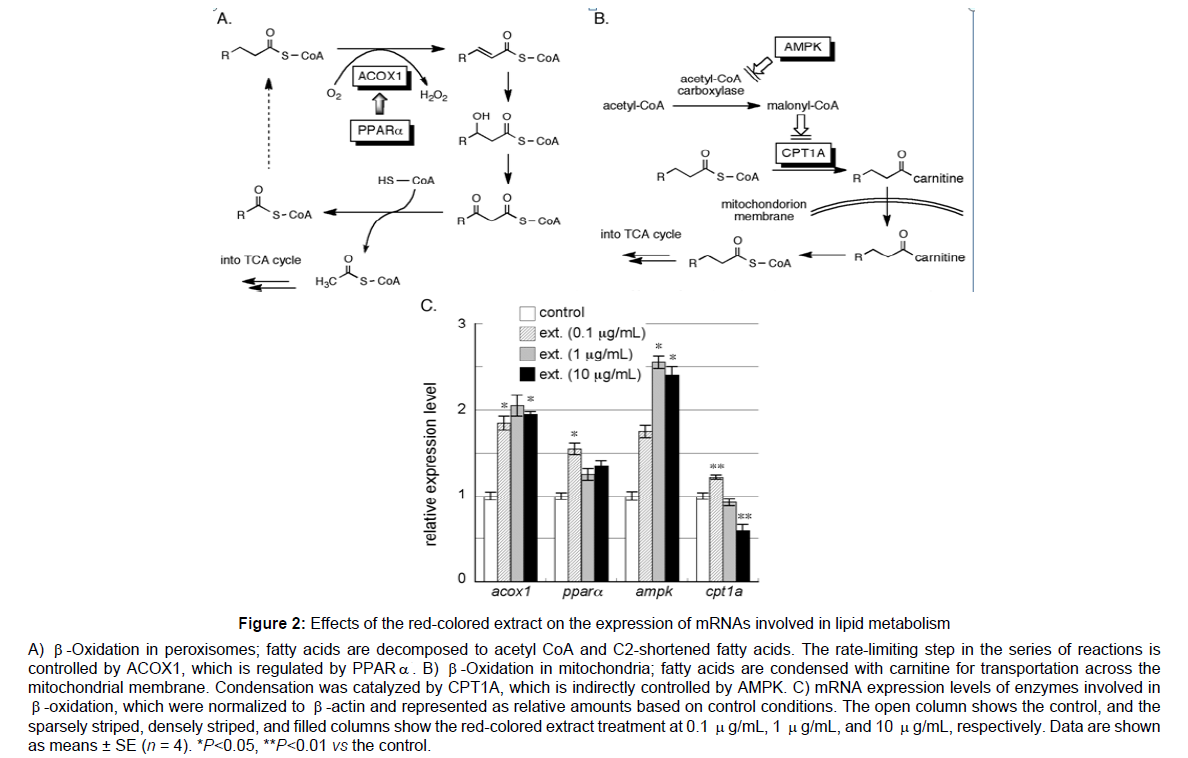
To elucidate the mechanisms by which the red-colored extract from red rice reduced TG levels, we examined changes in the expression of genes involved in the metabolism of fatty acids. β-Oxidation, one of the principal metabolic pathways for fatty acids, mainly occurs in peroxisomes and mitochondria, but through different mechanisms. In peroxisomes, fatty acids are initially converted to the corresponding acyl CoA and then oxidized by acyl CoA oxidase 1 (ACOX1) to form unsaturated bonds. The introduction of a hydroxyl group followed by oxidization yields β-keto acyl CoA, which ultimately renders acetyl CoA and C2-shortened acyl CoA. The resultant acetyl CoA is utilized in the TCA cycle to generate energy and C2-shortened acyl CoA is applied to the same cycle. ACOX1 is the rate limiting step in this series of reactions and is positively controlled by peroxisome proliferatoractivated receptor α (PPARα). The up-regulation of ACOX1 and/or PPARα enhances β-oxidation and activates lipid metabolism Figure 2A. In mitochondria, fatty acids must be condensed with carnitine by carnitine-acyl transferase 1A (CPT1A), which is the rate-determining step in β-oxidation, for transportation across the mitochondrial membrane. Moreover, CPT1A is negatively controlled by malonyl CoA, the production of which is altered by adenosine monophosphateactivated protein kinase (AMPK). The up-regulation of AMPK induces a decrease in malonyl CoA, which increases CPT1A, thereby accelerating lipid metabolism Figure 2B. Therefore, the expression levels of mRNAs encoding ACOX1, PPARα, AMPK, and CPT1A were examined. Pre-cultured HepG2 cells, a cell line of human hepatic carcinoma, were treated with the red-colored extract for 20 hr and total RNA was extracted after lysis. cDNA was prepared from total RNA by reverse transcription, and then subjected to quantitative real-time PCR to assess mRNA levels. As shown in Figure 2C, the red-colored extract up-regulated the expression of ACOX1, PPARα, and AMPK, but not CPT1A. Therefore, the red pigment in red rice was suggested to promote the β-oxidation of fatty acids mainly in peroxisomes. In regard to AMPK, apple proanthocyanidins enhance phosphorylation of AMPK in differentiated adipocytes leading adipose fat reduction [23]. Similar to our result, pine bark proanthocyanidins enhance AMPK activity and PPARα expression in obese mice. Reports regarding proanthocyanidins have been published on CPT1 expression. However proanthocyanin B2, dimer of catechin, in grape seeds enhances hepatic CPT1 expression in obese mice[24] In our experiment, no significant differences were observed in CPT1A levels between the control and high concentration ofred-colored extract-treated groups despite the higher level of AMPK, and this may have been due to the presence of other factors controlling the expression of CPT1A.Instead of CPT1, ACOX1 expression was clearly enhanced by the red-colored extract.
Figure 2: Effects of the red-colored extract on the expression of mRNAs involved in lipid metabolism
A) β-Oxidation in peroxisomes; fatty acids are decomposed to acetyl CoA and C2-shortened fatty acids. The rate-limiting step in the series of reactions is
controlled by ACOX1, which is regulated by PPAR. B) β-Oxidation in mitochondria; fatty acids are condensed with carnitine for transportation across the
mitochondrial membrane. Condensation was catalyzed by CPT1A, which is indirectly controlled by AMPK. C) mRNA expression levels of enzymes involved in
β-oxidation, which were normalized to β-actin and represented as relative amounts based on control conditions. The open column shows the control, and the
sparsely striped, densely striped, and filled columns show the red-colored extract treatment at 0.1 μg/mL, 1 μg/mL, and 10 μg/mL, respectively. Data are shown
as means ± SE (n = 4). *P<0.05, **P<0.01 vs the control.
Proanthocyanin B1 has been reported to enhance mRNA expression of ACOX1 in TSOD obese mice[25]. However, our result that the redcolored extract enhances ACOX1 is seemed first observation.
Separation of fractions increasing ACOX1 levels from the red-colored extract
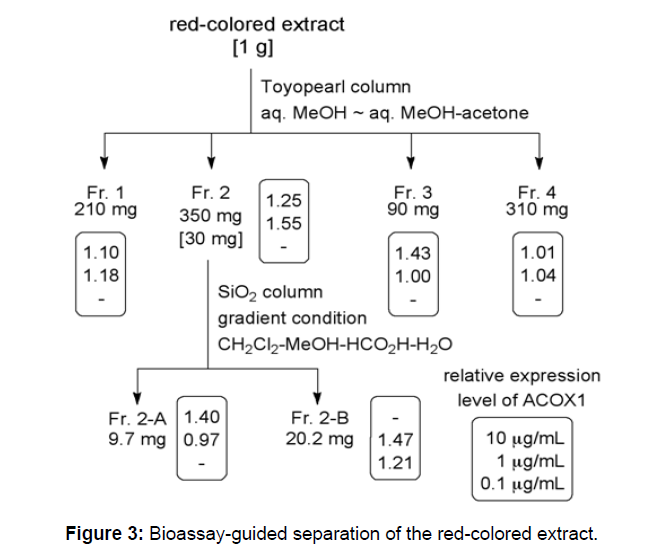
As described above, the red-colored extract prepared from red rice bran up-regulated the mRNA expression of ACOX1. Therefore, we searched for the active fraction increasing ACOX1 using bioassayguided separation. A solution of the red-colored extract in 50% aqueous MeOH was fractionated by size-exclusion chromatography using Toyopearl with 50% aqueous MeOH and a mixture of 50% aqueous MeOH-acetone as elution solvents to provide four fractions, Fr. 1-4. The second fraction, Fr. 2, which exhibited the strongest activity among the four fractions, was subjected to further separation by normal phase HPLC with the linear gradient CH2Cl2-MeOH-HCOOH-H2O solvent system[26] as the mobile phase to give two fractions, Fr. 2-A and 2-B. Both of these fractions up-regulated the expression of ACOX1 in a concentration-dependent manner Figure 3. The activity of Fr. 2-B was stronger than Fr. 2-A, because Fr. 2-B enhanced ACOX1 expression at 0.1 μg/mL.
Structural analysis of the active fraction increasing ACOX1 levels
1H NMR spectra of the fraction Fr. 2-A with fat metabolizing activity showed broad signals at 6.3~7.3 ppm (suggesting aromatic protons), at 5.5~6.0 ppm (oxymethine protons binds to the aromatic ring), and at 3.5~4.8 ppm (protons on the carbons binding to oxygen or aromatic ring). Furthermore, signals were rarely observed around 1.2 ppm, indicating alkyl protons; therefore, most of the components of Fr. 2-A were presumed to be proanthocyanidins Figure 4. The components of Fr. 2-B were also suggested to mainly be proanthocyanidins, but with contamination by other compounds because this fraction was obtained from an even broader range of elution. Therefore, the structural analysis of proanthocyanidins in red rice was performed using Fr. 2-A.
Figure 4: Gross structures of proanthocyanidins. The typical basic structure of proanthocyanidins in Fraction 2A it is shown. Some of the linkages between catechin units are presumed to be the C4-C6 connection. This structure may contain B-rings bearing hydroxy and methoxy moieties. The relative configuration of C2-C3 is shown as cis, epicatechin type, because epicatechins are mostly found in the catechin family in nature.
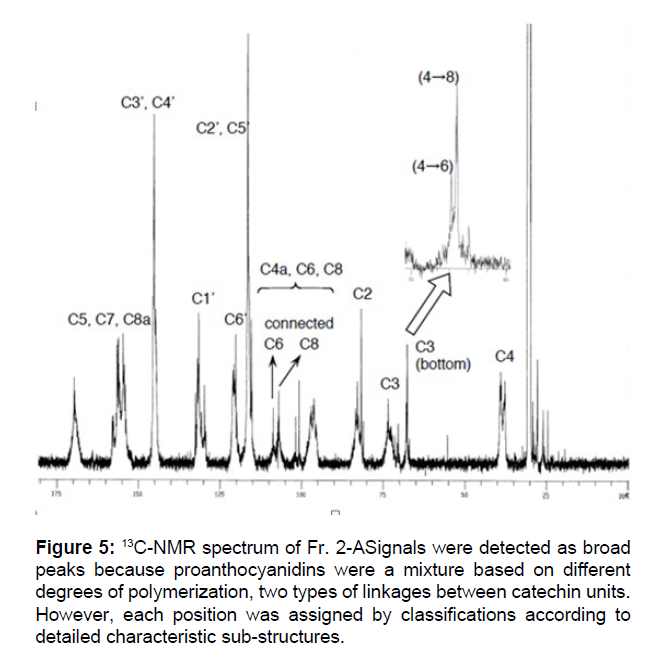
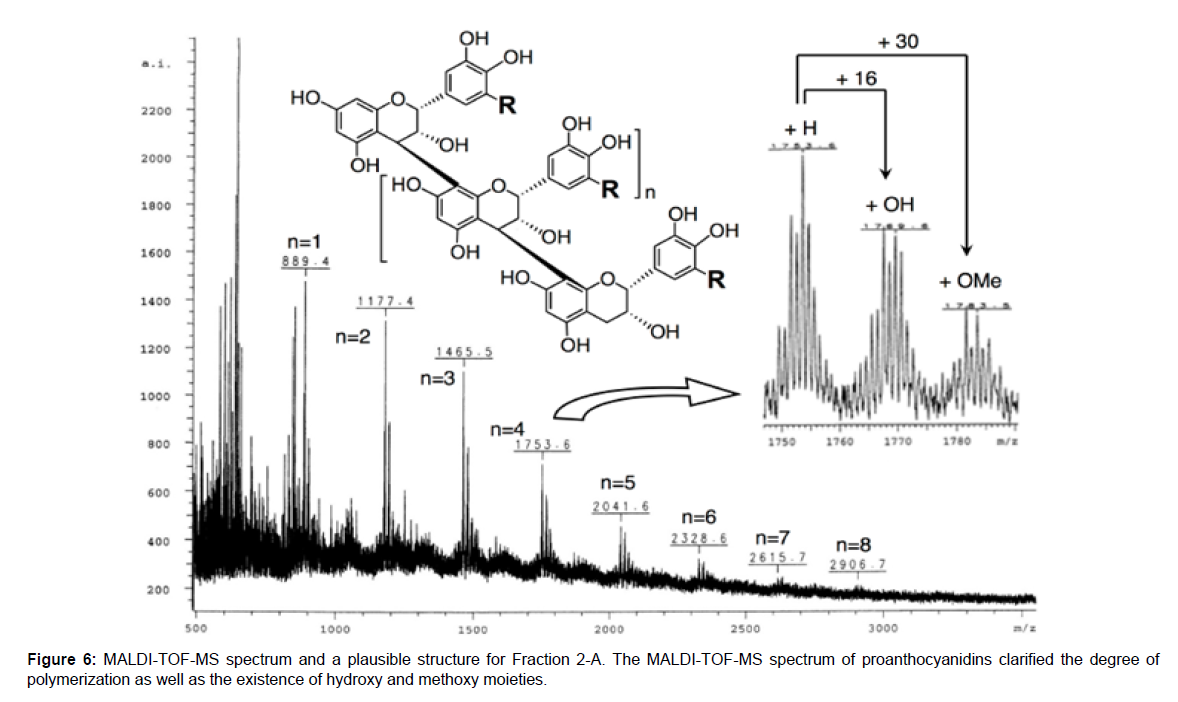
The 13C NMR and MALDI-TOF-MS of Fr. 2-A were performed to elucidate structures in more detail. Several broad signals were detected in the 13C NMR spectrum shown in Figure 5, which were assigned in accordance with previously reported data[27-30].Peaks were detected around 150 ppm, meaning aromatic carbons binding oxygen, and 90~130 ppm, meaning other aromatic carbons. The C-2 and C-3 positions were found at 82~84 ppm and 73~75 ppm, respectively, and the C-4 position of the bottom units was observed at 37~40 ppm. The relative configurations of C3-C4 were suggested to be trans because signals at 79~80 ppm, which were derived from the C-2 position of the 3,4-cis configuration, were rarely observed. On the other hand, there may be two different connection patterns between the catechin units, C4- C6 and C4-C8. At around 110 ppm, C-6 binding to C-4 was detected at a lower field than C-8 binding to C-4. Furthermore, among the signals of C-3 of the bottom units found around 68 ppm, those of the C4-C6 connection were observed at a slightly lower field than those of C4-C8. Based on comparisons of signal intensities, about two- or three-fold more of C4-C8 coupling patterns were suggested to be involved than C4- C6 couplings in the proanthocyanidins structure of Fr. 2-A Figure 5. In the MALDI-TOF-MS spectrum, signals were detected at every m/z=288 corresponding to one unit of catechin or epicatechin until 10-mer. In detail, the larger m/z signals than ordinary signals by 16 and 30 dalton were observed. These two signals seemed to be derived from proanthocyanidin derivatives possessing an additional hydroxy moiety at the B-ring and a further methylated hydroxy moiety, respectively Figure 6.
Figure 5: 13C-NMR spectrum of Fr. 2-ASignals were detected as broad peaks because proanthocyanidins were a mixture based on different degrees of polymerization, two types of linkages between catechin units. However, each position was assigned by classifications according to detailed characteristic sub-structures.
The active fraction Fr. 2-A from the red-colored extract from red rice bran that up-regulated the expression of ACOX-1 mainly consisted of proanthocyanidins, polymers of catechins and/or epicatechins. The degree of polymerization was suggested to be 3- to 10-mer and some units were presumed to possess hydroxy groups on the B-ring and possess one or more methyl moieties on any hydroxy group. Furthermore, C4-C8 linkages were estimated to be two- or three-fold more frequent than C4-C6 linkages as the bonds between monomer units, and the relative configuration between C3 and C4 was proposed to be almost or completely trans. The relative configuration between C2 and C3 currently remains unclear; however, 2,3-cis, epicatechins, were assumed to be the major components because they are mostly found in the catechin family in nature.Proanthocyanidins have not been reported to enhance ACOX1 expression, thus this is a first report describing that red rice proanthocyanidins enhance ACOX1 expression.
In summary, we revealed that red-colored extract from red rice bran reduced TG levels in the serum and liver of mice. Furthermore, this extract up-regulated the expression of mRNA encoding ACOX 1, which accelerates the consumption of fatty acids by β-oxidation, thereby preventing hyperlipidemia. Through bioassay-guided separation, proanthocyanidins were identified as the active components up-regulating the expression of ACOX-1. The structural features of these active proanthocyanidins were examined in detail by analyzing 13CNMR and MALDI-TOF-MS spectra. Red rice bran containing proanthocyanidins has potential in the treatment of obesity by reducing TG levels and promoting β-oxidation through increases in ACOX-1 levels.
Conclusion
We purifiedcolor pigment of red rice bran and identified a proanthocyanidin which enhances hepatic ACOX1 expression leading to cytosolic lipid β-oxidation. The structure was 3- to 10-mer of catechins and/or epicathechins including a 3,4-cis configuration, more C4-C8 than C4-C6 linkages, and the existence of a B-ring bearing three hydroxy groups with/without a methyl moiety.
Acknowledgments
The present study was supported by the “Challenging business supporting subsidies for small and medium companies and bencher companies in 2006 by Ministry of Economy”.
References
- Wu LC, Chen YC, Ho JA, Yang CS (2003) Inhibitory effect of red koji extract on mushroom tyrosinase. J Agri Food Chem 51: 4240-46.
- Ichikawa H, Ichiyanagi T, Xu B, Yoshii Y, Nakajima M, et.al (2001) Antioxidant activity of anthocyanin extract from purple black rice. J Med Food 4: 211-18.
- Deng GF, Xu XR, Zhang Y, Li D, Gan RY, et.al (2013) Phenolic compounds and bioactivities of pigmented rice. Crit Rev Food Sci Nutr 53: 296-306.
- Galli F (2007) Interaction of polyphenolic compounds with drug disposition and metabolism. Curr Drug Metab 8: 830-38
- Khan N, Mukhtar H (2007) Tea polyphenols for health promotion. Life Sci 81: 519-33.
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH (2006) A review of the effects and mechanism of polyphenolics in cancer. Crit Rev Food Sci Nutr 46: 161-83.
- Foo LY, Lu Y, Howell AB, Vorsa N (2000) The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Photochemistry 54: 173-81.
- Ghasemzadeh A, Karbalaii MT, Jaafar HZE, Rahmat A (2018) Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem Cent J 12: 17
- Ito VC, Lacerda LG (2019) Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem 301: 125304.
- Tamura S, Yan K, Shimoda H, Murakami N (2010) Anthocyanins from Oryza sativa L. subsp. indica. Biochem Syst Ecol 38: 438-40.
- Pereira-Caro G, Watanabe S, Crozier A, Fujimura T, Yokota T, et al. (2013) Phytochemical profile of a Japanese black-purple rice. Food Chem 141:2821-27.
- Chen MH, McClung AM, Bergman CJ (2016) Concentrations of oligomers and polymers of proanthocyanidins in red and purple rice bran and their relationships to total phenolics, flavonoids, antioxidant capacity and whole grain color. Food Chem 208: 279-87.
- Boue SM, Daigle KW, Chen MH, Cao H, Heiman ML (2016) Antidiabetic potential of purple and red rice (Oryza sativa L.) bran extracts. J Agric Food Chem 64: 5345-53.
- Ling WH, Cheng QX, Ma J, Wang T (2001) Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J Nutr 131: 1421-26.
- Sinthorn W, Chatuphonprasert W, Chulasiri M, Jarukamjorn K (2016) Thai red rice extract provides liver protection in paracetamol- treated mice by restoring the glutathione system. Pharm Biol 54: 770-79.
- Limtrakul P, Yodkeeree S, Pitchakarn P, Punfa W (2016) Anti-inflammatory effects of proanthocyanidin- rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages. Nutr Res Pract 10: 251-58.
- Limtrakul P, Yodkeeree S, Punfa W, Srisomboon J (2016) Inhibition of the MAPK Signaling Pathway by Red Rice Extract in UVB- irradiated Human Skin Fibroblasts. Nat Prod Commun 11:1877-82.
- Pintha K, Yodkeeree S, Limtrakul P (2015) Proanthocyanidin in red rice inhibits MDA-MB-231 breast cancer cell invasion via the expression control of invasive proteins. Biol Pharm Bull 38: 571-81.
- Upanan S, Yodkeeree S, Thippraphan P, Punfa W, Wongpoomchai R, et al. (2019) The proanthocyanidin- rich fraction obtained from red rice germ and bran extract induces HepG2 hepatocellular carcinoma cell apoptosis. Molecules 24: 813.
- Subkamkaew C, Limtrakul Dejkriengkraikul P, Yodkeeree S (2019) Proanthocyanidin rich fractions from red rice extract enhance TNF-α-induced cell death and suppress invasion of human lung adenocarcinoma cell A549. Molecules 24: 3393.
- Yin M, Zhang P, Yu F, Zhang Z, Cai Q, et al. (2017) Grape seed procyanidin B2 ameliorates hepatic lipid metabolism disorders in db/db mice. Mol Med Rep16: 2844-50.
- Bang CY, Choung SY (2014) Enzogenol improves diabetes related metabolic change in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. J Pharm Pharmacol 66: 875-85.
- Zou T, Wang B, Li S, Liu Y, You J (2020) Dietary apple polyphenols promote fat browning in high-fat diet-induced obese mice through activation of adenosine monophosphate-activated protein kinase β. J Sci Food Agric 100: e10248.
- Kallithraka S, Garcia-Viguera C, Bridle P, Bakker J (1995) Survey of solvents for the extraction of grape seed phenolics. Phytochem Anal 6: 265-67.
- Shimada T, Tokuhara D, Tsubata M, Kamiya T, Kamiya-Sameshima M, et al. (2012) Flavangenol (pine bark extract) and its major component procyanidin B1 enhance fatty acid oxidation in fat-loaded models. Eur J Pharmacol 677: 147-53.
- Rigaud J, Escribano-Bailon MT, Prieur C, Souquet JM, Cheynier V (1993) Normal-phase high-performance liquid chromatographic separation of procyanidins from cacao beans and grape seeds. J Chromatog A 654: 255-60.
- Newman RH, Porter LJ, Foo LY, Johns SR, Willing RI (1987) High-resolution 13C NMR studies of proanthocyanidins polymers (condensed tannins). Magn Reson Chem25: 118-24.
- Foo LY, Karchesy JJ (1989) Procyanidin dimers and trimers from douglas fir inner bark. Phytochemistry 28: 1743-47.
- Lou H, Yamazaki Y, Sasaki T, Uchida M, Tanaka H, et al. (1999) A type proanthocyanidins from peanut skins. Phytochemistry 51: 297-308.
- Fu C, Loo AEK, Chia FPP, Huang D (2007) Oligomeric proanthocyanidins from mangosteen pericarps. J Agri Food Chem 55: 7689-94.
Citation: Tamura S, Yan K, Kawano T, Ohnishi-Kameyama M, Tanaka J, et al. (2020) Identification of Chemical Structures of Hypolipidemic Proanthocyanidins in Red Rice Enhancing Acyl CoA Oxidase Expression. J Rice Res 8: 213. DOI: 10.4172/2375-4338.1000213
Copyright: © 2020 Tamura S, et al., This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2773
- [From(publication date): 0-2020 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2037
- PDF downloads: 736