Research Article Open Access
Identification of a Proteinaceous Alpha Amylase Inhibitor from a Medicinal Herb Oxalis corniculata L. (Oxalidaceae)
| Jyothi KSN1 , Shailaja M2, Viveni J2 and Suresh C3* | |
| 1Acharya Nagarjuna University, Guntur, AP, India | |
| 2Osmania University College for Women, Koti, Hyderabad, Telangana, India | |
| 3Department of Biochemistry, National Institute of Nutrition, Tarnaka, Hyderabad, Telangana, India | |
| Corresponding Author : | Suresh C Scientist and Assistant Director Department of Biochemistry National Institute of Nutrition Indian Council of Medical Research Department of Health Research Govt. of India, Hyderabad-500007, India Tel: +91-40-27197349 Fax: +91-40-27019074 E-mail: sureshnin2000@gmail.com |
| Received August 11, 2014; Accepted August 25, 2014; Published August 28, 2014 | |
| Citation: Jyothi KSN , Shailaja M, Viveni J, Suresh C (2014) Identification of a Proteinaceous Alpha Amylase Inhibitor from a Medicinal Herb Oxalis corniculata L. (Oxalidaceae). J Homeop Ayurv Med 3:165. doi: 10.4172/2167-1206.1000165 | |
| Copyright: © 2014 Suresh C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Health management through traditional medicine is a promising approach worldwide as it represents a multifaceted approach to health care than conventional medicine. The heightened importance to validate the efficacy and standards of traditional herbal medicine is the thrust area of present day research. The present study is one such attempt to evaluate the alpha amylase inhibitory potential and identify the candidate inhibitor from Oxalis corniculata L, a potent indigenous medicinal plant. Sequential solvent extraction of the leaves of O. corniculata was performed in previous work and the aqueous extract, showing maximum inhibition against porcine pancreatic alpha amylase against starch as substrate (IC50 value 68.08+0.06), was selected for purification using ammonium sulphate precipitation. The pellet obtained at 40%-80% precipitation was further subjected to ion exchange chromatography on DEAE-cellulose and gel filtration chromatography using Sephadex G-100. The molecular weight of the proposed amylase inhibitor named AI-1 was estimated to be about 30 kda on SDS-PAGE. Temperature sensitivity and pH stability of the inhibitor was studied. The AI-1 protein was stable up to 400 C and was totally destroyed beyond 700 C and showed maximum activity at pH 6. Further characterization of the AI-1 protein and its sequence determination will help in discovering a novel proteinaceous alpha amylase inhibitor from O. corniculata. Research of this kind will help to usher the identification of active bioconstituents from plants with great medicinal activities.
The last decade has witnessed a rapid increase in diseases caused by improper lifestyle among mankind. These diseases pose a major threat as their treatment by conventional drugs is not possible either due to decreasing efficacy of synthetic drugs or their increasing contraindications [5]. Since the traditional medicine system works in accordance with mind-body complex and has a holistic approach towards disease treatment, phytotherapy or plant based medication is now the most sought to alleviate suffering and disease [6]. Diabetes mellitus is one such lifestyle disorder, a multifactorial disease which cannot be completely cured by any one medication [7,8]. A large number of medicinal plants with potential to curb diabetes in various ways are being studied widely as they contain a large number of bioconstituents that are effective against diabetes [9]. Alpha amylase inhibitors from plants possess the ability to lower post prandial hyperglycemia and can be used in supplementary treatment of diabetes. Hence there is a great scientific urge to extract them from plants and standardize them clinically [10,11]. An extensive progress has been made in the last decade in the research on the physicochemical properties, nutritional and physiological role of plant alpha amylase inhibitors [12]. While proteinaceous inhibitors have been isolated from cereals and legumes, [13,14] tubers and some leafy vegetables, [15,16] little is known about the existence of such proteins in small herbs and other medicinal plants. The present work is one such kind, to describe the extraction and purification of a proteinaceous alpha amylase inhibitory protein from a small herb Oxalis corniculat. L belonging to the family Oxalidaceae. The alpha amylase inhibitors characterized so far from various plants were grouped into six different classes based on their tertiary structure and ranging in molecular weight from 5-60 kda [17,18]. In this study we report the isolation of a 30 kda protein from the leaves of O. corniculata which was active against porcine pancreatic alpha amylase.
Oxalis corniculata L is a small herbaceous plant of the family Oxalidaceae, indigenous to tropical and subtropical regions of the world. In Indian traditional medicine, the plant is widely used as anti-inflammatory, antibacterial, antiviral, anti-implantation and abortifacient, diuretic, and digestive agent [19,20]. The plant is well known for its medicinal value as a good appetizer and as a remover of anemia, dyspepsia, cancer, dementia, convulsion, and piles [21]. There is extensive study on the phytochemical analysis of the plant which showed the presence of flavonoids, tannins, phytosterols, glycosides, fatty acids, sterols, and amino acids [22]. Many of its therapeutic uses have been validated in vivo and in-vitro but no studies have been reported so far on the alpha amylase inhibitory potential of this plant. The preliminary evaluative studies have been conducted to analyze the alpha amylase inhibitory potential of the leaf extracts of this plant against porcine pancreatic alpha amylase using starch as substrate [23]. Further research was carried out to extract and purify the proposed alpha amylase inhibitor from the leaves of O. corniculata using ammonium sulphate precipitation, dialysis, ion exchange chromatography, and gel filtration chromatography. The molecular weight of the proteinaceous inhibitor was characterized by SDS-PAGE. Temperature sensitivity and pH stability of the inhibitor was also studied.
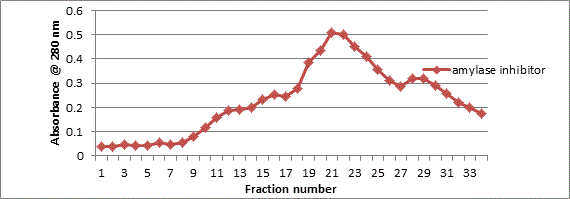
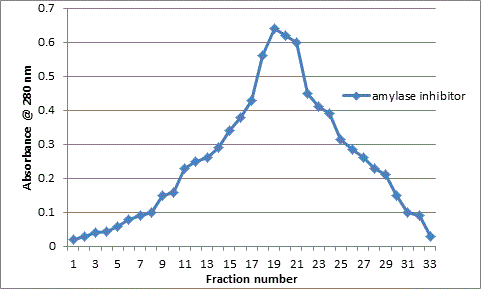
The major peak from the DEAE cellulose chromatography was then separated on a gel column using Sephadex G-100. The resulting peak from gel chromatography column as shown in Figure 2 also presents one major peak with specific activity of 3.1 U/mg protein and 13 fold purification as shown in Table 1.
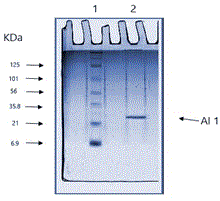
The purified AI-1 showed a molecular weight of 30 kda approximately as estimated using SDS-PAGE by comparison with the standard protein marker as shown in Figure 3.
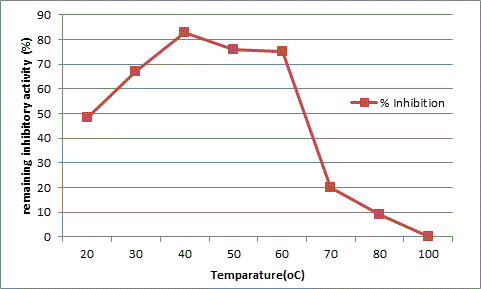
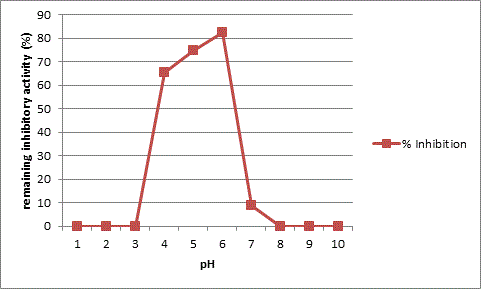
The results obtained from the studies on the effect of temperature and pH on the inhibitory activity as shown in Table 2 and 3 indicates that the AI-1 inhibitory protein has temperature optima at 40°C and is active up to 60°C. It shows a total loss of activity at 80°C as seen in Figure 4. The purified protein presents optimum activity at pH 6.0 and declining activity beyond pH 8 as seen in Figure 5.
The inhibitor AI-1 isolated from O. corniculata, shows a molecular weight of 30kda on SDS-gel electrophoresis. The inhibitor has an optimum temperature range of 40-60°C and is fully inactive at higher temperatures. The inhibitor is effective in the pH range of 4-6 and inactive in alkaline pH. There is extensive evidence that these kinds of alpha amylase inhibitors, obtained from plants, are a good source for controlling postprandial hyperglycemia, a major problem in type-II diabetes [31,32]. The present work is a contribution towards the identification of a novel alpha amylase inhibitor from a small medicinal herb O. corniculata. Further structural elucidation needs to be done on the inhibitor identified in this study in order to relate it to a specific class of amylase inhibitors identified so far from different plants [33]. Phytotherapy has gained great importance in the treatment of various lifestyle disorders like diabetes, obesity, heart diseases etc. [34,35]. Complete structural elucidation of this inhibitor might prove important in developing a natural plant based medication for the treatment of type-II diabetes.
References
- Houghton PJ (1995)The Role of Plants in Traditional Medicine and Current Therapy. J. Altern and Compement Med 1:131-143.
- Akerele O (1993) Nature's Medicinal Bounty: Don't Throw it Away. World Health Forum4: 390-395.
- Kong JM, Goh NK, Chia LS, et al.(2003) Recent Advances in Traditional Plant Drugs and Orchids. ActaPharmacol Sin 24:7-21.
- ArunRasheed, Sravya Reddy B, Roja C (2012) A Review on Standardisation of Herbal Formulation.Inter. J. of Phytotherapy2: 74-88.
- Boussageon R, Supper I, Bejan-Angoulvant T, Kellou N, Cucherat M, et al.(2012) Reappraisal of Metformin Efficacy in the Treatment of Type 2 Diabetes: A Meta-Analysis of Randomised Controlled Trials. PLoS Med. 9:4.
- Mohamed E, Debprasad C,Vincenzo DF, William CC (2013) Medicinal Plants in the Prevention and Treatment of Chronic Diseases. Evidence-Based Complementary and Alternative Medicine; VolumeArticle ID 180981, 3 pages.
- lin Y, sun Z (2010) Current Views on Type 2 Diabetes. Journal of Endocrinology 204: 1–11
- Peter G, Henrik LA, Hans HP, Oluf P (2010) Effect of a Multifactorial Intervention on Mortality in Type 2 Diabetes. N Engl J Med 358: 580-591
- Patel D, Prasad S, Kumar R,Hemalatha S (2012) An Overview on Antidiabetic Medicinal Plants Having Insulin Mimetic Property. Asian Pac J Trop Biomed 4: 320–330.
- Khan V, Najmi AK, Akhtar M, Aqil M, Mujeeb M, et al.(2012) A Pharmacological Appraisal of Medicinal Plants with Antidiabetic Potential. J Pharm BioalliedSci 4: 27-42.
- Melzig MF, Funke I (2007) Inhibitors of Alpha-amylase from Plants:a Possibility to Treat Diabetes Mellitus Type II by Phytotherapy. Wien Med Wochenschr 157:320-324.
- Franco OL, Rigden DJ, Melo FR, Grossi-De-Sá MF (2002) Plant Alpha-amylase Inhibitors and their Interaction with Insect Alpha-amylases. Eur J Biochem 269: 397-412.
- Khan N(2011) In Vitro Effects of Protienaceous Alpha Amylase Inhibitors on Red Flour Beetle, Triboliumcastaneum. Sci. Res. Rept 1: 101 - 104
- Xiaoyan H, Jiangui L, Qiughua S, Jusong Z, Xiaoling H, et al.(2009)Characterization of a Novel Legumin Alpha Amylase Inhibitor from Chickpea (Cicerarietinum L.) Seeds. Biosci. Biotechnol. Biochem 73:1200-1202.
- Ewan RM, MadivhaRP, Djarova T, Oyedeji OA, A. R. Opoku(2010) Alpha-amylase Inhibitor of Amadumbe (Colocasiaesculenta): Isolation, Purification andSelectivity Toward - Amylases from Various Sources. Afr J Biochem Res 4:220-224.
- ChagollaLA, BlancoLA, Patthy A, Sánchez R, Pongor S (1994) A novel alpha-amylase inhibitor from amaranth (Amaranthushypocondriacus) seeds. J BiolChem 38: 23675-23680.
- Bonavides KB; Pelegrini PB; Laumann RA; Grossi-de-Sá MF; Bloch C; Melo JA; Quirino BF; Noronha EF; Franco OL (2007) Molecular Identification of Four Different a-amylase Inhibitors from Baru ( Diptyrexalata) Seeds with Activity toward Insect Enzymes. J BiochemMolBiol 40:1-7.
- Hoover R, Sosulski F (1984) Characteristics and concentrations of amylase inhibitor in Phaseolus vulgaris biotypes. Starch/Starke 36: 246-250
- Ashwani K, Niketa, Sapna R and Somiya S (2012) An Absolute Review on Oxalis corniculata Linn, Int J Pharm Biomed Res 3:1179-1188.
- Hemant B, Mukesh KS, Deepa T, Tapan KG, TripathiDK (2011) The Botany, Chemistry, Pharmacological and Therapeutic Application ofOxalis corniculata Linn.International Journal of Phytomedicine3: 01-08.
- Ibrahim M, Hussain I, Imran M, Hussain N, Hussain A, et al.(2013)Corniculatin A, a New FlavonoidalGlucoside fromOxalis corniculata. Rev. bras. Farmacogn 23:630-634.
- Goid, Rashmi S, Rajkumar HG(2011) Preliminary Phytochemical Analysis and In Vitro Evaluation of Antifungal Activity of Five Invasive Plant Species against MacrophominaPhaseolina (Tassi). International Journal of Plant Research 1: 11-15.
- Jyothi KSN, Hemalatha P, Challa S(2011) Evaluation of a-amylase Inhibitory Potential of Three Medicinally Important Traditional Wild Food Plants of India.Int. j. green pharm5: 95-99.
- Jyothi KSN, Hemalatha P, AvanthiA, Suresh C (2013) A Comparative Analysis on the Alpha Amylase Inhibitory Potential of Six Ornamental Medicinal Plants. J. Nat. Prod. Plant Resource 3: 1-6.
- Miller GL (1959) Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem 31: 426-428.
- Asmaa ALI, AbdulwahidShamkhi Jabir, Raghda S. Abdulaziz 2012 Purification and Characterization of Amylase Inhibitor Extracted from White Kidney Bean (Phaseolus vulgaris). Cell & Plant Sci3: 17-21.
- Laemmli EK (1970) Cleavage of Structural Proteins: During the Assembly of the Head of Bacteriophage T4, Nature. 227: 680- 685
- Hames BD, and D. Rickwood(1990) Gel Electrophoresis of Proteins: A Practical Approach. IRL, OxfordPP 383
- Sales PM, Souza PM, Simeoni LA, Silveira D (2012) a-Amylase Inhibitors: a Review of Raw Material and Isolated Compounds from Plant Source. J Pharm PharmSci 15: 141-183.
- Kirtikar KR, Basu BD (1975) Indian Medicinal Plants Vol 1. 3rd Ed. New Delhi: MS periodical experts. page 437.
- Yoshikawa H, Kotaru M, Tanaka C, Ikeuchi T, Kawabata M (1999) Characterization of Kintoki Bean (Phaseolus Vulgaris) Alpha-amylase Inhibitor: Inhibitory Activities against Human Salivary and Porcine Pancreatic Alpha-amylases and Activity Changes by ProteolyticDigestion. J NutrSciVitaminol 45: 797-802.
- Etxeberria U, de la Garza AL, Campión J, Martínez JA, Milagro FI (2012)Antidiabetic Effects of Natural Plant Extracts Via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert OpinTher Targets 16:269-297.
- Octavio L and Rigden D(2002) Plant a-amylase Inhibitors and their Interaction with a-amylase. Eur. J. Biochemistry 269: 397-412.
- Desseaux V, Koukiekolo R, Moreau Y, Santimone MandMourenGM (2002)Mechanism of Porcine Pancreatic a-amylase:inhibition of Amylose and Maltopentaose Hydrolysisby Various Inhibitors. Biologia, Bratislava.57: 163-170
- Fugh-Beerman A (2000) Herb-drug Interactions. Lancet355: 134-138.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 19614
- [From(publication date):
December-2014 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 14985
- PDF downloads : 4629
