Hypoxic Ischemic Encephalopathy MRI Findings and Patterns Review, Revisited
Received: 03-Dec-2022 / Manuscript No. roa-22-83330 / Editor assigned: 05-Dec-2022 / PreQC No. roa-22-83330 (PQ) / Reviewed: 19-Dec-2022 / QC No. roa-22-83330 / Revised: 23-Dec-2022 / Manuscript No. roa-22-83330 (R) / Published Date: 30-Dec-2022 DOI: 10.4172/2167-7964.1000415
Abstract
Hypoxic ischemic encephalopathy, HIE, despite advances in healthcare and imaging modalities, is still a major cause of death and a leading cause of neonatal encephalopathy, neurodevelopmental disabilities, and seizures. It is associated with a high incidence of morbidity and mortality, which is significantly higher in developing countries. Early diagnosis and detection of the pattern and severity of brain injury aid to predict prognosis, select for appropriate therapy options like hypothermia therapy, and, therefore, improving the clinical outcome and prognosis. MRI brain with DWI is the investigation of choice for diagnosis, MRI is considered a predictor factor in addition to clinical findings and biomarkers. The four patterns of MRI findings in HIE are: the basal ganglia, thalamus, and posterior limb of internal capsule pattern, the watershed predominant pattern affecting the white matter in the cortex and subcortical zones, the minimal focal/multifocal injury of white matter, and the extensive injury of the entire brain. These patterns are determined by many factors combined including brain maturation, time, duration, and strength of the cerebral blood flow impairment. This mini review will discuss the pathophysiology of HIE and MRI findings patterns in premature and term neonates.
Keywords
MRI; Diffusion weighted; Encephalopathis; Hypoxic ischemic; Brain hypoxic ischemia; Ischemia; Neonatal; Hypoperfusion
Incidence and complications
Hypoxic ischemic encephalopathy, HIE is a leading cause of neonatal encephalopathy and can cause a wide range of neurodevelopmental disabilities ranging from seizures and ending with severe motor disability, cerebral palsy, or death. The HIE incidence in developed countries is 1.5-2.5 per 1000 live births [1]. However, the incidence is much higher in developing countries as it was 10.9% in a study in Northern Tanzania in 2014-2015 [2], and in a study in Tanzania in 2007 about 30.9% [3]. HIE is a leading cause of neonatal encephalopathy [4]. Furthermore, it is considered a major cause of significant neurologic disabilities and even death of term or near-term newborns, fifth to half of the new-borns with HIE die in early as infants, and a quarter of survivals experience long-term neurologic sequelae and disabilities; learning and intellectual disabilities, seizures, and cerebral palsy [5, 6].
Aetiology and mechanism
The main cause of HIE is an event of impaired blood flow to the brain that results in hypoperfusion and insufficient oxygen supply to neuronal cells [7]. A few factors affect the outcome and determine which structures of the brain are affected and how far the ischemia is. These factors include the number and frequency of the hypoperfusion events, their severity, and duration. Another important key factor is the maturation of the brain at the time of the incident if it is fully mature in term neonates or immature in preterm neonates [8]. The obstetric and perinatal history, generally, includes some of the predisposing factors that affect maintaining sufficient oxygen supply to the brain. The major vascular compromise is either maternal or foetal including placenta previa or abruption, cord prolapse or avulsion, rupture of uterus or maternal haemorrhage, nuchal cord, shoulder dystocia, and delayed long labour [9]. It is important to analyse the perinatal history and associated events including any sign of foetal distress. After delivery, checking 1 minute and 5 minutes APGAR is an important predictive factor along with any resuscitation attempts. Acidosis can be confirmed if the PH value in cord blood is less than 7. The neurological physical examination can assess neurological deficits early in life. Although HIE is the leading cause of neonatal encephalopathy, however, it is important to consider other non-ischemic causes such as metabolic, congenital, infectious, and trauma that can result as well in neonatal encephalopathy [10].
The mechanism of injury is summarized by asphyxia causing hypoperfusion (decreased cerebral blood flow) and hypoxia (impaired oxygen supply to the brain), these in turn initiate a series of cellular changes including acidosis, secretion of inflammatory mediators, and release of radicals. These biochemical changes affect the autoregulation of brain tissue and lead finally to the death of the neuronal cells [11,12]. Although, the imaging features of HIE are classified based on the severity of the event, however, in a given patient, features of both severe and mild to moderate can be seen [13].
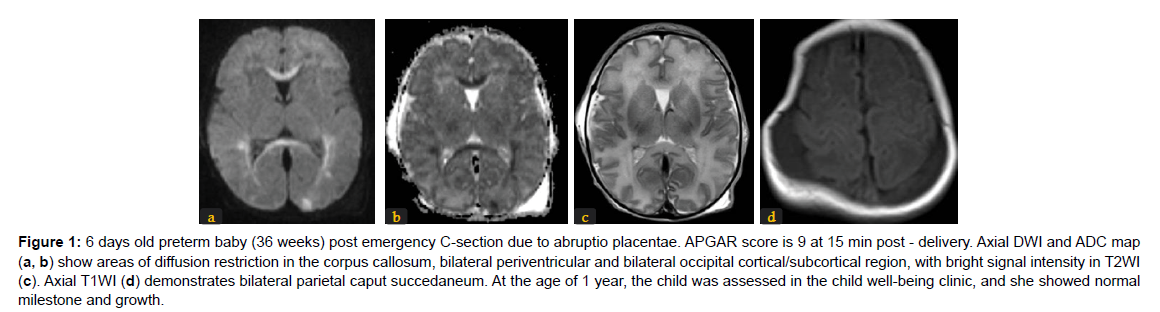
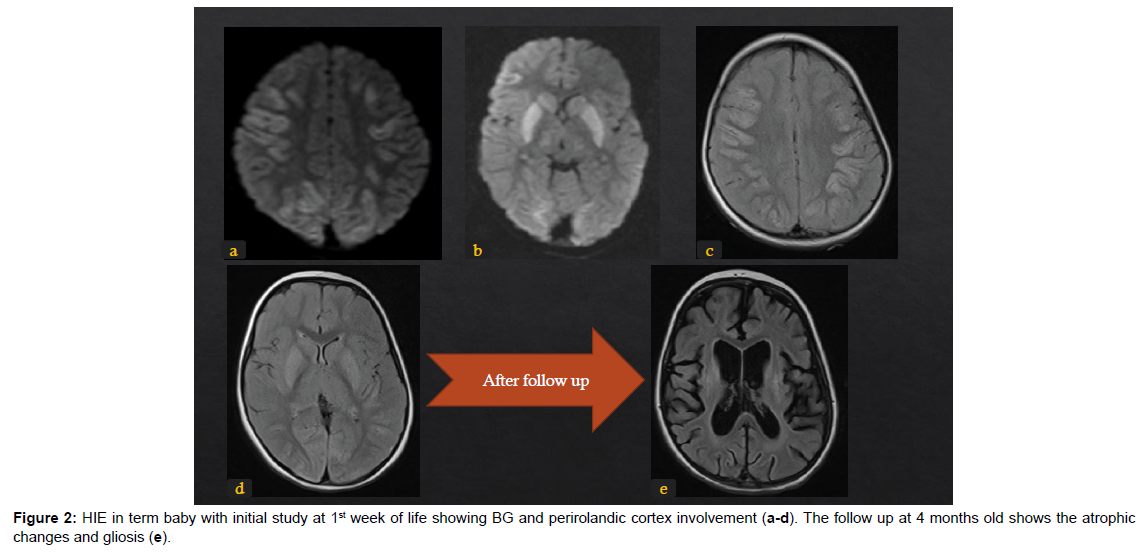
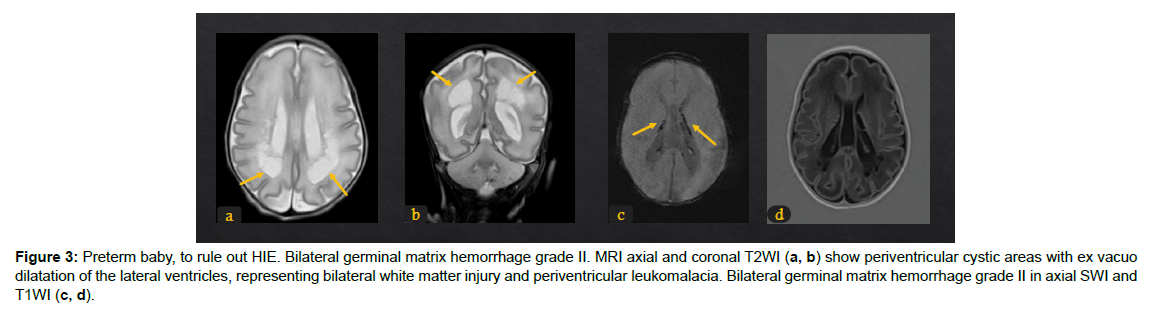
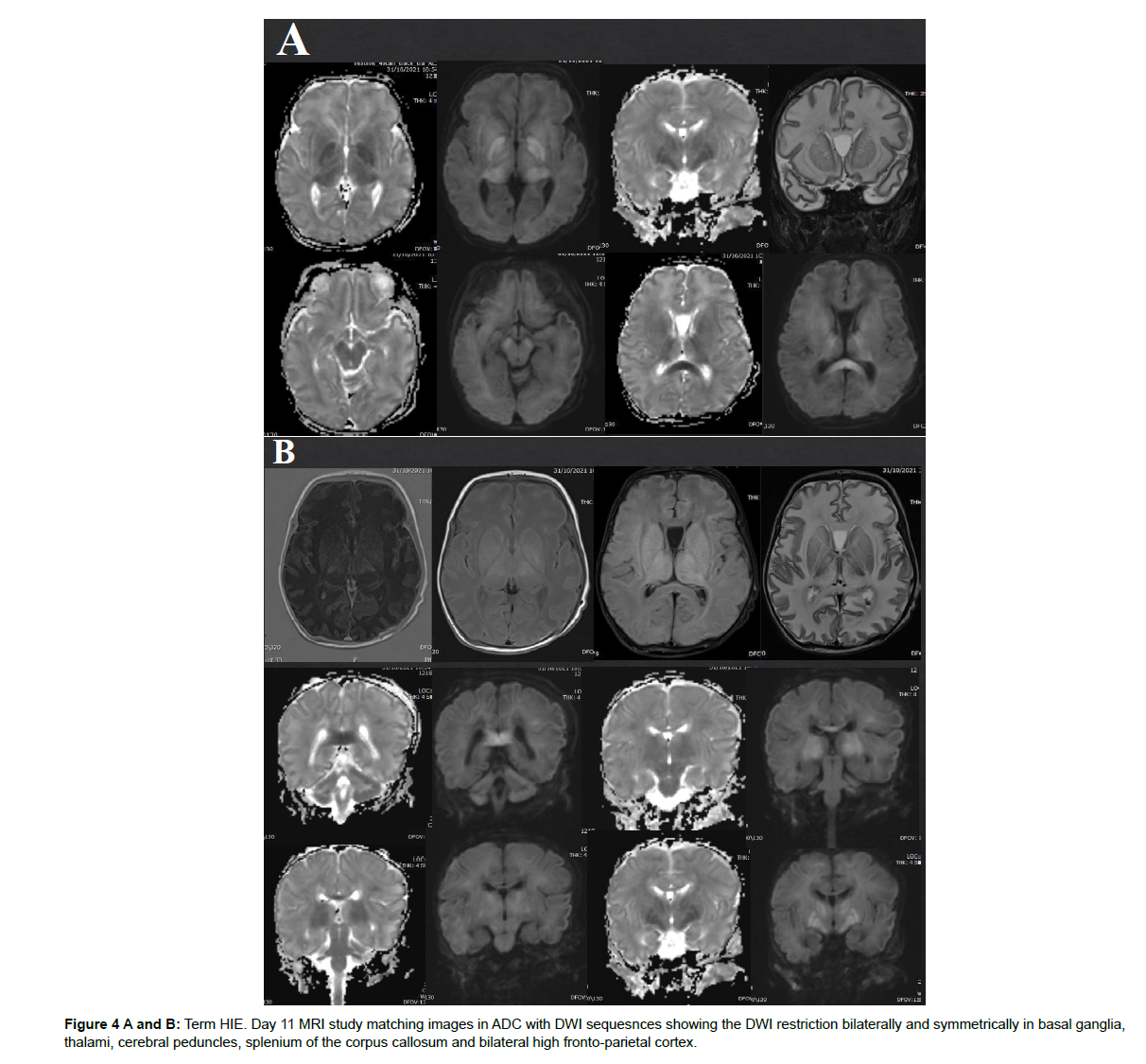
The different patterns of HIE changes are related to 2 major factors: the causing event-related factors, and the age of the neonates or its brain maturation at the time of exposure to hypoperfusion event. The severity, duration, and repetition of injury result in different parts of the affected brain. If the injury is mild, chronic, and repetitive, the cerebral blood flow is restored during the recovery between repeated injuries, hence, the deep structures like deep grey matter, basal ganglia, and thalami are spared from ischemic changes. Hereby, MRI signal changes are mainly in white matter and/or cortex (Figure 1). The nuchal cord is an example of this partial prolonged injury pattern. On the other hand, cases of uterine rupture or placenta abruption will lead to profound acute hypoperfusion that does not allow time for cerebral blood flow redistribution. As a result, ischemia targets the highmetabolic demanding cranial structures including the basal ganglia and corticospinal tracts with spread into the perirolandic white matter and cortex [14]. (Figure 2). The stage of brain maturation at the time of the hypoperfusion event would affect the anatomical distribution of hypoxia in the brain. In preterm neonates, if the hypoperfusion is mild it would result in periventricular injury (Figure 3); however, if hypotension is severe, it causes deep structures’ infarction (deep grey matter, brainstem, and cerebellum). In term neonates, mild hypoperfusion leads to damage to the cortex and subcortex in parasagittal zones; however, lateral thalamus, posterior putamen, hippocampus, corticospinal tract, and sensorimotor cortex injury is typical in severe hypoperfusion [13], (Figure 4). The four patterns of MRI findings in HIE are: the basal ganglia, thalamus, and posterior limb of internal capsule pattern, the watershed predominant pattern affecting the white matter in the cortex and subcortical zones, the minimal focal/multifocal injury of white matter, and the extensive injury of the entire brain [15, 16] (Figures 1-4).
Figure 1: 6 days old preterm baby (36 weeks) post emergency C-section due to abruptio placentae. APGAR score is 9 at 15 min post - delivery. Axial DWI and ADC map (a, b) show areas of diffusion restriction in the corpus callosum, bilateral periventricular and bilateral occipital cortical/subcortical region, with bright signal intensity in T2WI (c). Axial T1WI (d) demonstrates bilateral parietal caput succedaneum. At the age of 1 year, the child was assessed in the child well-being clinic, and she showed normal milestone and growth.
Figure 3: Preterm baby, to rule out HIE. Bilateral germinal matrix hemorrhage grade II. MRI axial and coronal T2WI (a, b) show periventricular cystic areas with ex vacuo dilatation of the lateral ventricles, representing bilateral white matter injury and periventricular leukomalacia. Bilateral germinal matrix hemorrhage grade II in axial SWI and T1WI (c, d).
Role of Imaging
The imaging modality of choice and standard of care is performing brain MRI with diffusion-weighted DWI/ADC images, and if possible MR spectroscopy. MRI is a predictive factor of morbidity and mortality in HIE [6]. In developing countries or primary centres and depending on the patient’s condition, screening cranial ultrasound can sometimes add valuable information to guide clinicians towards early diagnosis of HIE [17,18].
Trans fontanelle cranial ultrasound
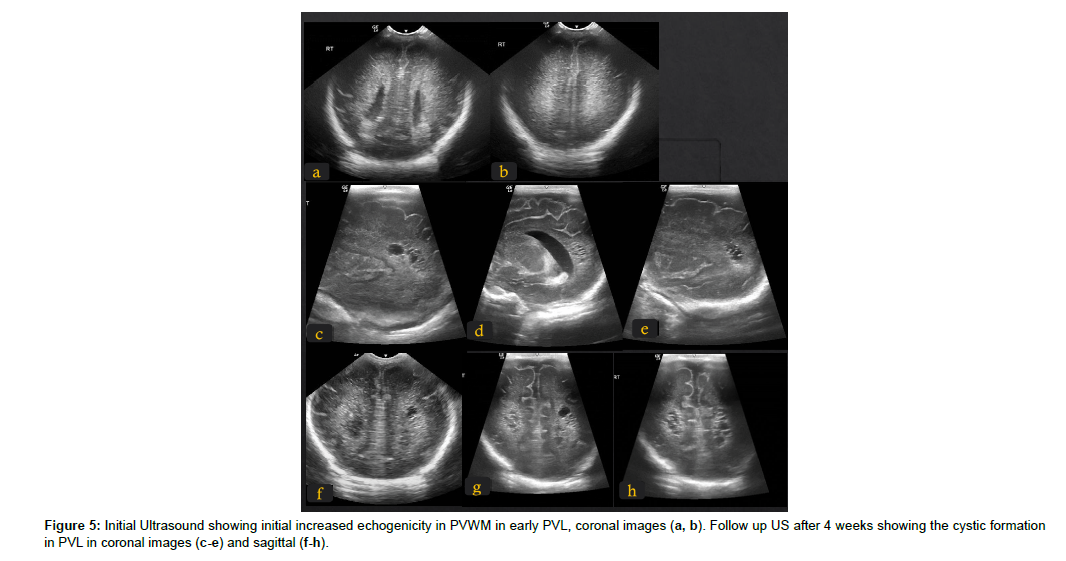
The cranial ultrasound is an easily available bedside portable noninvasive and not expensive tool to investigate suspicion of HIE. It can rule out intracranial haemorrhage and detect cystic periventricular leukomalacia (PVL), (Figure 5), hydrocephalus, and many intracranial structural anomalies. Doppler study of the MCA detects the increased resistance index, RI, in severe asphyxia. The ultrasound, however, is limited due to difficulty in assessing the superficial cranial structures such as the cortex, the operator dependence, and the inter-observership variations [19].The sonographic signs of HIE and echogenicity changes in white matter and basal ganglia might take 2-3 days to be visible by ultrasound [20, 21], by that time, DWI and MRI are more sensitive and can detect changes earlier (Figure 5).
Magnetic Resonance Imaging, DWI, and MR Spectroscopy
MRI assessment in suspected HIE requires full knowledge of normal myelination pattern variation as per gestational age of the neonate if less or more than 37 weeks. Therefore, the interpretation of the brain MRI of an HIE-suspected patient should be done side by side with a completely normal MR brain study of the same neonatal age. The MRI conventional study protocol includes the following sequences and planes: a volumetric sagittal T1- weighted, axial, and coronal T2-weighted, DWI, diffusion-weighted imaging, susceptibility-weighted imaging, a volumetric coronal inversion recovery, axial T1-weighted. The FLAIR axial should be added after age 6 months. MR Spectroscopy in the early neonatal period can add important information and helps differentiate HIE from other mimics including metabolic or infectious causes. However, it is subjective to availability [22]. Each of the MRI sequences plays an important role to detect findings and diagnose HIE. Myelination in the first 6 months of age is best assessed on T1WI which can detect ischemia and subacute haemorrhage as well. Axial T2WI is not used before 6 months of age to check myelination, however, it detects the signal changes in white matter due to good grey matter to white matter contrast [23, 24]. The fluid Attenuation Inversion Recovery, FLAIR, sequence is very sensitive in detecting the T2 signal changes, however, it is less sensitive in differentiating gliosis from not yet myelinated white matter. It can detect cystic and glial increased T2 signal intensity [23, 24]. The susceptibility-weighted images (SWI) are crucial to differentiate grey matter ischemia, haemorrhage, and astrogliosis [23, 25]. The gold standard sequence, DWI with ADC maps, shows the restricted diffusion (summarized by bright signal intensity on DWI and dark signal intensity on ADC) a sign of cytotoxic oedema in acute injury. The restricted diffusion is a timely sign, its peak is almost 3-5 days after an ischemic event, but can be positive from 24 hours to 7 days, (Figure 6). After that period, the DWI/ADC images are negative. Therefore,in the case of antenatal ischemic events, brain DWI after delivery will show negative DWI and refers to a late sequel of ischemia, and cell death [23, 24]. MR spectroscopy (MRS) if available can add important signs, however, should be interpreted with MRI and DWI. MRS is more sensitive to detect HIE in the initial 24 hours after the insult. It can detect the increased release of lactate (a sign of anaerobic metabolism) in acute ischemia and the increased release of lipids in infarction. It is important to know that the preterm brain has a high level of lactate which does not help to detect acute ischemia, unlike the term neonate’s brain in which the lactate peak is identified by the characteristic double peak at 1.3 ppm. The lactate peak is superimposed on lipids, therefore, using the intermediate TE like 144 ms, will show the inverted lactate peak and helps isolate lactate from lipids (Figure 7). Some MRS signs to suggest poor neurologic outcome are elevated lactate-to-creatinine and lactate-to-choline ratios along with decreased NAA and choline in term neonates at levels of the basal ganglia [26].
MRI Patterns
The HIE brain MRI changes are divided into the following 4-5 categories: (Table 1).
| Pattern | Severity of injury | Areas/structures affected | Clincal outcome |
|---|---|---|---|
| Basal ganglia–thalamus pattern (BGT) | acute sentinele; ‘acute near total asphyxia’ | ventrolateral thalami and posterior putamina and perirolandic cortex | Severe motoneuronal abnormalities |
| Watershed predominant pattern of injury (WS) | Mild to moderate and prolonged repetitive event ‘prolonged partial asphyxia’ | Vascular watershed zones | Seizures, intellectual and cognitive impairment often normal outcome |
| Mixed pattern | Prolonged mild event followed by acute profound one | Both areas of 1 and 2 | |
| 4-Extensive injury, “white cerebellum” [23]/ Multilobar cystic encephalomalacia/ multicystic encephalopathy [31] | very severe and prolonged hypoxia or total anoxia “near total injury” or “global injury” | subcortical WM and cortex sparing PVWM and central GM, cerebral infarction | Fatal or multicystic encephalomalacia |
| Punctate white matter lesions | Mild in preterm | Periventricular white matter | Mild encephalopathy mild seizures |
| Perinatal arterial ischaemic stroke (PAIS), perinatal haemorrhagic stroke (PHS) and sinovenous thrombosis | Perinatal asphyxia | Follows arterial/vascular territory, usually MCA | Seizures, atrophy |
Table 1: Brain MRI findings patterns in HIE [21-23, 31].
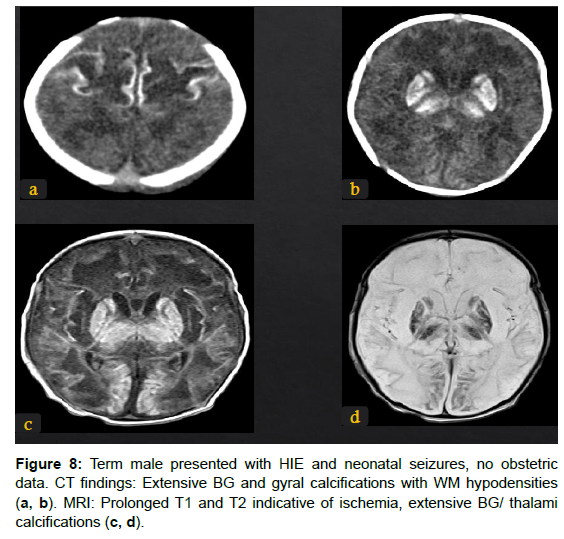
1. Acute profound ischemia results from a sudden profound hypoxic event and affects deep nuclei, perirolandic cortical and subcortical WM, and the hippocampus. This is called the “Basal Ganglia Pattern” (Figure 4, 8).
2. The partial prolonged ischemia results from prolonged or intermittent mild to moderate hypoperfusion events and will affect the cerebral watershed areas. It is called “Watershed Pattern”.
3. The “Mixed Pattern” combines the former two findings and results from severe, however, short ischemia, or combined the repetitive followed by a profound event.
4. The cystic encephalomalacia is an end stage of “Global Ischemia” in which the two forms: type 1 and type 2 affect the cerebral cortex and white matter, with basal nuclei involvement (in type 2) or without (in type1), it is a result of severe ischemia, either prolonged in type 1 or profound in type 2 [22] (Figures 6-8).
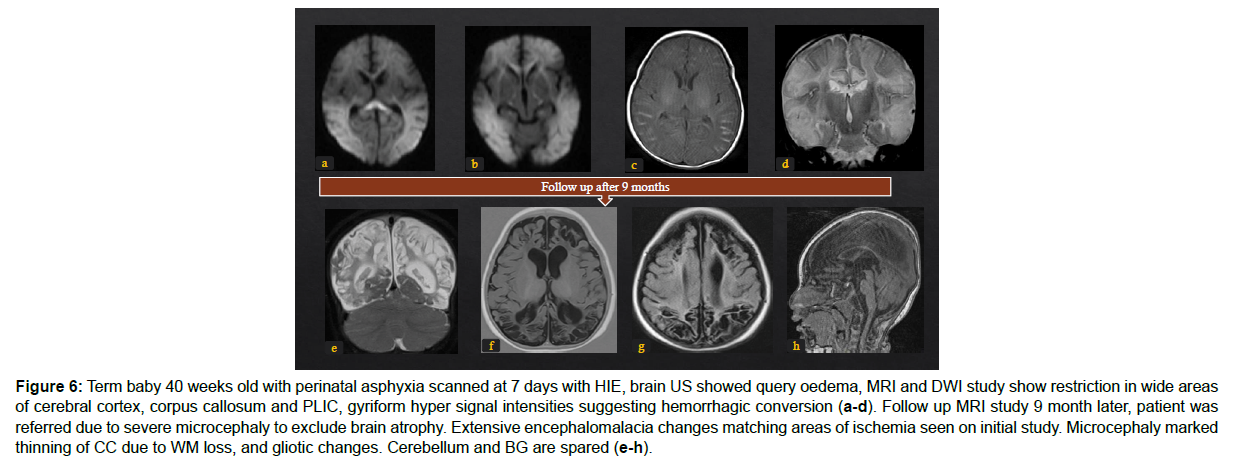
Figure 6: Term baby 40 weeks old with perinatal asphyxia scanned at 7 days with HIE, brain US showed query oedema, MRI and DWI study show restriction in wide areas of cerebral cortex, corpus callosum and PLIC, gyriform hyper signal intensities suggesting hemorrhagic conversion (a-d). Follow up MRI study 9 month later, patient was referred due to severe microcephaly to exclude brain atrophy. Extensive encephalomalacia changes matching areas of ischemia seen on initial study. Microcephaly marked thinning of CC due to WM loss, and gliotic changes. Cerebellum and BG are spared (e-h).
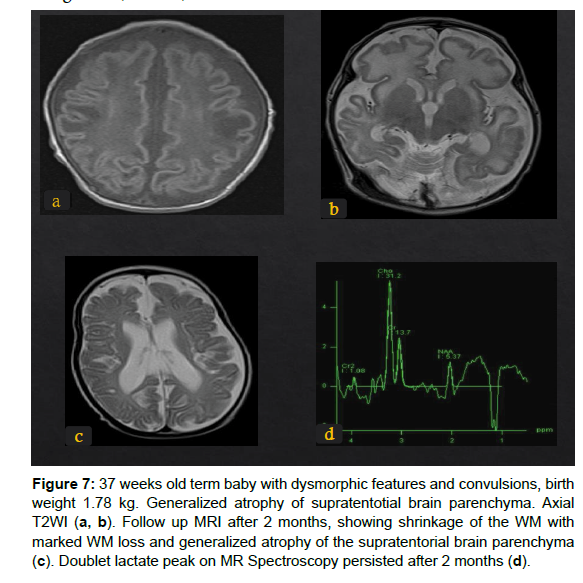
Figure 7: 37 weeks old term baby with dysmorphic features and convulsions, birth weight 1.78 kg. Generalized atrophy of supratentotial brain parenchyma. Axial T2WI (a, b). Follow up MRI after 2 months, showing shrinkage of the WM with marked WM loss and generalized atrophy of the supratentorial brain parenchyma (c). Doublet lactate peak on MR Spectroscopy persisted after 2 months (d).
Preterm HIE patterns
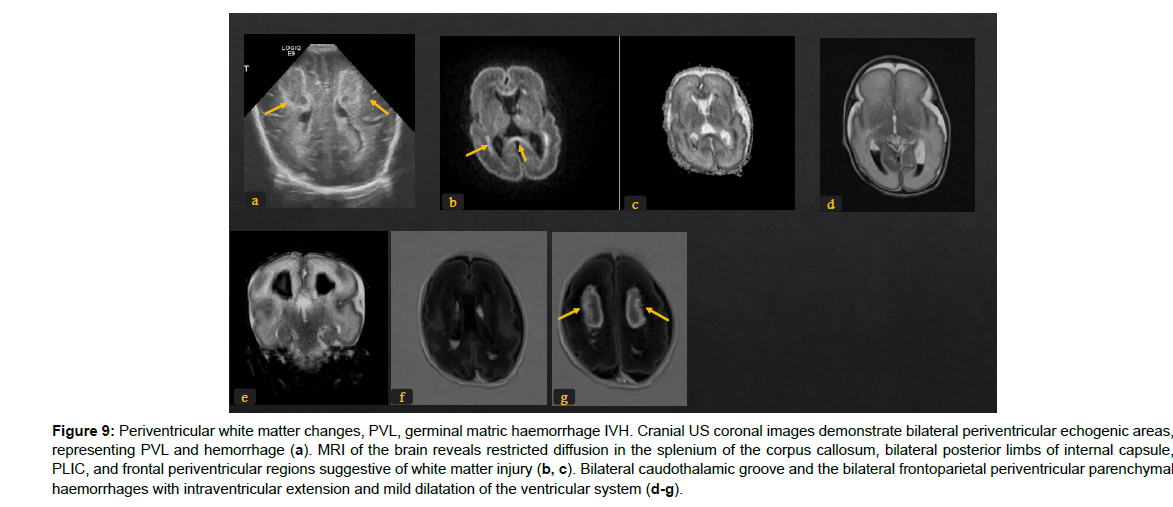
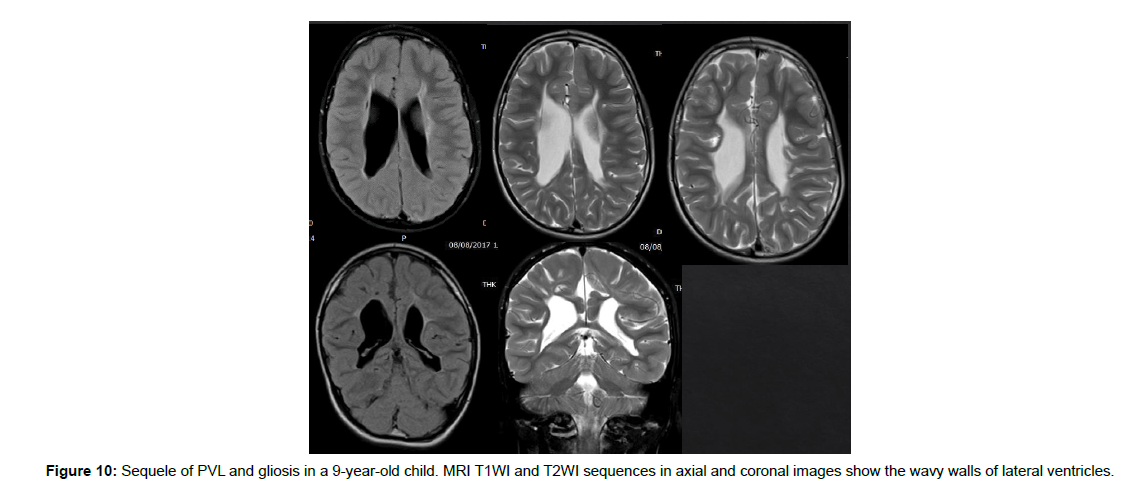
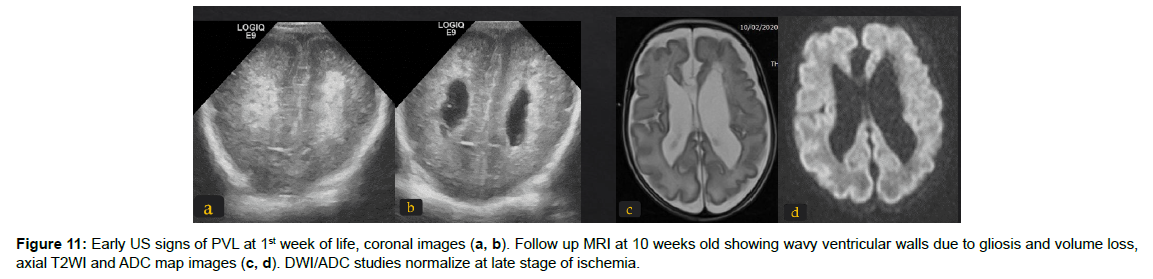
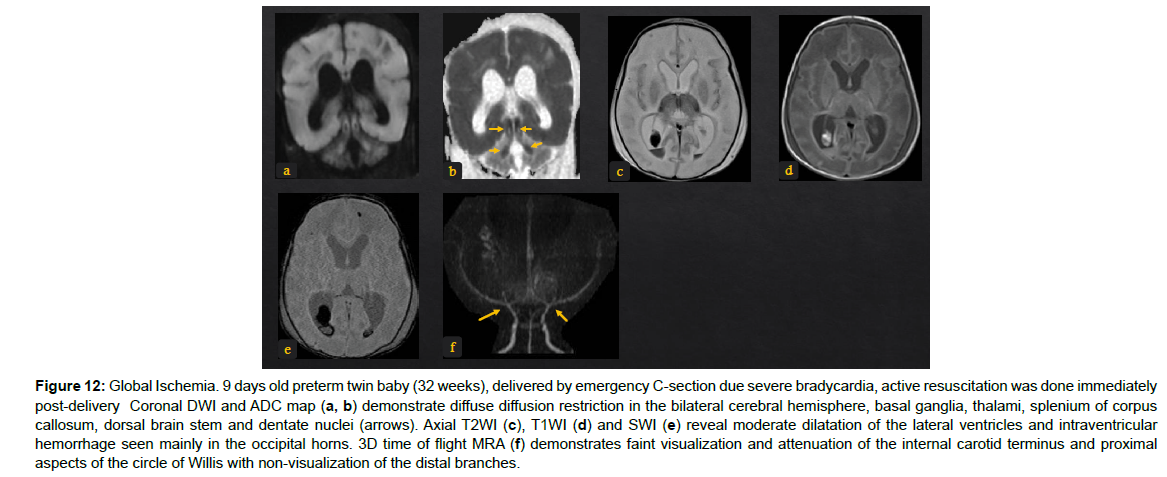
The immature brain in preterm neonates has periventricular white matter (PVWM) very susceptible to HIE injury. Initially in mild or moderate events, focal injury to periventricular white matter known as white matter injury of prematurity (WMIP) or periventricular leukomalacia (PVL), (Figure 9). The PVL starts as necrosis that cavitates and forms cysts. Later, the small cysts collapse and end as gliotic changes which is the end stage of PVL associated with complete loss of the periventricular white matter [23], (Figure 3). The gliosis and volume loss of white matter in the periventricular zone and centrum semi-ovale manifest as the thinned corpus callosum and dilated trigon and body of lateral ventricles [12], (Figure 3, 9), the wavy ventricular contour is characteristic [23], (Figure 9-11). On the other hand, severe hypoperfusion in an immature brain usually targets the highly metabolic deep structures which were myelinated early including the thalamus, dorsal brain stem, and anterior vermis [12]. The areas that are less involved generally are the basal ganglia, hippocampus, perirolandic cortex, and corticospinal tract (Figure 12). The distribution is linked to the myelination that occurs early around 24-25 weeks of gestation and appears as high signal intensity in T1WI in globus pallidum and late (after 35-36 weeks of gestation) in the caudate, putamen, and perilolandic cortex [23]. Concurrent HIE changes of both a mildmoderate event and a profound event can be seen in the same patient [12, 23] (Figures 9-12).
Figure 9: Periventricular white matter changes, PVL, germinal matric haemorrhage IVH. Cranial US coronal images demonstrate bilateral periventricular echogenic areas, representing PVL and hemorrhage (a). MRI of the brain reveals restricted diffusion in the splenium of the corpus callosum, bilateral posterior limbs of internal capsule, PLIC, and frontal periventricular regions suggestive of white matter injury (b, c). Bilateral caudothalamic groove and the bilateral frontoparietal periventricular parenchymal haemorrhages with intraventricular extension and mild dilatation of the ventricular system (d-g).
Figure 12: Global Ischemia. 9 days old preterm twin baby (32 weeks), delivered by emergency C-section due severe bradycardia, active resuscitation was done immediately post-delivery Coronal DWI and ADC map (a, b) demonstrate diffuse diffusion restriction in the bilateral cerebral hemisphere, basal ganglia, thalami, splenium of corpus callosum, dorsal brain stem and dentate nuclei (arrows). Axial T2WI (c), T1WI (d) and SWI (e) reveal moderate dilatation of the lateral ventricles and intraventricular hemorrhage seen mainly in the occipital horns. 3D time of flight MRA (f) demonstrates faint visualization and attenuation of the internal carotid terminus and proximal aspects of the circle of Willis with non-visualization of the distal branches
Term HIE patterns
In term babies, mild or moderate HIE affects initially the cortical and subcortical white matters in parasagittal watershed areas (the areas between vascular supply territories of the anterior cerebral artery and the middle cerebral artery, and between the middle cerebral artery and the posterior cerebral artery) [12]. If perfusion is severely impaired, it involves lentiform nuclei (posterior and lateral) and thalami (ventrolateral) (Figure 2, 4, 8). Further, the ischemic changes extend (if hypoperfusion persists) to affect all basal ganglia and medial thalami. If the HIE is more extensive, it affects the caudad structures along the midbrain, pons, and medulla (dorsal zones). This stage of HIE carries a poor prognosis and a high mortality rate [12, 16], (Figure 13). If the posterior limb of the internal capsule, PLIC, was affected by HIE, (Figure 14), it was found to have unflavoured motor impairment [27, 28]. Therefore, special attention is paid to checking the degree of normal myelination of the posterior third or half of PLIC that is normally evident at age 37 gestational weeks and beyond [23]. The basal ganglia pattern is more common than the parasagittal watershed pattern and it carries the worse prognosis [12]. Studies found that cerebral palsy and early death were associated with basal ganglia injury [29-31]. It is important to monitor the suspected HIE in cases of normal initial MRI study, chronic HIE can manifest as multi cystic encephalomalacia and profound thinned atrophied cerebral cortex [12] (Figure 13 and 14).
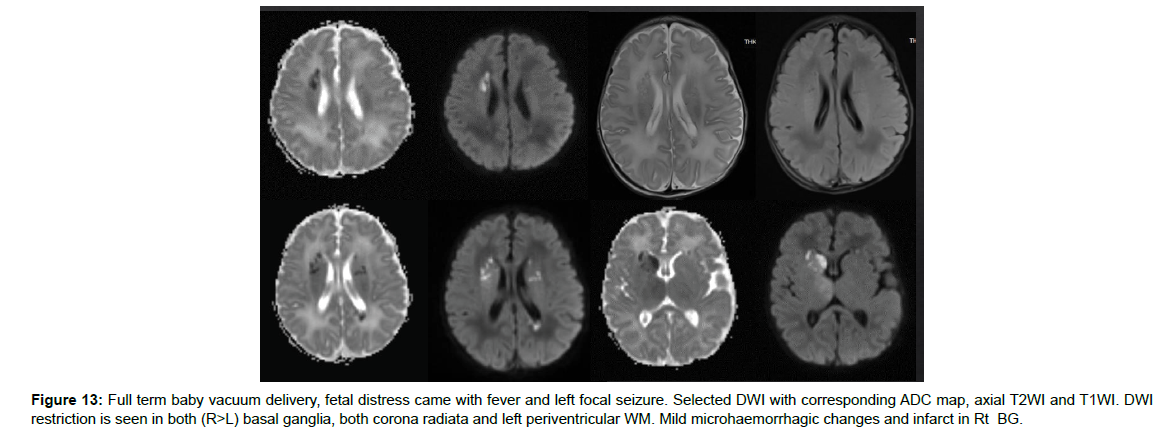
Figure 13: Full term baby vacuum delivery, fetal distress came with fever and left focal seizure. Selected DWI with corresponding ADC map, axial T2WI and T1WI. DWI restriction is seen in both (R>L) basal ganglia, both corona radiata and left periventricular WM. Mild microhaemorrhagic changes and infarct in Rt BG.
Figure 14: Term HIE, neonatal seizures status-post hypothermia therapy. MRI study shows bilateral symmetrical white matter T2WI/FLAIR high signal intensity in the occipital cortex, posterior limb of internal capsules, and bilateral medial aspect of the cerebellar hemispheres. DWI restriction with high signal intensity in genu and selenium of the corpus callosum. Scattered micro haemorrhagic foci in supratentorial and infratentorial distribution involving both occipital subcortical WM and the subependyma of the occipital horn of the right lateral ventricle.
What can mimic ischemia?
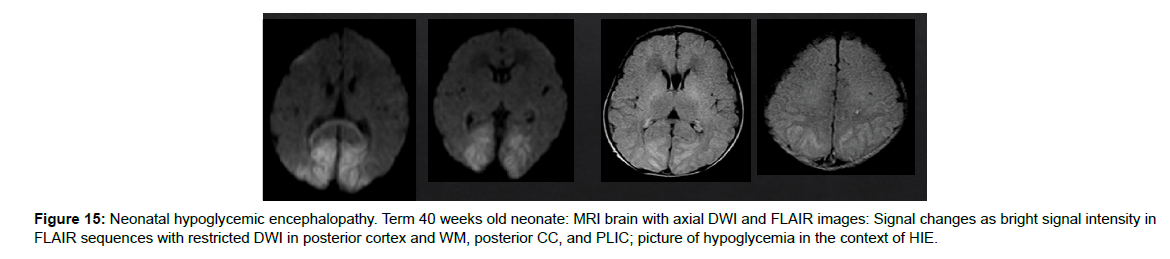
Many non-ischemic disorders can mimic HIE clinical presentation and MRI findings. Therefore, neonatal encephalopathy does include in the differential diagnosis list the metabolic disorders, congenital malformations, vascular causes, neuronal infection, and trauma [12], (Figure 15).
Conclusion
Brain MRI and DWI are the standards of care and predictive factors in the diagnosis of HIE. Signal changes affect various structures according to the brain maturation and severity, duration, and timing of the event resulting in hypo perfusion and ischemia. The diagnostic signs on DWI, conventional MRI, and MRS are not constant and vary depending on the time of insult, therefore, the timing of the neuroimaging is crucial to detect HIE. The four patterns of MRI findings in HIE are the basal ganglia pattern, the watershed pattern, the mixed pattern, the global ischemic pattern, and other vascular and white matter multifocal lesions. These patterns help the radiologist to reach the diagnosis and help the clinician to predict the clinical outcome and start the treatment as early as possible. Despite the tremendous advances in healthcare standards of care and imaging modalities and techniques, HIE is unfortunately still a major cause of morbidity and mortality in developing countries.
References
- Long M, Brandon DH (2007) Induced hypothermia for neonates with hypoxic-ischemic encephalopathy. J Obstet Gynecol Neonatal Nurs 36: 293-298.
- Simiyu IN, Mchaile DN, Katsongeri K, Philemon RN, Msuya SE (2017) Prevalence, severity and early outcomes of hypoxic ischemic encephalopathy among newborns at a tertiary hospital, in northern Tanzania. BMC Pediatr 17: 131.
- Juma A (2007) Prevalence and immediate outcomes of hypoxic ischemic encephalopathy among infants admitted at the neonatal Ward of Muhimbili National Hospital in Dar es Salaam Tanzania. Official publication of the Tanzania Medical students’ association 15.
- Blair E, Stanley FJ (1988) Intrapartum asphyxia: A rare cause of cerebral palsy. J Pediatr 112: 515-519.
- Sarnat HB, Sarnat MS (1976) Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol 33: 696-705.
- Shim GH (2021) Which factors predict outcomes of neonates with hypoxic-ischemic encephalopathy following therapeutic hypothermia?. Clin Exp Pediatr 64: 169-171.
- Kurinczuk JJ, White-Koning M, Badawi N (2010) Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 86: 329-338.
- Douglas-Escobar M, Weiss MD (2012) Biomarkers of hypoxic-ischemic encephalopathy in newborns. Front Neurol 3: 144.
- Shankaran S (2009) Neonatal Encephalopathy: Treatment with Hypothermia. J Neurotrauma 26: 437-443.
- Salas J, Tekes A, Hwang M, Northington FJ, Huisman TAGM (2018) Head Ultrasound in Neonatal Hypoxic-Ischemic Injury and Its Mimickers for Clinicians: A Review of the Patterns of Injury and the Evolution of Findings Over Time. Neonatology 114: 185-197.
- Johnston MV, Trescher WH, Ishida A, Nakajima W (2001) Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res 49: 735-741.
- Bano S, Chaudhary V, Garga UC (2017) Neonatal Hypoxic-ischemic Encephalopathy: A Radiological Review. J Pediatr Neurosci 12: 1-6.
- Chao CP, Zaleski CG, Patton AC (2006) Neonatal Hypoxic-Ischemic Encephalopathy: Multimodality Imaging Findings. RadioGraphics 26: S159-S172.
- Moore KR, Barkovich AJ, Grant E, Jones BV, Vézina G, et al. (2007) Diagnostic Imaging: Pediatric Neuroradiology. Am J Neuroradiol 29: e36.
- Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, et al. (2008) Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121: 906-914.
- Mao J (2017) Patterns of brain injury in neonatal hypoxic-ischemic encephalopathy on magnetic resonance imaging: recommendations on classification. Chin J Contemp Pediatrics 19: 1225-1233.
- Ahearne CE, Boylan GB, Murray DM (2016) Short and long term prognosis in perinatal asphyxia: an update. World J Clin Pediatr 5: 67-74.
- Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, et al. (2012) White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr 161: 799-807.
- Barkovich AJ (2007) Pediatric Neuroimaging. (4th edn), Philadelphia, PA: Lippincott Williams & Wilkins; AJNR Am J Neuroradiol 28: 192-193.
- Annink KV, De Vries LS, Groenendaal F, Vijlbrief DC, Weeke LC, et al. (2020) The development and validation of a cerebral ultrasound scoring system for infants with hypoxic-ischaemic encephalopathy. Pediatr Res 87: 59-66.
- Parmentier CEJ, de Vries LS, Groenendaal F (2022) Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics (Basel) 12: 645.
- Misser SK, Barkovich AJ, Lotz JW, Archary M (2020) A pictorial review of the pathophysiology and classification of the magnetic resonance imaging patterns of perinatal term hypoxic ischemic brain injury – What the radiologist needs to know….. SA J Radiol 24: 1915.
- Varghese B, Xavier R, Manoj VC, Aneesh MK, Priya PS, et al. (2016) Magnetic resonance imaging spectrum of perinatal hypoxic-ischemic brain injury. Indian J Radiol Imaging 26: 316-327.
- Rutherford MA (2002) The asphyxiated term infant. MRI of the neonatal brain, London. 99: 128.
- Shroff MM, Soares Fernandes JP, Whyte H, Raybaud C (2010) MR Imaging for Diagnostic Evaluation of Encephalopathy in the Newborn. Radiographics 30: 763-780.
- Boichot C, Walker PM, Durand C, Grimaldi M, Chapuis S, et al. (2006) Term neonate prognoses after perinatal asphyxia: Contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology 239: 839-848.
- Lakatos A, Kolossváry M, Szabó M, Jermendy Á, Barta H, et al. (2019) Neurodevelopmental effect of intracranial hemorrhage observed in hypoxic ischemic brain injury in hypothermia-treated asphyxiated neonates - an MRI study. BMC Pediatr 19: 430.
- Goergen SK, Ang H, Wong F, Carse EA, Charlton M, et al. (2014) Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: Interobserver agreement and MRI predictors of outcome at 2 years. Clin Radiol 69: 72-81.
- Rutherford M, Martinez Biarge M, Allsop J, Counsell S, Cowan F (2010) MRI of perinatal brain injury. Pediatr Radiol 40: 819-833.
- de Vries LS, Groenendaal F (2010) Patterns of neonatal hypoxic–ischaemic brain injury. Neuroradiology 52: 555-566.
- Counsell SJ, Rutherford MA (2002) Magnetic resonance imaging of the newborn brain. Current Paediatrics 12: 401-413.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Tabban HA, Hassan IA, ABHS-R, Salem KY, Salem TA, Ibrahim S (2022) Hypoxic Ischemic Encephalopathy MRI Findings and Patterns Review, Revisited. OMICS J Radiol 11: 415. DOI: 10.4172/2167-7964.1000415
Copyright: © 2022 Tabban HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 7582
- [From(publication date): 0-2022 - Nov 26, 2025]
- Breakdown by view type
- HTML page views: 6984
- PDF downloads: 598