Research Article Open Access
Hyperuricemia Obstructs the Effect of Spinal Cord Stimulation on Peripheral Arterial Occlusive Diseases: The Retrospective Analysis of Eleven Cases
| Sumihisa Aida1*, Zen’ichiro Wajima2,3, Toshiya Shiga4, Kanta Kido5 and Eiji Masaki5 | |
| 1Department of Pain Medicine, Nippori-Jogu Hospital, Tokyo, Japan | |
| 2Department of Anesthesiology, Shioya Hospital, International University of Health and Welfare, Tochigi, Japan | |
| 3Department of Anesthesiology, International University of health and Welfare Hospital, Tochigi, Japan | |
| 4Department of Anesthesiology, Kaken Hospital, International University of Health and Welfare, Chiba, Japan | |
| 5Division of Dento-oral Anesthesiology, Tohoku University Graduate School of Dentistry, Sendai, Japan | |
| Corresponding Author : | Sumihisa Aida Nippori-Jogu Hospital Tokyo 204-0023, Japan Tel: +81-3-5827-0176 Fax: +81-3-5827-0176 E-mail: aida.sum@gmail.com |
| Received November 12, 2014; Accepted November 20, 2014; Published November 22, 2014 | |
| Citation: Aida S, Wajima Z, Shiga T, Kido K, Masaki E (2014) Hyperuricemia Obstructs the Effect of Spinal Cord Stimulation on Peripheral Arterial Occlusive Diseases: The Retrospective Analysis of Eleven Cases. J Pain Relief 3:164. doi: 10.4172/2167-0846.1000164 | |
| Copyright: © 2014 Aida S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Objective: The indication of spinal cord stimulation (SCS), a treatment for intractable pain, has been expanded to include pain due to peripheral arterial occlusive disease (PAOD). However, its effectiveness may be influenced by the presence of some medical backgrounds. Then, the influences were analyzed in the current study, which identifyed an obstacle for effectiveness of SCS on PAOD.
Methods: Eleven patients with PAOD underwent implantation of a SCS device. The Fontaine’s stages (FSs) of all patients before the implantation (pre-FS) were III (4 cases) or IV (7 cases). The relationship between FS 6 months post implantation (post-FS) and the patients’ background, including age, duration of SCS introduction, chronic renal failure (CRF), diabetes mellitus (DM), hypertension, hyperuricemia (HU), hypercholesterolemia (HC), height, and body weight were retrospectively assessed.
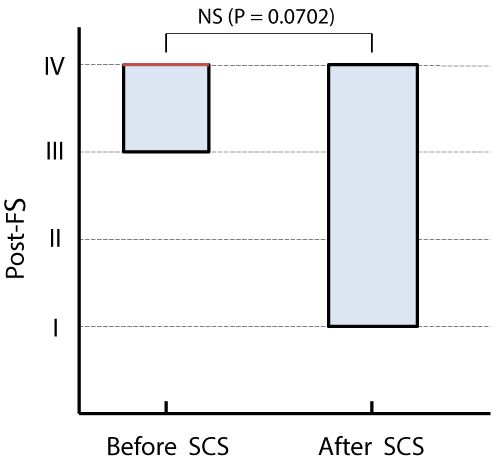
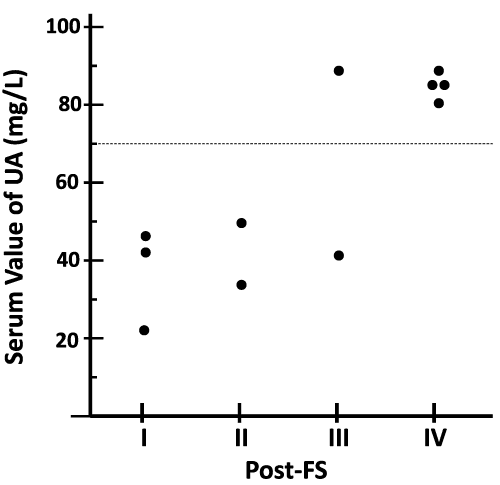
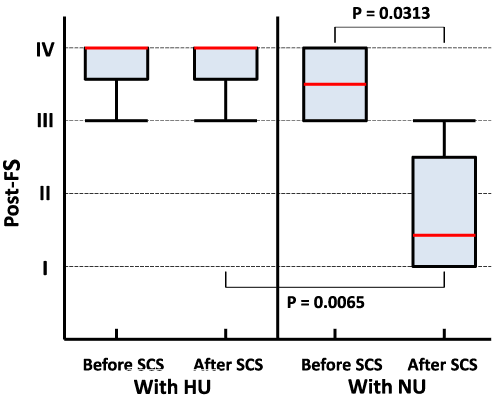
Results: The effect of SCS on PAOD varied among the 11 patients and was not significant. However, a significant Spearman correlation (r=0.7144, p=0.0182) between post-FS and the serum value of uric acid (UA) was demonstrated. Furthermore, the effectiveness of SCS was significant (p=0.0313) in the 6 patients with normouricemia (NU) when comparing the pre- vs. post-FS, and both post-FSs differed significantly (p=0.0065) when comparing NU vs. HU patients. In contrast, the improvement was null in the other 5 patients with HU. There were neither significant changes nor correlations between post-FS and all of the other background characteristics that were assessed.
Conclusion: Considering that SCS improved FS, both pain scores and tissue blood flow were improved. SCS is an effective treatment for patients with PAOD; however, the results differed depending on the presence of NU or HU. Thus, UA is suggested to be a marker of PAOD or a predictor of its prognosis.
Methods: Eleven patients with PAOD underwent implantation of a SCS device. The Fontaine’s stages (FSs) of all patients before the implantation (pre-FS) were III (4 cases) or IV (7 cases). The relationship between FS 6 months post implantation (post-FS) and the patients’ background, including age, duration of SCS introduction, chronic renal failure (CRF), diabetes mellitus (DM), hypertension, hyperuricemia (HU), hypercholesterolemia (HC), height, and body weight were retrospectively assessed.
Results: The effect of SCS on PAOD varied among the 11 patients and was not significant. However, a significant Spearman correlation (r=0.7144, p=0.0182) between post-FS and the serum value of uric acid (UA) was demonstrated. Furthermore, the effectiveness of SCS was significant (p=0.0313) in the 6 patients with normouricemia (NU) when comparing the pre- vs. post-FS, and both post-FSs differed significantly (p=0.0065) when comparing NU vs. HU patients. In contrast, the improvement was null in the other 5 patients with HU. There were neither significant changes nor correlations between post-FS and all of the other background characteristics that were assessed.
Conclusion: Considering that SCS improved FS, both pain scores and tissue blood flow were improved. SCS is an effective treatment for patients with PAOD; however, the results differed depending on the presence of NU or HU. Thus, UA is suggested to be a marker of PAOD or a predictor of its prognosis.
The indication of SCS has recently expanded to include the treatment for peripheral arterial occlusive diseases (PAOD) [5-8]. SCS has been found not only to control pain but also to salvage the extremities from amputation due to an increase in tissue blood flow [8-12]. The pain relief attributed to SCS results from reduced sympathetic tonus, because SCS itself inhibits sympathetic activity. In addition, the antidromic nerve impulses from SCS induce the secretion of vasodilators including calcitonin-gene-related peptide (CGRP), prostaglandins, and substance P [12-14].
On the other hand, patients with PAOD often have medical backgrounds that include chronic renal failure(CRF), diabetes mellitus(DM), hypertension, hyperuricemia(HU), or hypercholesterolemia(HC). These conditions with SCS may reflect the effectiveness of SCS in PAOD. In the current study, the influences of patients’ backgrounds on the effectiveness of SCS for PAOD were analyzed.
Bipolar electric stimulation with square pulses produced by the implanted generator was delivered continuously throughout the day to patients via the electrode. Patients could adjust the pulse pattern by themselves within a frequency of 2.1–130 Hz, a voltage of 0–10.5 V, and a width of 60–450 μs to achieve an optimal level of pain reduction.
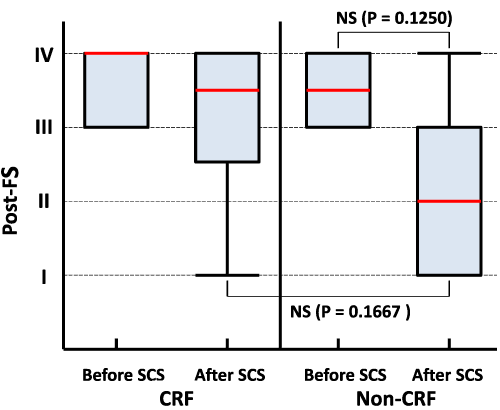
Statistically insignificant trends towards improvement were detected on comparison of the pre- vs. post-FS in the NU patients (p=0.125) and on comparison of both post-FSs in the CRF vs. non-CRF patients (p=0.167; Figure 4).
There were neither significant changes nor correlations between post-FS and age, duration of SCS introduction, DM, control of hypertension, HC, height, or body weight.
The results of previous studies have already shown that SCS produces vasodilatation and promotes peripheral circulation [8-12]. For the evaluation of PAOD severity, FS rather than pain score was utilized in the current study. Therefore, the observed decreases in FS indicated a pain relief effect as well as a circulation-promoting effect attributable to SCS. Although the findings are limited within the patients with NU only in the current study, these findings support results from previous studies indicating that SCS allowed for the salvage of necrotic extremities [8-14]. The safety and efficacy associated with SCS preceding surgical treatments should lead to its more frequent use as opposed to amputation or arterial grafts.
Chemical substances, such as UA, adenosine triphosphate (ATP), DNA, high mobility group–box chromosomal protein-1 (HMGB-1), and SAP130, have recently been implicated as important mediators in the pathologic state of tissue necrosis. In the process of necrosis due to ischemia, UA is released from necrotic tissue in order to signal danger and triggers an acute inflammatory response to sterile cell death [15]. Subsequently, macrophages, followed by neutrophils, infiltrate into necrotic tissues receiving the danger signal from these chemical substances [16]. In our current patients with HU, therefore, massive tissue necrosis had been advancing to more severe condition despite of the SCS treatment. Thus, the effect of SCS in the patients with HU was obstructed. As such, UA is suggested to be a marker of PAOD or a predictor of its prognosis.
CRF is associated with abnormal phosphorus metabolism, which induces secondary hyperthyroidism and promotes vessel calcification [17-19]. These metabolic changes may increase arterial stiffness and offset the positive effects of SCS. Then, the influence of CRF may be stronger than that of other medical backgrounds assessed in the current study. Consequently, CRF may boost the severity of PAOD, and at a glance seemed to be a risk factor for PAOD [20]. However, CRF does not usually induce HU; rather HU is a risk factor for CRF [21-23]. In a study that included a larger sample size, the offsetting effect seemed to be significant. If so, however, this result may be of pseudo-significance. Overall then, HU is ranked upstream [21,22].
In addition, UA has already been also demonstrated to be a risk factor for atherosclerosis [24-26], cardiovascular events [25,27], metabolic syndrome [23,28], and stroke [29]. Taking these findings into account, HC, DM, and body weight are likely to influence the effectiveness of SCS on PAOD. However, none of these medical backgrounds showed even minimal effects on the effectiveness, because UA is not released due to these medical backgrounds and HU is also ranked upstream. Furthermore, an increasing effect of these medical backgrounds on arterial stiffness appears still weaker than that of CRF. HU is usually caused by over-uptake or under-excretion of purine compounds, and acts as a risk factor for the aforementioned medical backgrounds. In PAOD, however, UA is released from the necrotic tissue and acts as a danger signal. Therefore, the clinical utility of assessing HU differs depending on the patient's medical background (with or without PAOD).
In the current study, most patients (90.9%) receiving SCS were males. This phenomenon may be worthy of note, and may be attributable to a gender difference [30], and/or a life-style gap between genders. In conclusion, SCS has been shown to improve pain and increase in the tissue blood flow due to PAOD. In the treatment of PAOD, the current study identified an obstacle, UA, which is suggested to be a marker of PAOD, or a predictor of its prognosis.
References
- Shealy CN, Mortimer JT, Reswick JB (1967) Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. AnesthAnalg 46: 489-491.
- Shimoji K, Higashi H, Kano T, Asai S, Morioka T (1971) [Electrical management of intractable pain]. Masui 20: 444-447.
- Shimoji K, Hokari T, Kano T, Tomita M, Kimura R (1993) Management of intractable pain with percutaneous epidural spinal cord stimulation: differences in pain-relieving effects among diseases and sites of pain. AnesthAnalg 77:110-116
- Grabow TS, Tella PK, Raja SN (2003) Spinal cord stimulation for complex regional pain syndrome: an evidence-based medicine review of the literature. Clin J Pain 19: 371-383.
- Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E (1976) Vascular disease of extremities. Electric stimulation of spinal cord and posterior roots. N Y State J Med 76: 366-368.
- Spincemaille GH, de Vet HC, Ubbink DT, Jacobs MJ (2001) The results of spinal cord stimulation in critical limb ischaemia: a review. Eur J VascEndovascSurg 21: 99-105.
- Mingoli A, Sciacca V, Tamorri M, Fiume D, Sapienza P (1993) Clinical results of epidural spinal cord electrical stimulation in patients affected with limb-threatening chronic arterial obstructive disease. Angiology 44: 21-25.
- Kumar K, Toth C, Nath RK, Verma AK, Burgess JJ (1997) Improvement of limb circulation in peripheral vascular disease using epidural spinal cord stimulation: a prospective study. J Neurosurg 86: 662-669.
- Augustinsson LE, Carlsson CA, Holm J, Jivegård L (1985) Epidural electrical stimulation in severe limb ischemia. Pain relief, increased blood flow, and a possible limb-saving effect. Ann Surg 202: 104-110.
- Horsch S, Claeys L (1994) Epidural spinal cord stimulation in the treatment of severe peripheral arterial occlusive disease. Ann VascSurg 8: 468-474.
- Amann W, Berg P, Gersbach P, Gamain J, Raphael JH (2003) Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the europian peripheral vascular diseases outcome study (SCS-EPOS) EurVascEndovasc Surg;26:280-286.
- Tiede JM, Huntoon MA (2004) Review of spinal cord stimulation in peripheral arterial disease. Neuromodulation 7: 168-175.
- Hilton SM, Marshall JM (1980) Dorsal root vasodilatation in cat skeletal muscle. J Physiol 299: 277-288.
- Linderoth B, Herregodts P, Meyerson R (1994) Sympathetic medication of peripheral vasodilatation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery 35:711-719.
- Kono H, Chen CJ, Ontiveros F, Rock KL (2010) Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest 120: 1939-1949.
- Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279-289.
- Goodman WG, London G, Amann K, Block GA, Giachelli C, et al. (2004) Vascular calcification in chronic kidney disease. Am J Kidney Dis 43: 572-579.
- London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H (2003) Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18:1731-1740.
- Shanahan CM (2005) Mechanisms of vascular calcification in renal disease. ClinNephrol 63: 146-157.
- O'Hare AM, Hsu CY, Bacchetti P, Johansen KL (2002) Peripheral vascular disease risk factors among patients undergoing hemodialysis. J Am SocNephrol 13: 497-503.
- Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, et al. (2002) A role for uric acid in the progression of renal disease. J Am SocNephrol 13: 2888-2897.
- Feig DI (2009) Uric acid: a novel mediator and marker of risk in chronic kidney disease? CurrOpinNephrolHypertens 18: 526-530.
- See LC, Kuo CF, Chuang FH, Shen YM, Ko YS, et al. (2011) Hyperuricemia and metabolic syndrome: associations with chronic kidney disease. ClinRheumatol 30: 323-330.
- Iwashima Y, Horio T, Kamide K, Rakugi H, Ogihara T, et al. (2006) Uric acid, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertension 47: 195-202.
- Ofori SN, Odia OJ (2014) Serum uric acid and target organ damage in essential hypertension. Vasc Health Risk Manag 10: 253-261.
- Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, et al. (2006) Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension 48: 1037-1042.
- Storhaug HM, Norvik JV, Toft I, Eriksen BO, Løchen ML (2013) Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: a gender specific analysis from The Tromsø Study. BMC CardiovascDisord. 13:115.
- Borges RL, Ribeiro AB, Zanella MT, Batista MC (2010) Uric acid as a factor in the metabolic syndrome. CurrHypertens Rep 12: 113-119.
- Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, et al. (2009) Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum 61: 885-892.
- Wang SF, Shu L, Wang S, Wang XQ, Mu M, et al. (2014) Gender difference in the association of hyperuricemia with hypertension in a middle-aged Chinese population. Blood Press 23: 339-344.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 13615
- [From(publication date):
November-2014 - Jul 18, 2025] - Breakdown by view type
- HTML page views : 9076
- PDF downloads : 4539
