Research Article Open Access
Hyperdry Human Amniotic Membrane (HD-AM) is Supporting Aciclovir Included Device of Poly-N-p-Vinyl-Benzyl-D-Lactonamide (PVLA) Sphere for Treatment of HSV-1 Infected Rabbit Keratitis Model
Motonori Okabe1*, Toshiko Yoshida1, Michiko Suzuki2, Mitsuaki Goto3, Masahiro Omori2, Masato Taguchi4, Ayaka Toda1, Takuma Suzuki5, Kazuaki Nakagawa2, Fumitaka Hiramoto2, Toyohiko Ushijima2, Hiroki Waki6, Etsuko Furuichi1, Kenichi Arai7, Kaixuan Zhou1, Moustafa Fathy Omar8, Makoto Nakamura7, Yoshihiro Nomura5, Toshio Kasama2, Kiyoshi Katou6, Shigeru Saito9and Toshio Nikaido1
1Department of Regenerative Medicine, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Japan
2The Research and Development Division, TOA Pharmaceuticals Co., Ltd., Japan
3Molecular Engineering Institute, Kinki University, Japan
4Department of Clinical Pharmacokinetics, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Japan
5Applied Protein Chemistry, Faculty of Agriculture, Tokyo University of Agriculture and Technology, Japan
6Department of Obstetrics and Gynecology, Takaoka City Hospital, Japan
7Department of Life Sciences and Bioengineering, Graduate School of Science and Engineering for Research (Engineering), University of Toyama, Japan
8Biochemistry Department, Faculty of Pharmacy, Minia University, Egypt
9Department of Obstetrics and Gynecology, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Japan
- *Corresponding Author:
- Okabe M
Department of Regenerative Medicine
Graduate School of Medicine and Pharmaceutical Sciences
University of Toyama, Sugitani 2630, Toyama City
Toyama Pref., 930-0194, Japan
Tel: +81-76-434- 7212
Fax: +81-76-434-5011
E-mail: okabe@med.u-toyama.ac.jp
Received date: November 09, 2016; Accepted date: December 23, 2016; Published date: December 30, 2016
Citation: Okabe M, Yoshida T, Suzuki M, Goto M, Omori M, et al. (2016) Hyperdry Human Amniotic Membrane (HD-AM) is Supporting Aciclovir Included Device of Poly-N-p-Vinylbenzyl-D-Lactonamide (PVLA) Sphere for Treatment of HSV-1 Infected Rabbit Keratitis Model. J Biotechnol Biomater 6:251. doi: 10.4172/2155-952X.1000251
Copyright: ©2016 Okabe M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The human amniotic membrane (AM) has been widely used in ophthalmic surgery, including in ocular surface reconstruction. However, whether AM is effective for the release of impregnated drugs is unknown. Hyperdry-AM (HD-AM), glutaraldehyde cross-linked HD-AM (Glu HD-AM) and a solvent of poly-N-p-vinylbenzyl- D-lactonamide (PVLA) did not cause irritation to the rabbit eye. Although the concentration of residual glutaraldehyde in Glu HD-AM was approximately 40 ppm (dry-weight basis), no eye irritation was observed. Acyclovir (ACV) containing PVLA-bearing spheres (ACV/PVLA) was loaded into HD-AM (ACV/PVLA HD-AM), and then the therapeutic efficacy of ACV/PVLA HD-AM was compared with that of ACV/PVLA solution in a herpes simplex virus-1 (HSV-1) eye infection model. The former was more effective against ocular infection, and its efficacy was dose- and volume-dependent. Thus, ACV/PVLA HD-AM sheets were very effective as a curative treatment for eye infection with HSV-1. HD-AM can be function as drug delivery system. In the future, it may be possible to combine HD-AM and PVLA in a device that can control the release of hydrophobic medicines.
Keywords
Hyperdry Human Amniotic Membrane (HD-AM); Drug storage; Herpes simplex virus-1 (HSV-1); Rabbit keratitis model; Aciclovir (ACV); Poly-N-p-vinylbenzyl-D-lactonamide (PVLA)- spheres; Drug delivery.
Introduction
Herpes simplex virus (HSV) is one of the main infectious causes of corneal blindness worldwide. A variety of clinical manifestations of both infectious and immunologic diseases affects all levels of the cornea in HSV keratitis. Acyclovir (ACV) is the main therapeutic regime for corneal ulcer [1] because HSV-1 is sensitive to ACV [2]. The common regimen for corneal herpes in Japan is administration of ACV eye ointment (ACV-O). However, because ACV-O must be administered 5 times daily and shows poor water solubility and thus low bioavailability, alternative delivery approaches are needed to increase the therapeutic potential of ACV.
Poly-N-p-vinylbenzyl-D-lactonamide (PVLA) is a superior surface-coating material for culturing hepatocytes [3,4]; it adheres to hepatocytes through a highly specific interaction between its galactose moiety and cell surface asialoglycoprotein receptors [5]. PVLA has been shown to form polymeric micelles in water because of the presence of a hydrophobic polystyrene backbone and hydrophilic sugar moieties [6]. A saline solution containing PVLA may be an effective solvent for ACV.
The human amniotic membrane (AM) has been used as a graft and covering material for patients undergoing reconstructive surgery [7] or treatment for ulcerations [8] or burns [9] because of its antiinflammatory and antimicrobial effects [10-12], anti-fibroblastic activity and anti-angiogenic properties [10] and potential to reduce scarring [13]. The AM can also produce a wide array of growth factors [14], provide a healthy new substrate suitable for re-epithelialization, and promote epithelial healing [15]. In the field of ophthalmology, the AM has been used with varying degrees of success to treat numerous ocular surface disorders [16-21].
Until recently, the AM was cryopreserved by deep-freezing to −80°C, but there was no reliable method for sterilization. To overcome these limitations, we developed a hyperdry-AM (HD-AM) under vacuum conditions using far-infrared rays and microwaves, then conducted sterilization by gamma-ray (γ) irradiation. The HD-AM is thin, safe and effective new biological material for clinical use that can be stored at 2–8°C [22] and maintains its growth factors and nutrients because of treatment with our patented hyperdry method [23]. In addition, we developed glutaraldehyde cross-linked HD-AM (Glu HD-AM) as an alternative HD-AM product. Glu HD-AM is a useful substrate for corneal perforations and tissue adhesive to conjunctival defects following multiple trabeculectomy, as it is resistant to collagenases [24,25]. The characteristics of the HD-AM and benefits of Glu HDAM have been described in clinical studies and case reports [24-32]. In addition, the primary constituents of the AM are a stromal matrix composed mainly of collagen and an epithelial layer [33]. In studies of drug delivery systems (DDS), collagen [34], gelatin [35], alginate [36] and polymer matrices such as ethylene-vinyl acetate copolymer [37] were reported to carry an aqueous solution of basic fibroblast growth factor. The HD-AM and Glu HD-AM are new biological materials made from collagen.
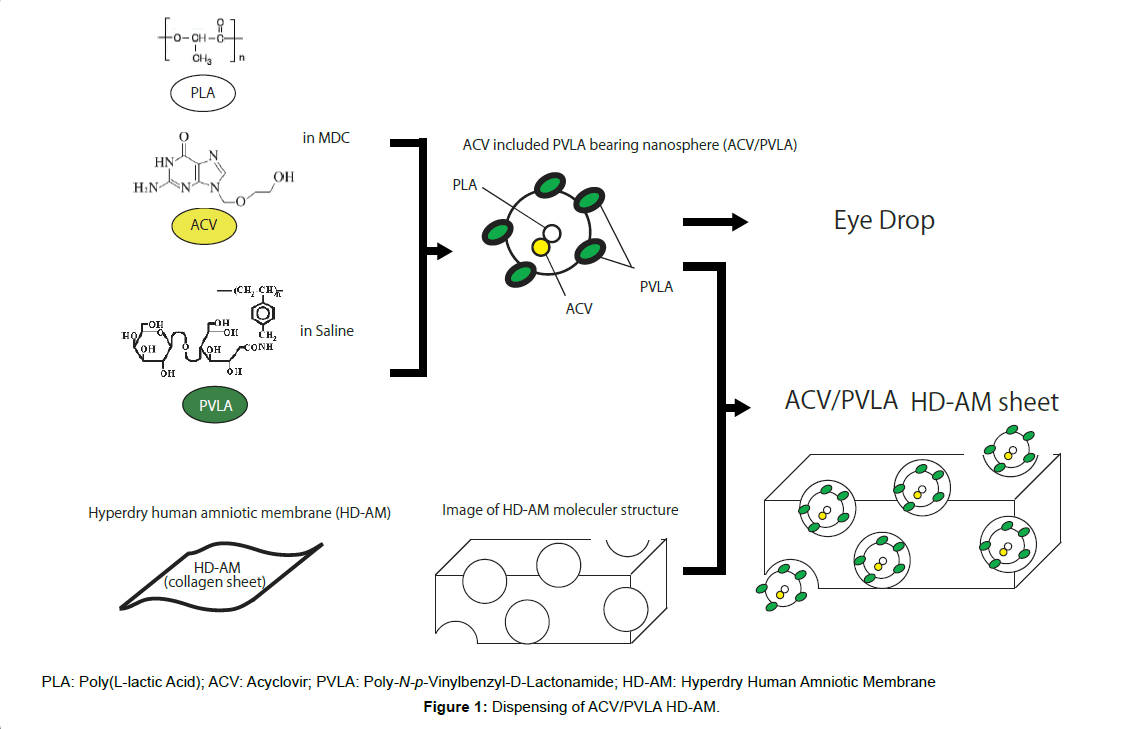
In this present study, we combined two different materials, PVLA and HD-AM, as a new DDS material for ACV. We then evaluated the 1) safety of HD-AM and associated products in an eye irritation test; 2) level of residual glutaraldehyde in HD-AM; 3) therapeutic effects of ACV-containing PVLA-bearing spheres (ACV/PVLA) impregnated in HD-AM (ACV/PVLA HD-AM) sheets compared to treatment with ACV eye drops; and 4) dose- and volume-dependent efficacy of treatment with an ACV/PVLA HD-AM device in a rabbit model of ocular infection with HSV-1. The results showed that PVLA and HDAM was effective and showed advantages as a DDS for ACV treatment (Figure 1).
Materials and Methods
Preparation of HD-AM and Glu HD-AM
AMs were obtained during elective caesarean sections from volunteers who were serologically negative for human immunodeficiency virus, hepatitis virus types B and C, syphilis and human T-lymphotropic virus-1. AMs were washed several times with sterile 0.01 M phosphatebuffered saline (pH 7.2–7.8, D5652-1L; Sigma-Aldrich, St. Louis, MO, USA) or sterile physical saline (pH 6.4, Otsuka Normal Saline; Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan).
Hyperdry AMs (HD-AMs) were prepared as reported previously [22]. AMs were placed on cooking sheets (Toyo Aluminum Ekco Products, Tokyo, Japan), and dried under 1) a vacuum (approximately 0.4 kPa), 2) far-infrared rays using a 0.4 kW heater to keep the chamber at 50°C, 3) 0.1 kW microwave irradiation (after the air pressure was increased from 0.4 to 4.6 kPa) for 1–3 min, and 4) a hyperdrying device (which again decreased the air pressure to 0.4 kPa on the wet sample). After several repetitions of this cycle of air pressure changes, the samples were dried completely but not frozen. Following this drying treatment, the boiling temperature of the AM samples was decreased to approximately 30°C at 4.60 kPa.
According to the method described by Spoerl et al. [38], the AM was cross-linked with 0.1% glutaraldehyde (20% glutaraldehyde solution; Wako Pure Chemical Industries, Ltd., Osaka, Japan) solution for 30 min. The cross-linked AM was then washed thoroughly with sterile physical saline or water for injection (pH 5.66, Otsuka Water for Injection; Otsuka Pharmaceutical Factory Inc., Tokushima, Japan), and dried with a hyperdrying device. γ-irradiation (25 kGy) was used to sterilize HD-AM and Glu HD-AM.
Judgments of eye irritation
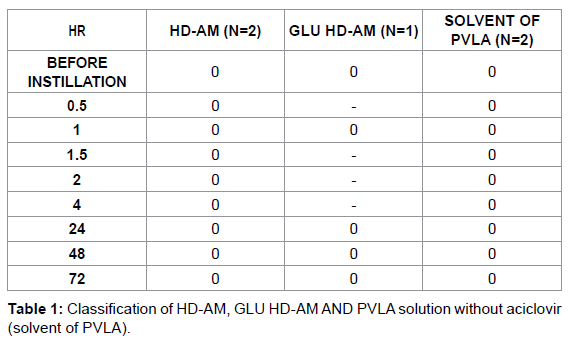
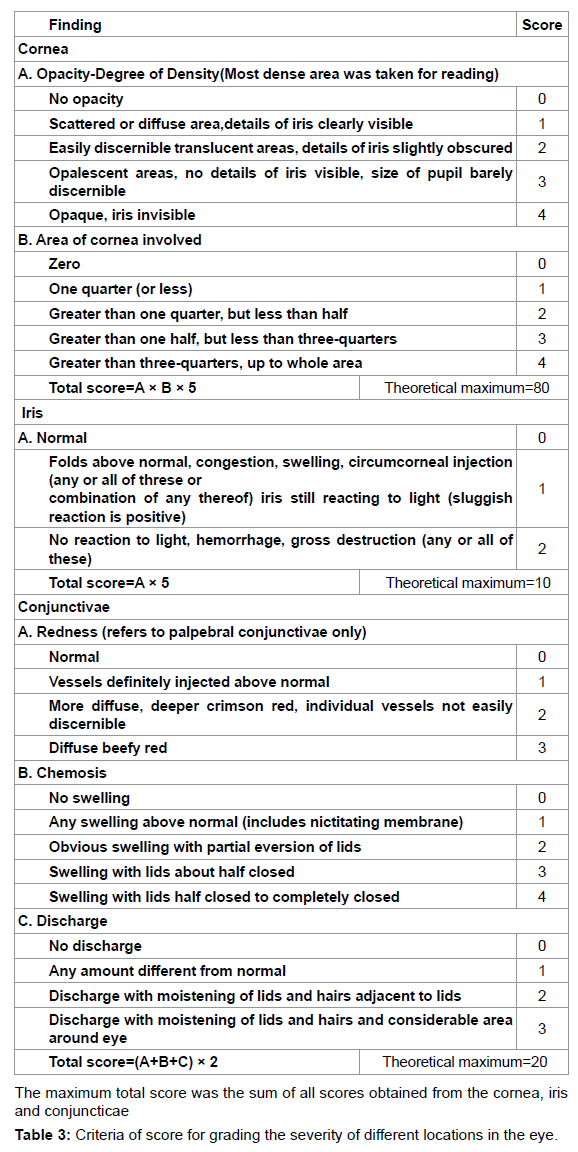
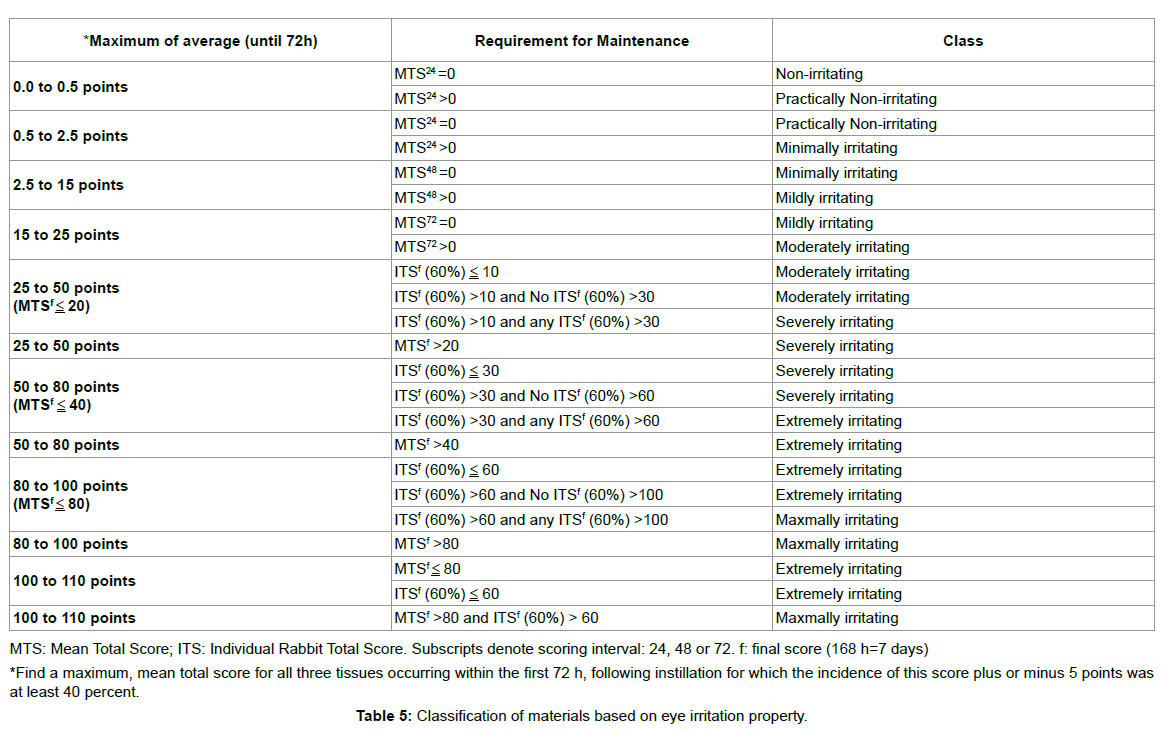
The experimental procedures used to assess eye injury were based on the scoring systems described by Draize et al. [39] and Kay et al. [40] with the following modifications. Injuries of the cornea, conjunctiva and palpebral mucosa, and iris were scored separately. For scoring, 100 μL aliquots of the tested materials (the liquid used to dilute the PVLA sphere solution and extract solutions containing homogenated HDAM or Glu HD-AM) were applied to the conjunctival sac. The time intervals were 0.5, 1, 1.5, 2, 4, 24, 48 and 72 h (3 days) after instillation of the tested materials into the eye and evaluation was conducted as shown in Tables 1-3. As a control, saline was instilled into the other eye and evaluated in the same manner.
Assay of glutaraldehyde
The residual concentration of glutaraldehyde in Glu HD-AM was determined by reversed-phase high performance liquid chromatography (HPLC) after derivatization with 2,4-dinitrophenylhydrazine (DNPH). For this procedure, 0.1 g of Glu HD-AM was soaked in 3 mL of extraction fluid containing 0.1 M glacial acetic acid and NaOH (pH 4.93), and the sample was gently stirred overnight at 4°C following homogenization. After removal of the solid residues by centrifugation, the extract was buffered to pH 3.0 with 3 mL of 1 M citrate buffer and reacted with 0.1 mL of 70% DNPH reagent (w/w in acetonitrile) at 40°C for 1 h. The DNPH-derivatized compound was serially extracted twice with 2 mL of dichloromethane. The organic phase was then evaporated to dryness with a SpeedVac® system (Savant, Farmingdale, NY, USA), the residue was dissolved in 500 μL of acetonitrile, and 100 μL of this solution was injected onto the column. The HPLC system consisted of an LC-10ATvp Liquid Chromatograph Series (Shimadzu, Kyoto, Japan) with a model SPD-10Avp ultraviolet detector (Shimadzu) and Inertsil® ODS-3 column (15 cm × 4.6 mm i.d.; 3 μm particle size; GL Sciences, Tokyo, Japan). The mobile phase was acetonitrile and water (70:30 v/v). The flow rate of the mobile phase was 1.2 mL/min and the column temperature was 40°C. Peaks were monitored at a wavelength of 360 nm and the retention time was 4.6 min for glutaraldehyde-DNPH. The quantitation limit for glutaraldehyde-DNPH was an extract concentration of 50 nM. The coefficient of intra-day variations of this assay were 2.8% and 4.3% at fluid concentrations of 1.25 and 12.5 μM, respectively.
Preparation of PVLA-bearing ACV spheres (final 0.1% and 0.5% ACV/PVLA solution)
PVLA-bearing poly(L-lactic acid) (PLA) (M=1 × 104; Wako Pure Chemicals Industries, Ltd., Osaka, Japan) spheres provided by Celagix, Inc. (Tokyo, Japan) were prepared by the modified emulsification/ solvent evaporation method as described previously [41]. Briefly, an aqueous solution (0.5 mg/mL in 20 mL) of PVLA was placed in a 30 mL sample tube in an ice bath and stirred with a magnetic stirrer. PLA (10 mg) was dissolved in 1 mL of methylene dichloride and added dropwise to the aqueous solution. The mixture was sonicated by a probe-type sonicator (UD-200; TOMY, Tokyo, Japan) for 10 min at 4°C for 1 min intervals interspersed with 1 min rests, then evaporated to remove the methylene dichloride. The formed spheres were collected by centrifugation at 17,500x g for 30 min, and then passed through a 0.2 μm filter for sterilization and adjustment of the diameter. To prepare PVLA-PLA spheres, ACV was mixed into the organic phase.
The 0.5% ACV solution was produced by adding ACV to an ACV solution at a final concentration of 0.1% ACV to yield 0.5% ACV with sonication at 40–45°C for 90 min.
Preparation of ACV/PVLA HD-AM sheets
To prepare ACV/PVLA HD-AM sheets, 100 μL of 0.1% or 0.5% ACV/PVLA solution and a single square (400 mm2) from an HD-AM sheet were incubated in a microtube at 37°C for 1 h. Following this treatment, approximately 50 μL of ACV/PVLA solution remained in the microtube.
Culture of HSV-1
The viruses used in this experiment were generated on a background of the HSV-1 strain McKrae as described by Myles et al. [42] with the following modifications. Viral stocks were propagated in culture dishes containing a confluent monolayer of Vero cells (ATCC CCL-81; Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) in Dulbecco’s Modified Eagle’s Medium (GIBCO 11965-092; Life TechnologiesTM Japan, Tokyo, Japan) containing 5% fetal bovine serum (GIBCO 26140-079; Life TechnologiesTM Japan, Tokyo, Japan) at a multiplicity of infection (MOI) of 0.01. Viral titers were generated using Vero cells.
HSV-1-infected rabbit keratitis model
Male New Zealand white (NZW) rabbits (specific pathogen-free) weighing 2 kg were obtained from Kitayama Labesu, Inc. (Nagano, Japan), handled and maintained in accordance with the tenets established by TOA Pharmaceuticals Co., Ltd. (Toyoma, Japan). HSV-1 infection was conducted as described by Myles et al., with the following modifications [42]. Rabbits were inoculated in one eye with 1.0 × 103 pfu/eye of either HSV-1 in a 10 μL suspension. Prior to inoculation, the corneas were lightly scarified in a circle from edge to center using a crosshatch pattern with a cotton swab. The selected virus was then placed into the lower conjunctival cul-de-sac of each eye, and the lower eyelid was gently massaged over the eye for 30s. After inoculation, the rabbits were treated from days 4–6, and the ACV/PVLA HD-AM sheet was administered 3 times per day at 3 h intervals. Acute infection was confirmed by slit lamp biomicroscopy on post-inoculation (PI) days 4, 5, 6 and 7.
Administration protocol
1) Eye drops (final concentration 0.5%).
Beginning on the 4th day after the inoculation procedure, ACV/ PVLA solution was administered 3 times per day at 3 h intervals. Two rabbits were used as the no-treatment group. Treatment with the 0.5% ACV/PVLA HD-AM sheet group and treatment with the 0.5% ACV/ PVLA solvent eye drop group were scored as the mean ± S.E.M. for n=three rabbits.
2) Sustained-release effect of the ACV/PVLA HD-AM device
Four days after the inoculation procedure, the ACV/PVLA HD-AM sheet was administered 3 times per day at 3 h intervals by incubating the sheet in the conjunctival sac for 1 h. For high-dose administration, 1 or 3 pieces of the 0.5% ACV/PVLA HD-AM sheet were kept in the conjunctival sac, while for low-dose administration, 1 piece of the 0.1% ACV/PVLA HD-AM sheet was kept in the conjunctival sac. One rabbit was not treated. The 0.5% ACV/PVLA HD-AM sheet group and 0.1% ACV/PVLA HDAM sheet group were scored as the mean ± S.E.M. for n=three rabbits.
3) Criteria for treatment assessment.
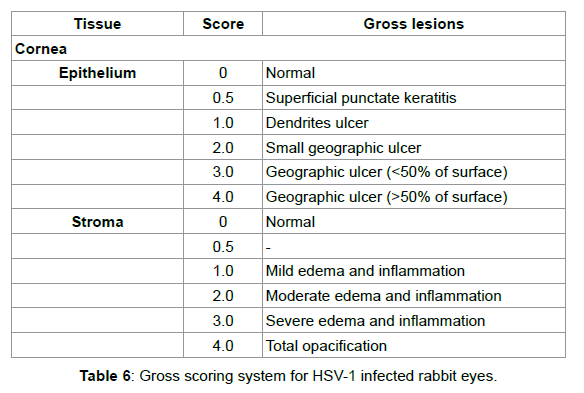
NZW rabbits were observed daily for signs of corneal change or ocular infection. Infection progress was followed by slit-lamp biomicroscopy and using fluorescein to visualize epithelial defects. Ocular lesions were scored as described by Nauss et al. [43], with some modifications as shown in Table 4.
Statistical analysis
Data were evaluated as the mean values from three rabbits (triplicate assays). A total of three (n=3) data was used in each analysis. Student’s t-test for independent samples was performed using SPSS version 22 software for Mac (IBM Japan, Ltd., Tokyo, Japan). Comparisons were two-sided with significance levels of 5% (*) and 1% (**).
Results
Residual glutaraldehyde
In the present study, we successfully detected residual glutaraldehyde from the two HD-AM samples, although no studies have measured glutaraldehyde via derivatization with 2,4-DNPH. The extract concentrations of glutaraldehyde were 3.29 μM and 3.73 μM for the No. 1 and No. 2 samples, respectively (Table 6). Thus, the mean glutaraldehyde remaining in the HD-AM was approximately 40 ppm on a dry weight basis.
Development of experimental rabbit model for studying ocular infections with HSV-1
Four days after ocular administration of HSV-1, signs of infection appeared on the cornea (Figure 2). As the infection progressed, epithelial deficiency and stromal edema/inflammation were observed by fluorescein staining (Figures 2C and 2D).
Controlled release effect of ACV/PVLA HD-AM
The treatment effects of ACV were observed for both 0.5% ACV/ PVLA eye drops and 0.5% ACV/PVLA HD-AM sheets. The slit lamp examination score of 0.5% ACV/PVLA HD-AM was significantly lower than that of 0.5% ACV/PVLA eye drops (Figure 3a).
Eye drop treatment resulted in geographic ulcer in two rabbits and dendritic ulcer in one rabbit. In three rabbits, dendritic ulcer was observed after ACV/PVLA HD-AM treatment. The diameter (�?) or rectangular area of the epithelium deficiency in the group receiving the eye drop treatment was 1.0–2.0 mm. The �? or rectangular area of epithelium deficiency in the group receiving ACV/PVLA HD-AM treatment was 0.5–1.0 mm. The area of epithelium deficiency following eye drop treatment was much greater than that after ACV/PVLA HD-AM treatment on day 7 (Figure 3b).
ACV concentration-dependently inhibited the progress of HSV-1 infection in rabbit eyes
Following treatment of one 0.1% ACV/PVLA HD-AM sheet or three 0.5% ACV/PVLA HD-AM sheets for one-time administration, the progress of HSV-1 infection was decreased in a dose-dependent manner (Figure 4a). Treatment with 0.5% ACV/PVLA HD-AM showed significantly strong inhibition on days 6 and 7.
Figure 3: Time course of SLE (Slit Lamp score) score treated by 0.5% ACV/PVLA solvent eye drop or 0.5% ACV/PVLA HD-AM sheet for ocular infection. a) ACV mitigated the symptoms of virus infection in rabbit eyes. The treatment was started from day 4 to 6 and the scores were taken after treatment on days 4, 5, 6 and 7. �?�: Control; �?�: 0.5% ACV/PVLA solvent eye drops; �?�: 0.5% ACV/PVLA HD-AM sheet (1 piece). The values shown are the means ± S.E.M. of triplicate assays.
Double and single asterisks (�? and �?�?) indicate significant differences at the level of P<0.05 and P<0.01 by Student’s t-test, respectively. b) Gross lesion score levels of rabbits treated with different method on day 7
Following treatment of one 0.1% ACV/PVLA HD-AM sheet or three 0.5% ACV/PVLA HD-AM sheets for one-time administration, the progress of HSV-1 infection was decreased in a dose-dependent manner (Figure 4a). Treatment with 0.5% ACV/PVLA HD-AM showed significantly strong inhibition on days 6 and 7.
Discussion
Ocular irritation tests for HD-AM and Glu HD-AM are important steps in ensuring safety in both the medical industry and medical technology. Irritation testing using rabbits has largely remained unchanged for many years. The Draize, and Kay and Calandra eye irritation tests [39,40] are governmentally endorsed methods described in the OECD testing guidelines (TG) 405 (OECD, 2002). Dreize and Kay set the time interval at 96 h to determine if residual injury was present, while Calandra conducted evaluated at 168 h (7 days). However, in our experiments, there was no score after 72 h, thus we modified the interval to 72 h. HD-AM, Glu HD-AM, and solvent of PVLA showed no eye irritation.
Figure 4: Time course of SLE score treated by a 0.1% ACV/PVLA HD-AM sheet (1 piece) or 0.5% ACV/PVLA HD-AM sheet (3 pieces). a) The treatment was started from day 4 to 6, and the scores were taken after treatment in the manner described in Figure 3. �?�: Control; �?�: 0.1% ACV/PVLA HD-AM sheet (1 piece); �?�: 0.5% ACV/PVLA HD-AM sheet (3 pieces).
The values shown are the means ± S.E.M. of triplicate assays. Single and double asterisks (�? and �?�?) indicate significant differences at the level of P<0.05 and P<0.01 by Student’s t-test, respectively. b) Gross lesion score levels of rabbits treated with different dose on day.
Denaturation or degradation of the implanted collagen over time is an important problem. In the past, these effects have been prevented by intermolecular cross-linkage by processing with glutaraldehyde solution [44]. These materials, made from porcine and bovine pericardium as pericardial bioprosthesis, are processed into the mitral valve, aortic valve, etc. Various aldehydes have been used for this purpose, including glyoxal, formaldehyde [45], dialdehyde starch, and glutaraldehyde [44], because of their reduced thrombogenicity in bioprosthetic heart valves.
The mean residual glutaraldehyde level in an extract from Glu HDAM was 3.51 μM, which corresponds to approximately 40 ppm (dryweight basis). Although the HD-AM and Glu HD-AM did not elicit eye irritation or inflammation, an additional process for decreasing residual aldehyde levels may be preferable prior to surgical implantation of the Glu HD-AM.
ACV [47], valacyclovir, and famciclovir [48,49] are common medical treatments against HSV-1 in Japan. A 3% ACV eye ointment (ACV-O) is often prescribed to patients with herpetic keratitis. An ACV-O medicine has also been developed to exploit the hydrophobic properties of ACV. However, eye ointment treatment can be inconvenient because ACV-O must be administered 5 times per day, and frequently causes blurred vision after administration. Therefore, new drugs like as eye drops etc. for herpetic keratitis are needed.
PVLA-related materials can encapsulate Z-Asp, which dissolve well in DMSO and caspase inhibitor, in its nanospheres and are specifically absorbed by hepatocytes [50]. In this experiment, we incorporated hydrophobic ACV into PVLA (ACV/PVLA), because of its amphipathic properties. We evaluated both an 1) ACV/PVLA solution as an eye drop-administered drug and 2) ACV/PVLA HD-AM sheet that holds the ACV/PVLA. An in vivo stable HSV-1-infected rabbit model was constructed, beginning on the 4th day after administration of HSV- 1 (Figure 2). The effect of treatment with ACV/PVLA HD-AM was significantly better than that of ACV/PVLA solvent administered as eye drops (Figure 3a). Evaluation on day 7 showed that all three indices examined, i.e., 1) the state of the gross lesion, 2) the diameter or rectangular area of epithelium deficiency, and 3) the number of epithelium-deficient regions, indicated that ACV/PVLA HD-AM treatment was better than eye drop treatment (Figure 3b). Thus, the drug-impregnated HD-AM was able to control the release capacity of the drug and achieve a therapeutic effect.
Following ACV/PVLA HD-AM administration, the progress of HSV-1 infection was decreased in a dose-dependent manner (Figure 4a). Moreover, all parameters at the 0.5% concentration of ACV were better than those at the 0.1% concentration (Figure 4b). This indicates that ACV/PVLA HD-AM showed a therapeutic effect against HSV-1. The effect of treatment with 0.5% PVLA sphere solution was approximately 80% of effect of treatment with 3% ACV-O (data not shown). Although the concentrations of ACV included in the PVLA spheres solution were lower than that in general ACV-O, PVLA sphere solution may exhibit controlled release of ACV. However, HD-AM may leave PVLA contain ACV to itself, which was effective for releasing PVLA particles (Figure 3). Based on these two effects, ACV may suppress HSV-1 infection grade in a rabbit model. Regulating drug delivery would increase therapeutic efficacy and decrease potential side effects.
Several beneficial effects of HD-AM have been confirmed in clinical evaluations. HD-AM shows good bone surface prevention [27- 29,31], greatly decreases pain [30], and decreases injury dehydration [28,29]. HD-AM is useful as a biomaterial substrate to treat glaucoma filtering bleb leak, corneal perforation [26], and recurrent pterygium[22]. Corneal perforation is often caused by HSV-1. HD-AM may be useful for both treatment of corneal perforation resulting from HSV-1 and controlled drug release with ACV, including PVLA.
Final concentrations of 0.1% and 1.0% ACV (ACV/PVLA solutions) were initially prepared, but both caused precipitation during laboratory-to-laboratory transfer. Increasing the temperature to 40– 45°C and sonication for 90 min resulted in a clear and fully dissolved 0.1% ACV/PVLA solution. This 0.1% ACV/PVLA solution was stable at room temperature for additional one month. We thus prepared a stable 0.5% ACV/PVLA solution at 37°C with this procedure. However, the 1.0% ACV included PVLA sphere solution did not remain clear.
Generally, 1 g of ACV will dissolve in approximately 800 mL of water (0.12%). Using PVLA, we increased the concentration of ACV up to 4-fold.
Conclusion
In summary, we demonstrated that 1) administration of HDAM, Glu HD-AM, or solvent of PVLA resulted in no eye irritation; 2) following Glu HD-AM administration, 3.51 μM of residual glutaraldehyde was present (approximately 40 ppm on a dry-weight basis), and no eye irritation was observed; 3) the ACV/PVLA HDAM sheet was much more effective for treatment in the HSV-1 eye infection rabbit model than the eye drop-administration method, as it also controlled PVLA particle release; and 4) the combination of HDAM and PVLA-bearing spheres was a very useful for drug delivery as a hydrophobic medicine requires continuous administration.
Acknowledgement
We thank the late Assoc. Prof. Kiyotaka Kitagawa (Department of Ophthalmology, Graduate School of Medicine and Pharmaceutical Sciences, Univ. of Toyama), Seishi Suzuki (Biocon (Japan) Ltd.), Takashi Nakaya (Biocon (Japan) Ltd.), Masahiko Arakawa (SAKURA SEIKI Co., Ltd.), Masatoshi Kawai (Koga Isotope Ltd.), Mitsugu Ishida (Nichi-Iko Pharmaceutical Co., Ltd.), Dr. Masamichi Iwama and Masaki Kashima (Toyama Prefectural Agricultural, Forestry & Fisheries Research Center) for their technical assistance.
This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Grant-in-Aid for Scientific Research (C), contract grant number: JP25460647 and JP16K08905 (to Okabe).
References
- Kaye S, Choudhary A (2006) Herpes simplex keratitis. Prog Retin Eye Res 25: 355-380.
- Field HJ, Bell SE, Elion GB, Nash AA, Wildy P (1979) Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob Agents Chemother 15: 554-561.
- Kobayashi A, Akaike T, Kobayashi K, Sumitomo H (1986) Enhanced Adhesion and Survival Efficiency of Liver-Cells in Culture Dishes Coated with a Lactose-Carrying Styrene Homopolymer. Makromol Chem-Rapid 7: 645-650.
- Tobe S, Takei Y, Kobayashi K, Akaike T (1992) Receptor-Mediated Formation of Multilayer Aggregates of Primary Cultured Adult-Rat Hepatocytes on Lactose-Substituted Polystyrene. Biochem Biophys Res Commun 184: 225-230.
- Kobayashi A, Goto M, Kobayashi K, Akaike T (1994) Receptor-mediated regulation of differentiation and proliferation of hepatocytes by synthetic polymer model of asialoglycoprotein. J Biomater Sci Polym Ed 6: 325-342.
- Goto M, Kobayashi K, Hachikawa A, Saito K, Cho CS, et al. (2001) Micellar behavior of sugar-carrying polystyrene in aqueous solution. Macromol Chem Phys 202: 1161-1165.
- Filipas D (2001) Vaginal reconstruction/fistulae. Curr Opin Urol 11: 267-270.
- Bennett JP, Matthews R, Faulk WP (1980) Treatment of chronic ulceration of the legs with human amnion. Lancet 1: 1153-1156.
- Maral T, Borman H, Arslan H, Demirhan B, Akinbingol G, et al. (1999) Effectiveness of human amnion preserved long-term in glycerol as a temporary biological dressing, Burns. Journal of the International Society for Burn Injuries 25: 625-635.
- Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F (2000) Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19: 348-352.
- Li J, Koike-Soko C, Sugimoto J, Yoshida T, Okabe M, et al. (2015) Human Amnion-Derived Stem Cells Have Immunosuppressive Properties on NK Cells and Monocytes. Cell Transplant 24: 2065-2076.
- Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schonheyder HC, et al. (2001) Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol 94: 224-229.
- Trelford JD, Trelford-Sauder M (1979) The amnion in surgery, past and present. Am J Obstet Gynecol 134: 833-845.
- Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, et al. (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20: 173-177.
- Graue-Hernez EO, Zuniga-Gonzalez I, Hernez-Camarena JC, Jaimes M, Chirinos-Saldana P, et al. (2012) Tectonic DSAEK for the Management of Impending Corneal Perforation. Case Rep Ophthalmol Med 2012: 916528.
- Gomes JA, dos Santos MS, Cunha MC, Mascaro VL, Barros JdeN, et al. (2003) Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology 110: 466-473.
- Taylan Sekeroglu H, Erdem E, Dogan NC, Yagmur M, Ersoz R, et al. (2011) Sutureless amniotic membrane transplantation combined with narrow-strip conjunctival autograft for pterygium. Int Ophthalmol 31: 433-438.
- Hick S, Demers PE, Brunette I, La C, Mabon M, et al. (2005) Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: A review of 33 cases. Cornea 24: 369-377.
- Espana EM, Grueterich M, Soval H, Solomon A, Alfonso E, et al. (2003) Amniotic membrane transplantation for bullous keratopathy in eyes with poor visual potential. Journal of cataract and refractive surgery 29: 279-284.
- Tseng SCG, Prabhasawat P, Lee SH (1997) Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol 124: 765-774.
- Tsubota K, Satake Y, Ohyama M, Toda I, Takano Y, et al. (1996) Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol 122: 38-52.
- Okabe M, Kitagawa K, Yoshida T, Suzuki T, Waki H, et al. (2014) Hyperdry human amniotic membrane is useful material for tissue engineering: physical, morphological properties, and safety as the new biological material. J Biomed Mater Res A 102: 862-870.
- Toda A, Okabe M, Yoshida T, Nikaido T (2007) The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 105: 215-228.
- Kitagawa K, Okabe M, Hayashi A, Nikaido T (2009) Combined use of a novel dried cross-linked amniotic membrane and tissue sdhesive to conjunctival defect following multiole trabeculectomy. Toyama Medical Journal 20: 1-3.
- Kitagawa K, Okabe M, Yanagisawa S, Zhang XY, Nikaido T, et al. (2011) Use of a hyperdried cross-linked amniotic membrane as initial therapy for corneal perforations. Jpn J Ophthalmo 55: 16-21.
- Kitagawa K, Yanagisawa S, Watanabe K, Yunoki T, Hayashi A, et al. (2009) A hyperdry amniotic membrane patch using a tissue adhesive for corneal perforations and bleb leaks. Am J Ophthalmol 148: 383-389.
- Tomita T, Hayashi N, Okabe M, Yoshida T, Hamada H, et al. (2011) New dried human amniotic membrane is useful as a substitute for dural repair after skull base surgery. J Neurol Surg B 73: 302-307.
- Shojaku H, Takakura H, Okabe M, Fujisaka M, Watanabe Y, et al. (2011) Effect of hyperdry amniotic membrane patches attached over the bony surface of mastoid cavities in canal wall down tympanoplasty. Laryngoscope 121: 1953-1957.
- Kanazawa Y, Shojaku H, Okabe M, Fujisaka M, Takakura H, et al. (2012) Application of hyperdry amniotic membrane patches without fibrin glue over the bony surface of mastoid cavities in canal wall down tympanoplasty. Acta Otolaryngol 132: 1282-1287.
- Arai N, Tsuno H, Okabe M, Yoshida T, Koike C, et al. (2012) Clinical application of a hyperdry amniotic membrane on surgical defects of the oral mucosa. J Oral Maxillofac Surg 70: 2221-2228.
- Tsuno H, Arai N, Sakai C, Okabe M, Koike C, et al. (2014) Intraoral application of hyperdry amniotic membrane to surgically exposed bone surface. Oral Surg Oral Med Oral Pathol Oral Radiol 117: e83-e87.
- Okabe M, Kitagawa K, Yoshida T, Koike C, Katsumto T, et al. (2013) Application of 2-octyl-cyanoacrylate for corneal perforation and glaucoma filtering bleb leak. Clin Ophthalmol 7: 649-653.
- Bourne GL (1966) The anatomy of the human amnion and chorion. Proc R Soc Med 59: 1127-1128.
- Gilbert DL, Kim SW (1990) Macromolecular release from collagen monolithic devices. J Biomed Mater Res 24: 1221-1239.
- Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y (2000) Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 21: 489-499.
- Edelman ER, Mathiowitz E, Langer R, Klagsbrun M (1991) Controlled and modulated release of basic fibroblast growth factor. Biomaterials 12: 619-626.
- Langer R, Folkman J (1976) Polymers for the sustained release of proteins and other macromolecules. Nature 263: 797-800.
- Spoerl E, Wollensak G, Reber F, Pillunat L (2004) Cross-linking of human amniotic membrane by glutaraldehyde. Ophthalmic Res 36: 71-77.
- Draize JH, Woodard G, Calvery HO (1944) Methods for the study of irritation and toxicity of substances applied especially to the skin and mucous membranes. J Pharmacol Exp Ther 82: 377-390.
- Kay JH, Calra JC (1962) Interpretation of eye irritation tests. J Soci Cosm Chem 13: 281-289.
- Maruyama A, Ishihara T, Adachi N, Akaike T (1994) Preparation of nanoparticles bearing high density carbohydrate chains using carbohydrate-carrying polymers as emulsifier. Biomaterials 15: 1035-1042.
- Myles ME, Alack C, Manino PM, Reish ER, Higaki S, et al. (2003) Nicotine applied by transdermal patch induced HSV-1 reactivation and ocular shedding in latently infected rabbits. J Ocul Pharmacol Th 19: 121-133.
- Nauss KM, Anderson CA, Conner MW, Newberne PM (1985) Ocular infection with herpes simplex virus (HSV-1) in vitamin A-deficient and control rats. J Nutr 115: 1300-1315.
- Carpentier A, Lemaigre G, Robert L, Carpentier S, Dubost C (1969) Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg 58: 467-483.
- McClurg WM, Lawford PV, Hughes H, Rogers S (1996) Formaldehyde replaces glutaraldehyde in porcine bioprosthetic heart valves. J Heart Valve Dis 5: 343-347.
- Dyas A, Das BC (1985) The activity of glutaraldehyde against Clostridium difficile. J Hosp Infect 6: 41-45.
- McCulley JP, Binder PS, Kaufman HE, O'Day DM, Poirier RH (1982) A double-blind, multicenter clinical trial of acyclovir vs idoxuridine for treatment of epithelial herpes simplex keratitis. Ophthalmology 89: 1195-1200.
- Gatchel S (1996) Use of famciclovir and valaciclovir in the treatment of viral keratitis, Clinical infectious diseases: An official publication of the Infectious Diseases. Society of America 22: 886.
- Vere Hodge RA, Sutton D, Boyd MR, Harnden MR, Jarvest RL (1989) Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-l-yl)guanine; penciclovir]. Antimicrob Agents Chemother 33: 1765-1773.
- Shibuya I, Akaike T, Watanabe Y (2000) Design of a temporally and spatially controlled drug delivery system for the treatment of liver diseases in mice. Hepatology 32: 1300-1308.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 6046
- [From(publication date):
March-2017 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 5069
- PDF downloads : 977