Humoral Immune Response of Vaccinated Employees against SARS-CoV-2 in a Tertiary Hospital in Germany: CALM-UKA (COVID-19 Antibody Level Monitoring University Hospital Augsburg)
Received: 15-Apr-2022 / Manuscript No. JIDT-22-60866 / Editor assigned: 20-Apr-2022 / PreQC No. JIDT-22-60866 (PQ) / Reviewed: 04-May-2022 / QC No. JIDT-22-60866 / Revised: 11-May-2022 / Manuscript No. JIDT-22-60866 (R) / Published Date: 18-May-2022 DOI: 10.4172/2332-0877.1000499
Abstract
Objectives: In the context of the SARS-CoV-2 pandemic, it is important to better understand whether and, more importantly, how long individuals who were administered a SARS-CoV-2 vaccination are protected from future infections. Healthcare workers are at permanent risk of exposure to SARS-CoV-2-safe and lasting vaccination response is therefore of immense importance. The aim of the present work is to record antibody titer against SARS-CoV-2 in a large cohort of vaccinated employees at the university hospital over time and to identify possible factors influencing antibody formation.
Methods: We retrospectively analyzed a cohort of 1045 employees who received vaccination against SARS-CoV-2 during the course with BNT162b2 vaccine from Biontech/Pfizer. Blood was drawn from the employees at predefined time points and analyzed for the presence of SARS-CoV-2 antibodies (IgG) against the Receptor Binding Domain (RBD) of the Spike protein (S). In addition, all participants completed a questionnaire.
Results: A total of 863 females (82.6%) and 182 males (17.4%) were screened. The mean antibody titer was 69127.8 BAU/ml (SD=69319.6 BAU/ml) in female participants and 60867.8 BAU/ml (SD=65249.6 BAU/ml) in male participants. There was no significant difference concerning gender and antibody levels (p=0.071). In a multivariate analysis, we found a significant influence of age on the antibody formation (p<0.001); the older the participants, the lower the antibody level. The highest antibody levels were detected in study participants 30-50 days after their first vaccination. 50 days after vaccination only lower antibody titer in proband could be detected.
Conclusion: In the large cohort of 1045 health care workers, a high heterogeneity of antibody titers was observed. There is a significant influence of age on antibody levels-older employees had a lower antibody titer. 50 days after vaccination only lower antibody titer in proband could be detected.
Keywords: Immune response; SARS-CoV-2; Antibodies; Vaccination
Introduction
COVID-19 is a viral disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), first described in 2019 [1]. Most patients present with a mild course of disease with fever and cough as the most common symptoms. However, a smaller proportion of patients suffers from severe disease courses and requires inpatient or even intensive care treatment with mainly respiratory insufficiency and failure. More than three million people in Germany have been infected with the coronavirus, and about 10 % of the infected patients required hospitalization [2]. Medical personnel are particularly exposed due to their close contact with patients with a known or suspected infection. Therefore, it is essential that this group receives an early vaccination and, if possible, a favorable response to the vaccine. However, so far, studies have shown very heterogeneous results regarding the antibody status of medical staff in the inpatient sector [3-6].
Following infection with SARS-CoV-2, the extent and duration of pathogen-specific cellular and humoral immune responses vary widely. Often, SARS-CoV-2 antibodies are detectable in patients` blood for months after surviving the disease; however, several studies have also shown that in a number of patients suffering from SARS-CoV-2, detectable antibody levels persist for only a few months [6-8].
Currently, data on the kinetics of the immune response after vaccination are mainly known from registration studies of vaccines. However, the exact mechanisms of protection against infection after vaccination or preceding infection are currently unclear. Therefore, the detection of a specific immune response does not allow a reliable conclusion on actual protection against infection after vaccination. Nevertheless, high antibody titers are believed to be associated with a good response to vaccination followed by protection or a milder course of disease.
Since January 2021 medical staffs in Germany have been vaccinated systematically as part of the prioritization process due to their high risk of exposure. Thus, a large number of employees at Augsburg University Hospital (UKA) have already received two vaccinations. In addition, all employees of the UKA were vaccinated with the BNT162b2 vaccine from Biontech/Pfizer.
The aim of the present study was to analyze the kinetics of the humoral immune response after vaccination against SARS-CoV-2. In addition, the effect of potential influencing variables on the immune response was analyzed to understand interindividual differences of the immune response of vaccinated individuals.
Materials and Methods
Study design and methods
Study design: This single-center study had a retrospective observational design and was carried out in the University Hospital Augsburg, Germany. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice. In addition, the study was approved by the Ethics Committee of the Ludwig- Maximilians-University in Munich (21-0530).
The COVID-19 registry of University Hospital Augsburg (COKA registry) was established to collect data regarding infection with SARS- CoV-2 and COVID-19 vaccinations. The collection of data from employees was voluntary and pseudonymized.
The data analyzed in the present study were included in the COKA registry between 01/01/2021 and 05/19/2021. After given written informed consent, a standardized blood sample was collected from employees and analyzed on the same day. Employees introduced themselves at several predetermined times, the blood samples were taken prospectively and results were registered in the COKA registry. Later, the COKA registry data were retrospectively analyzed. In total 1045 employees took part in this study.
In addition, the study participants received an accompanying questionnaire containing questions on age, sex, immunosuppressive medication, chronic diseases or immunodeficiency, allergies and clinical symptoms after vaccination. Afterwards, the questionnaires were reviewed by a physician and the reported previous diseases were classified into relevant (e.g. adipositas or diabetes mellitus) and irrelevant (e.g. alopecia) previous diseases. Relevant previous diseases are those which are already known to affect the immune system in a relevant way. Regarding immunosuppressive therapy, common immunosuppressive drugs were listed as checkbox responses. All additional drugs mentioned by the study participants were also checked by a physician and classified on the basis of studies whether they could have a relevant effect on the immune system or not.
.In addition, the questionnaire asked whether multiple vaccinations were needed in other vaccinations in the past. It was explained to the study participants that a need for multiple vaccinations exists if more vaccination doses than usual had been necessary in the past to obtain adequate protection (for example, multiple vaccinations against hepatitis B in case of an insufficient titer).
Participating employees were recruited into two groups: the high- resolution and low-resolution groups. In the high-resolution group blood samples were taken from the included employees at the baseline before vaccination. Two further blood collections took place at fixed dates, namely on days 14 and 35 after the first vaccination. As all included employees of the high-resolution cohort received their second vaccination on day 21 after the initial vaccination, the study participants were thus fully vaccinated at their third visit.
We assumed that an adequate response to the vaccination would be detectable two weeks after the second vaccination. The primary purpose was to analyze the early antibody response and its course shortly after vaccination.
In the low-resolution group, vaccinated employees were recruited regardless of the first and second vaccination dates. The day of the second vaccination in this group was not always on the 21st day after the first vaccination due to personal preferences or capacity reasons. The primary purpose of this cohort was to evaluate the development of the humoral immune response in the months following complete vaccination. However, all participants denied having neither any symptoms nor a positive PCR test before enrollment in the study.
Test Method: The collected serum samples were tested in the in- house laboratory for the presence of SARS-CoV-2 antibodies (IgG) against the Receptor Binding Domain (RBD) of the Spike protein (S) using immunoassays Elecsys® Anti-SARS-CoV-2 (Roche Diagnostics, Germany). The quantitative result (sensitivity 99.85%, specificity 99.98% linear measuring range is between 8.72 and 54.500 BAU/ml) was evaluated according to the manufacturer`s specifications. Serum samples with results more than 54.500 BAU/ml were titrated by dilution analysis up to a maximum of 1:10. Antibody titers less than 8.72 BAU/ ml cannot be detected according to the manufacturer's instructions.
Statistical evaluation: Data were initially analyzed descriptively with categorical variables presented as absolute frequencies and percentages. Continuous variables were described with means and standard deviations. Dependency between categorical variables was carried out using Chi-Squared Test. A comparison of means was done using Mann-Whitney-U Test. Multivariate analysis of the high- resolution group was performed using one-factor ANOVA with repeated measures with study visit, gender, age, steroidal medication, necessity for multiple vaccination in previous vaccinations, chronic disease, and side effects after first dose of vaccine as covariate to compare the average change in antibody titer. The analysis of antibody level over time was performed using time difference between the first vaccination and antibody titer measurement, as well as time difference between both vaccinations. Age, gender, chronic disease, intake of steroidal medication, intake of immunosuppressants, necessity for multiple vaccination in previous vaccinations, and side effects after the first and second vaccination as influencing variables. Multivariate analysis of the data from the low-resolution group was performed using multiple linear regressions. The IBM SPSS (Statistical Package for the Social Sciences) version 27 program was used for data analysis.
Results
Study population
One thousand forty-five employees were included between 01/01/2021 to 05/19/2021, and the level of antibody titers against the Receptor-Binding Domain (RBD) of the Spike protein (S) of SARS- CoV-2 was measured. All employees included in the study received vaccination against SARS-CoV-2 with the BNT162b2 vaccine from Biontech/Pfizer. 17.4% (n=182) of participants were male and 82.6% (n=863) were female. The largest proportion of included employees was between 50 and 59 years old (28.0%, n=292), followed by employees within 40 and 49 years (22.9%, n=239). 12.7% (n =133) of study participants reported suffering from a chronic disease or immune deficiency.
4.5% (n=47) of employees took steroid medications, and another 1.1% (n=12) were on immunosuppressants. 0.5% (n=5) were taking biologicals, 0.5% (n=5) were taking immunomodulators, 0.1% (n=1) were taking methotrexate, 0.1% (n=1) reported therapy with tacrolimus. 41.5% (n=434) indicated a known allergy with 58.3% (n=609) having no allergies. 8.9% (n=93), 81.9% (n=856) and 9% (n=93) of participants reported lower immune responses to previous other vaccinations (e.g. to hep. B vaccination) in the past, so that additional doses had to be adminiseterd. 2% (n=21) of employees reported increased vaccination reactions in the past. Details are listed in Table 1. Symptoms after the first SARS-CoV-2 vaccination were affirmed by 30.8% (n=322) and denied by 38.9% (n=406). 30.3% (n=317) made no statement.
| Dimension | Number | % |
|---|---|---|
| Number of employees (n) | 1045 | |
| Gender | ||
| male | 182 | 17.40% |
| female | 863 | 82.60% |
| Age (years) | ||
| 20 - 29 | 213 | 20.40% |
| 30 - 39 | 186 | 17.80% |
| 40 - 49 | 239 | 22.90% |
| 50 - 59 | 292 | 28.00% |
| 60 - 82 | 114 | 10.90% |
| not specified | 1 | 0.10% |
| Chronic diseases/ immunodeficiency | ||
| yes | 133 | 12.70% |
| no | 910 | 87.10% |
| Steroid medication | ||
| yes | 47 | 4.50% |
| no | 998 | 95.50% |
| Immunosuppressants | ||
| Methotrexat | 1 | 0.10% |
| Azathrioprin | 0 | 0.00% |
| Cyclophosphamid | 0 | 0.00% |
| Sirolimus | 0 | 0.00% |
| Tacrolimus | 1 | 0.10% |
| Biologicals | 5 | 0.50% |
| Immunmodulators | 5 | 0.50% |
| none | 1033 | 98.90% |
| Known allergies | ||
| No | 609 | 58.30% |
| Yes | 434 | 41.50% |
| Unknown | 2 | 0.20% |
| More vaccination doses needed than usual | ||
| No | 856 | 81.90% |
| Yes | 93 | 8.90% |
| unknown | 94 | 9.00% |
| Previous vaccination reactions | ||
| no | 1021 | 97.70% |
| yes | 21 | 2.00% |
| Side effects after 1st vaccination | ||
| no | 406 | 38.90% |
| yes | 322 | 30.80% |
| unknown | 317 | 30.30% |
| Side effects after 2nd vaccination | ||
| no | 134 | 12.80% |
| yes | 313 | 30.00% |
| unknown | 598 | 57.20% |
Table 1: Descriptive statistics of the whole collective - gender distribution, previous diseases, age distribution, steroidal medication, intake of immunosuppressants. Known allergies and side effects after the vaccinations are listed.
High-resolution group-early kinetics after the first and second vaccination
In total, 208 employees were recruited in the high-resolution group. They received the first blood sample on day 0 before vaccination. 208 (100.0%) staff members had complete data at baseline. 175 (84.1%) of employees came for the second study visit 14 days after the first vaccination and from 141 (67.8%) employees blood was taken on the 35th day after the first vaccination and therefore 15 (IQR: 15-18.25) days after the second vaccination. The second vaccination occurred 21 days after the first vaccination for all employees in the high-resolution group.
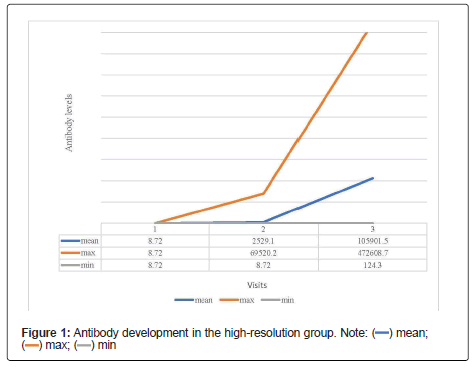
The descriptive data of the high-resolution group is shown in Table 2. Five (2.4%) of the 208 employees were not eligible and therefore excluded from further analysis due to an antibody titer level above 8.72 BAU/ml before being administered the first dose of vaccine. Consequently, the remaining 203 employees had no detectable antibody titer (<9.72 BAU/ml) upon first visit and therefore were found eligible for the inclusion in the data analysis. Second study visit taking place 14 days after the first vaccination was attended by 175 staff members. The average antibody titer upon second visit accounted for 2520.1 (SD=8349.4) BAU/ml. The maximum titer level measured was 69520.2 BAU/ml, whereas 6 study participants (2.9%) still did not have any detectable antibody titer (<8.72 BAU/ml). The third visit took place 36 days after the first vaccination and 14 days after the second vaccination. One hundred forty-one staff members showed up for the third study visit. The average antibody titer measured was 105900.0(SD=87904.1) BAU/ml. The lowest antibody measured was 124.26 BAU/ml and the maximum titer accounted for 472608.7 BAU/ml. The development of the antibody titers across three study visits is shown in detail in Table 3 and Figure 1.
| Dimension | Number | % |
|---|---|---|
| Number of employees (n) | 208 | |
| Excluded because of prior infection with SARS-CoV-2 | 5 | |
| Gender | ||
| male | 40 | 19.20% |
| female | 168 | 80.80% |
| Age (years) | ||
| 20 - 29 | 50 | 24% |
| 30 - 39 | 42 | 20.20% |
| 40 - 49 | 42 | 20.20% |
| 50 - 59 | 52 | 25.00% |
| 60 - 82 | 22 | 10.60% |
| Chronic diseases/ immunodeficiency | ||
| yes | 37 | 17.80% |
| no | 171 | 82.20% |
| Steroidal medication | ||
| yes | 12 | 5.80% |
| no | 196 | 94.20% |
| Immunosuppressants | ||
| Mycophenolat-Mofetil | 1 | 0.05% |
| Biologicals | 1 | 0.05% |
| Immunmodulators | 1 | 0.05% |
| none | 205 | 98.50% |
Table 2: Descriptive data from the high-resolution group including 208 employees - Gender distribution, previous diseases, age distribution, steroidal medication and the intake of immunosuppressants are listed.
| Visit | Number/ n | Days since vaccination - (median) | Mean of the antibodies in BAU/ml | Standard deviation | Maximum value in U/ml | Minimum value in U/ml |
|---|---|---|---|---|---|---|
| Visit 1 | 203 | 0 | <8.72 | 0 | <8.72 | <8.72 |
| Visit 2 | 175 | 14 | 2529.1 | 8349.4 | 69520.2 | <8.72 |
| Visit 3 | 141 | 36 | 105901.5 | 87904.1 | 472608.7 | 124.3 |
Table 3: Development of antibody titers over the course of the three visits in the high-resolution group - descriptive listing of the level of antibodies: the development of the antibodies is very heterogeneous.
In a serial measurement analysis, increase of antibody levels after vaccination was highly age-dependent (p<0.05). Study participants being 30 to 39 years old having the highest antibody titers and study participants above 60 years having the lowest antibody titers at the third visit. The change between the genders was also found significant, however only for the third visit. Therefore, antibody titers were significantly higher in females compared to males (p<0.05).
Low-resolution group observing a more prolonged time course of the antibody levels
Eight hundred twenty-nine employees were included in the low- resolution group. All employees had received at least one vaccination and had no known history of infection (Table 4).
| Number/ n | Mean of antibody titer in BAU/ml | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 3 | Visit 1 | Visit 2 | Visit 3 | Visit 1 | Visit 2 | Visit 3 | |
| Gender | |||||||||
| Male | 29 | 22 | 4063.5 | 63538.3 | 0.255 | 0.013 | |||
| Female | 146 | 119 | 2132 | 113732.8 | |||||
| Age (years) | |||||||||
| <29 | 41 | 29 | 2177.8 | 130512.2 | 0.035 | 0.057 | |||
| 30-39 | 33 | 26 | 1469.3 | 135720.3 | |||||
| 40-49 | 37 | 34 | 821.9 | 91348.5 | |||||
| 50-59 | 43 | 34 | 5824.9 | 92442.95 | |||||
| >60 | 20 | 18 | 409.8 | 76082 | |||||
| Steroidal medication | |||||||||
| no | 163 | 131 | 2576.8 | 107061.9 | 0.45 | 0.572 | |||
| yes | 11 | 10 | 610.4 | 90698.9 | |||||
| More vaccination doses needed than usual | |||||||||
| no | 145 | 118 | 2775.3 | 106211.8 | 0.552 | 0.948 | |||
| yes | 12 | 7 | 2775.1 | 96247 | |||||
| unknown | 15 | 14 | 889.4 | 101871.1 | |||||
| Chronic diseases/ immunodeficiency | |||||||||
| no | 160 | 127 | 2644.3 | 102791.4 | 0.504 | 0.207 | |||
| yes | 15 | 14 | 1137.9 | 1341 | |||||
| Side effects after 1st vaccination | |||||||||
| no | 70 | 67 | 3023.7 | 119644.9 | 0.644 | 0.876 | |||
| yes | 23 | 22 | 2031.8 | 123409.8 | |||||
| Side effects after 2nd vaccination | |||||||||
| no | 28 | 26 | 4131.1 | 101952.1 | 0.329 | 0.238 | |||
| yes | 66 | 64 | 2134.9 | 128816.2 | |||||
Table 4: Intermediate subject effects - Unidimension analysis of the high-resolution cohort comparing the three visits on day 0, 14 and 35.
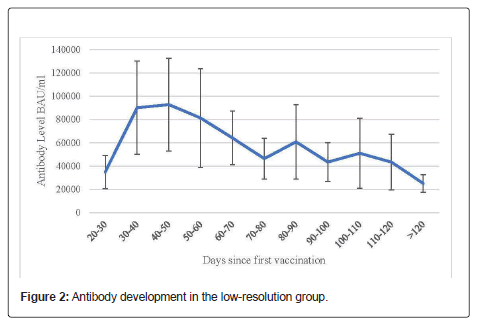
17 % (n=171) of the participants were male and 83% (n=658) were female. The largest proportion of included employees was within 50 and 59 years old (28.7%, n=238), followed by employees within 40 and 49 years (23.8%, n=197). 19.2% (n=159) suffered from chronic diseases or immunodeficiency. Details of the descriptive data of this group are listed in Table 5 and Figure 2.
| Dimension | Number | Percent |
|---|---|---|
| Number of employees(n) - low-resolution group | 829 | |
| Gender | ||
| Male | 171 | 17.00% |
| Female | 658 | 83.00% |
| Age (years) | ||
| 20 - 29 | 161 | 19.40% |
| 30 - 39 | 140 | 16.90% |
| 40 - 49 | 197 | 23.80% |
| 50 - 59 | 238 | 28.70% |
| Chronic diseases/ immunodeficiency | ||
| yes | 159 | 19.20% |
| no | 668 | 80.80% |
| Steroidal medication | ||
| yes | 35 | 4.20% |
| no | 793 | 95.70% |
| Immunosuppressants | ||
| Methotrexat | 2 | 0.20% |
| Tacrolimus | 1 | 0.10% |
| Biologicals | 4 | 0.50% |
| Immunmodulators | 4 | 0.50% |
| none | 818 | 98.70% |
Table 5: Descriptive data of the low-resolution group including 829 employees - Gender distribution, previous diseases, age distribution, steroidal medication and the intake of immunosuppressants are given.
A unidimensional comparison of antibody level across relevant characteristics is shown in Table 6. Since this group was heterogeneous in terms of days passed after the first vaccination, this variable was considered as confounder in the comparison of means. No significant difference in the levels of antibodies between men and women were observed (p=0.339).
| Category | Dimension | Mean in BAU/ml | SD in BAU/ml | p-value |
|---|---|---|---|---|
| Gender | Male | 60867.78 | 65249.58 | 0.339 |
| Female | 69127.8 | 69319.64 | ||
| =<29 | 97905.98 | 87110.62 | ||
| Age (years) | 30-39 | 68358.26 | 60891.76 | 0 |
| 40-49 | 55814.54 | 51875.28 | ||
| 50-59 | 59838.82 | 60137.48 | ||
| >=60 | 60257.38 | 80947.76 | ||
| Chronic diseases | no | 67457.92 | 62836.32 | 0.625 |
| yes | 68166.42 | 80496.5 | ||
| Steroidal medication | no | 67671.56 | 68048.7 | 0.851 |
| yes | 68696.16 | 82382.2 | ||
| Immunosuppressants | no | 68210.02 | 68953.4 | 0.037 |
| yes | 34045.06 | 31490.1 | ||
| More vaccination doses needed than usual | no | 67322.76 | 68792.08 | 0.421 |
| yes | 60266.1 | 48688.12 | ||
| unknown | 78381.9 | 82722.28 | ||
| Side effects after 1st vaccination | no | 62677.18 | 59147.76 | 0.153 |
| yes | 70019.42 | 65905.76 | ||
| Side effects after 2nd vaccination | no | 61537.04 | 64776.52 | 0.984 |
| yes | 61678.74 | 56448.92 |
Table 6: Unidimensional comparison of antibody level across relevant characteristics/influencing variables using mann-whitney-u test in the low resolution group: significant influence of age and immunosuppressants on the antibody formation.
Antibody levels detected in the low-resolution group 30-40 days after the first vaccination were significantly higher compared to earlier ones. The highest mean antibody level was determined 40-50 days after the first vaccination. Antibody levels determined later than 50 days after the first vaccination show a decreasing trend (Figure 2).
A unidimensional comparison of antibody level across relevant characteristics, and influencing variables using Mann-Whitney-U test groups is listed in Table 6. As this group was heterogeneous in terms of days elapsed since first vaccination, this variable was considered as a potential confounder in the comparison of means. There was no significant difference on the level of antibodies between men and women (p=0.339).
However, the age of the employees had a significant influence on the level of antibody titer formation (p<0.01). The older the employees were, the lower the level of antibody titers was.
In a univariate analysis the presence of a chronic disease had no significant effect on the level of titers (p=0.625). The use of steroid medication (p=0.851) also had no significant effect on antibody formation, whereas immunosuppressants showed a significant negative effect on the level of antibody titers in the univariate analysis (p<0.05).
No significantly lower antibody formation could be detected among employees stating that they needed more vaccination doses than usual formerly to obtain sufficient antibody levels (p=0.421). In a multivariate analysis, the time interval after the first vaccination in days and the difference between the two vaccinations in days were considered as potential confounding variables in addition to the confounders listed above.
Results of the multivariate analysis of antibody formation with time interval to vaccination as a potential confounder can be seen in Table 7.
Accordingly, the multivariate analysis confirmed significant influence of age on antibody formation. The older the study participants were, the lower their titer levels were (p<0.01). Furthermore, the interval between the first vaccination and the titer measurement had a significant influence on level of titer in blood (p<0.01). The more time had passed since vaccination, the lower the titers were. Increased interval between administration of two vaccination doses was significantly associated with higher levels of antibody titer (p<0.01). The use of immunosuppressive drugs was shown in the univariate analysis as inhibitor of antibody formation (p<0.05). In the multivariate analysis this finding could not be confirmed (p=0.337); however, the intake of steroidal medication had a significant influence on the antibody titer (p<0.05) (Table 7).
| Categories | Beta | p-value |
|---|---|---|
| Time difference between vaccination and blood samples | -0.361 | 0 |
| Time difference between vaccinations | 0.309 | 0 |
| Age | -0.221 | 0 |
| Gender | 0.015 | 0.776 |
| Chronic diseases | -0.033 | 0.552 |
| Steroidal medication | 0.109 | 0.038 |
| Immunsuppressants | -0.051 | 0.337 |
| More vaccination doses needed than usual | -0.015 | 0.776 |
| Side effects after 1st vaccination | -0.016 | 0.775 |
| Side effects after 2nd vaccination | 0.003 | 0.952 |
Table 7: Multivariate analysis of antibody formation in the low resolution group with time interval to vaccination as a potential confounder.
Side effects after vaccinations
30.8% (n=322) employees stated to have suffered from side effects after their first vaccination. 38.9% (n=406) declared to not have suffered side effects, 30.3% (n=419) employees did not make a statement (Table 8).
| 1st Vaccination | 2nd Vaccination | ||||
|---|---|---|---|---|---|
| Category | Dimension | N | % | N | % |
| Side effect | no | 406 | 38.9 | 134 | 12.8 |
| yes | 322 | 30.8 | 313 | 30 | |
| Myalgias | no | 487 | 46.6 | 290 | 27.8 |
| yes | 110 | 10.5 | 149 | 14.3 | |
| Fever | no | 568 | 54.5 | 328 | 31.4 |
| yes | 28 | 2.7 | 110 | 10.5 | |
| Limb pain | no | 523 | 50 | 263 | 25.2 |
| yes | 74 | 7.1 | 175 | 16.7 | |
| Redness injection site | no | 557 | 53.3 | 392 | 37.5 |
| yes | 41 | 3.9 | 45 | 4.3 | |
| Pain injection site | no | 371 | 35.5 | 251 | 24 |
| yes | 227 | 21.7 | 186 | 17.8 | |
| Fatigue | no | 470 | 45 | 243 | 23.3 |
| yes | 125 | 12 | 195 | 18.7 | |
| Nausea | no | 578 | 55.3 | 403 | 38.6 |
| yes | 15 | 1.4 | 33 | 3.2 | |
| Diarrhoea | no | 584 | 55.9 | 421 | 40.3 |
| yes | 8 | 0.8 | 15 | 1.4 | |
| Lymph node swelling | no | 571 | 54.6 | 407 | 38.9 |
| yes | 23 | 2.2 | 30 | 2.9 | |
Table 8: Descriptive statistics of complaints regarding the entire cohort.
After the first vaccination, myalgia occurred among 10.5% of employees (n=110), fever among 2.7% (n=28), limb pain among 7.1% (n=74), local redness at the injection site among 3.9% (n=41), pain at the injection site among 21.7% (n=227), fatigue among 12.0% (n=125), nausea and vomiting among 1.4% (n=15), diarrhea among 0.8% (n=8), and lymph node swelling among 2.2% (n=23). The data solely refer to the employees who made a statement about the symptoms.
After the second vaccination, 30.0% (n=313) employees had complaints, and 12.8% (n=134) had none. There was no response from 57.2% (n =700) employees. After the second vaccination, myalgia occurred among 14.3% of employees (n=149), fever among 10.5% (n=110), limb pain among 16.7% (n=175), local redness at the injection site among 4.3% (n=45), pain at the injection site among 17.8% (n=186), fatigue among 18.7% (n=195), nausea and vomiting among 3.2% (n=33), diarrhea among 1.4% (n=15), and lymph node swelling among 2.9% (n=30). Details on the occurrence of side effects after first and second vaccination are listed in Table 9.
| 1th Vaccination | 2nd Vaccination | ||||||
|---|---|---|---|---|---|---|---|
| N | % | p-value | N | % | p-value | ||
| Gender | male | 42 | 35.00% | 40 | 60.60% | 0.071 | |
| female | 280 | 46.10% | 0.026 | 273 | 71.70% | ||
| Age (years) | =<29 | 77 | 53.10% | 0.035 | 69 | 74.20% | 0.125 |
| 30-39 | 58 | 45.30% | 56 | 66.70% | |||
| 40-49 | 76 | 42.70% | 88 | 75.90% | |||
| 50-59 | 83 | 43.90% | 74 | 68.00% | |||
| >= 60 | 28 | 31.80% | 26 | 56.50% | |||
| Chronic diseases | no | 42 | 10.40% | 0.084 | 16 | 12.00% | 0.95 |
| yes | 47 | 14.60% | 37 | 11.80% | |||
| Allergies | no | 167 | 40.20% | 0.01 | 161 | 64.10% | 0.003 |
| yes | 155 | 49.80% | 150 | 77.30% | |||
| Previous vaccination reactions | no | 313 | 44.00% | 0.472 | 303 | 69.30% | 0.113 |
| yes | 8 | 53.30% | 8 | 100.00% | |||
Table 9: Occurrence of side effects after 1st and 2nd vaccination according to categories-data of the entre cohort: there is no influence of side effects on the level of antibodies. Known allergies had a significant influence on occurrence of side effects.
Females were significantly more likely to develop symptoms after the first (35.0% vs. 46.1%, p<0.05) vaccination than males, with this relationship balancing out after the second vaccination (60.6% vs. 71.7%, p=0.071). A known allergy significantly favored the occurrence of symptoms after both vaccinations. Thus, 49.8% of the employees developed symptoms after the first vaccination, whereas in non-allergic persons this proportion amounted to only 40.2% (p=0.01). The same trend was observed after the second vaccination, with 77.3% allergy sufferers and 64.1% non-allergy sufferers experiencing symptoms after vaccination (p<0.01).
Discussion
Our study investigated antibody formation over time in a large group of 1045 employees at University Hospital Augsburg after vaccination against SARS-CoV-2 with the BNT162b2 vaccine from Biontech/ Pfizer, as well as possible influencing factors.
The study participants were recruited in two different groups. In the high-resolution group, the early kinetics of the antibody development after the first and second vaccination was measured and examined. This questioning is of immense importance since there are only few publications dedicated to short term immune response on the vaccination against SARS-CoV-2. In the low-resolution group, the focus was on observing a more prolonged time course of antibody levels.
According to the results of the present study, upon second study visit the mean titer level accounted for 2521.82 BAU/ml (SD=8355.96). The increase of antibody titer after the second dose of vaccine was more prominent with a mean value of 105901.56 BAU/ml (SD=87906.17). Furthermore, in 6 (2.88%) study participants in the high-resolution arm no antibodies were detected 14 days after their first vaccination, whereas in employees administered the second dose of vaccine in each study participant an antibody level of at least 117.72 BAU/ml could be detected. In general, it is known that the presence of anti-spike or anti-nucleocapsid IgG antibodies is associated with a reduced risk of a SARS-CoV-2 infection in the following six months. Currently, there is still no general/ international recommendation concerning an exact antibody titer that might protect against further infections [9-11]. An antibody level of more than 218 BAU/ml is regarded as an adequate response to vaccination in our in-house guidelines. In this study no significant influence of chronic diseases or steroidal medication on the early kinetics of antibody response in the high-resolution group can be shown. Since only three study participants in the high-resolution group were taking immunosuppressants, this characteristic could not be considered as influencing variable, neither in unidimensional nor in multivariate analysis of the high-resolution group. In the pivotal studies people receiving immunosuppressive therapies were excluded [12,13].
In the low-resolution group the intake of steroid medications was detected in multivariate analysis as a significant factor influencing the formation of antibodies. When employees reported to take steroid medication, their antibody titer was slightly higher. Data show that systemic intake of steroids does not affect the level of antibodies after pneumococcal vaccination in COPD patients [14]. Currently, the data on the effect of corticosteroids in mRNA vaccines are limited but recent studies have shown an influence of steroids on the immune response after vaccination [15]. In the pivotal study of the Pfizer/Biontech and Moderna vaccine against SARS-CoV-2, the intake of 20 mg oral prednisolone dose was allowed [16-18]. Unfortunately, a subanalysis on this group is not available so far. A possible influencing factor not considered in our data was the dose of steroid therapy taken and the intake interval.
Overall, immunosuppressive therapies are known to affect and reduce the immune response after vaccination [19]. Regarding mRNA vaccines, the data situation is still limited. A small cohort study has shown that patients who received kidney transplants and subsequent immunosuppressive therapy showed an insufficient antibody formation after vaccination against SARS-CoV-2 [18]. The dose of immunosuppression was a major factor influencing the level of antibody titer in this case. Recent studies showed that a significantly lower antibody response could be detected within six months after first vaccination among participants with immunosuppressions [20-22]. In total, 11 members of the low-resolution cohort reported receiving immunosuppressive therapy. We could not detect significantly lower antibody titers in our immunosuppressed employees after accounting for the time interval to vaccination in the multivariate analysis. However, we also did not have data on the dose of immuno-suppression and the last time of intake.
Looking at the development of antibodies in the low-resolution group over a longer period of time, it becomes apparent that study participants with chronic disease did not have a significant different antibody level. However, numerous studies have shown association between chronic disease such as diabetes mellitus and reduced immune response after hepatitis B vaccination [23-25]. Various HLA antigens have also been found to have a negative impact on the humoral immune response and might be associated with chronic diseases. An inhibiting effect of chronic disease such as diabetes mellitus on the immune response after vaccination could be shown in other studies [24].
Gender-specific reactions on vaccines have already been shown in multiple studies; however, there are no data yet on their effect with mRNA vaccines against SARS-CoV-2. Overall, it could be shown that females develop a more robust reaction on cellular and humoral levels after infection or after vaccination. Thus, a significantly increased proportion of formed neutralizing antibodies could be measured in influenza vaccination in women [26,27]. Aside from genetic factors, this may also be explained by the level of sexual hormones and their effects on the immune system females with high levels of estrogen show a more robust humoral response. Conversely males with high testosterone levels showed a weaker formation of antibodies following vaccinations, as testosterone has an immunosuppressive effect in other studies [27].
Our study showed a gender dependent influence on the formation of antibodies at the third visit-35 days after the first vaccination was administered in the high-resolution group. Females showed a higher level of antibodies compared to men. The most likely assumption here is a short-term effect on early antibody formation, as no significance can be shown in either second visit in the high-resolution group or the low-resolution group.
Furthermore, female employees were significantly more likely to develop side effects after the first vaccination in our study population of the low-resolution group. After the first vaccination side effects occurred in 46% of woman and in 35% of men. According to a US study, women tend to develop increased symptoms after SARS-CoV-2 vaccination. Again, a possible influencing factor is the dampening effect of testosterone on the immune system.
It is well-known that human immune system undergoes profound changes with increasing age-the risk of infections increases and the effectiveness of vaccination decreases in elderly people [28,29]. The present study showed lower level of antibody in elderly study participants and higher levels in younger study participants. As the dependency between age and antibody titer levels was significant upon second and third study visit as well as in the low-resolution cohort, the effect of the age might be the strongest among those uncovered in the present study.
Additionally, a recent publication on antibody level after vaccination with mRNA vaccines against SARS-CoV-2 confirms that older people tend to develop less antibody titer than younger people [30]. Another current cohort study showed that people above the age of 80 years develop a significantly lower level of IgG antibody titer and neutralizing antibodies [31].
Employees older than 60 years had fewer symptoms after the first vaccination. However, the significant influence of the age and gender is no longer present after the second vaccination. Again, age-related limitations of the immune system may be a possible cause [18].
Regarding the cohort investigated in this manuscript, it should be noted that its participants were of working age. Therefore, the antibody response in people who are over 80 years could not be studied in our work. Nevertheless, it has been shown that age is one of the most important factors facilitating severe course of disease in individuals infected with SARS-CoV-2 [32,33].
If the employees stated that they suffered from an allergy of any kind, side effects were significantly more frequent after both the first and the second vaccination. There is currently no evidence that a pre- existing allergic diathesis leads to increased relevant side effects such as anaphylactic reactions [34,35].
In the low-resolution cohort, the focus was on observing a more prolonged time course of the antibody levels. After the second vaccination, there is a decrease in the level of titers starting from day 50 after the first vaccination. It is already known that mRNA vaccines induce B-cell responses a maximum two weeks after the booster vaccination. Afterwards the concentration of the measurable antibodies decrease until the B cells reach a sustained memory phase [36]. It also could be shown that after two doses of BNT162b1 the B-cells follow this pattern as well and the IgGs against the RBD started to decrease 43 days after the first vaccination. However, the level of neutralizing antibodies was still increasing 43 days after the initial vaccination [37]. Another study could show that SARS-CoV-2 mRNA-based vaccination induces a persistent B cell response and creates a robust humoral immunity despite increasing IgA and IgG antibodies [38].
Furthermore, the activity of neutralizing antibodies often correlates with protective immunity [39]. Recent studies have shown a decrease in antibody titers after six months. Data on this are becoming increasingly important in order to make a statement on the necessity of booster vaccination [40,41]. Based on our data, no statement can be made about immunity in the long-term course.
Limitations of our clinical study include that we did not analyse the level of neutralizing antibodies or the T-cell reactions in this study. Our cohort includes a significantly higher proportion of women and, on average, a young age of 44 years. Additionally, our study is a retrospective evaluation of registry data. Employees completed the questionnaire independently, and no further medical information was available. Another limitation is that although all participants denied having symptoms or a positive PCR test, no specific PCR testing of the participants was performed. Asymptomatic SARS-CoV-2 infections may therefore have affected antibody titers.
Conclusion
This study provides insight into the heterogeneity of IgG antibody formation after the first and second SARS-CoV-2 vaccination in a large collective of 1045 UKA employees. Overall, the response to the vaccination was good. There was a good response to the first vaccination and even more pronounced increase in antibody levels after the second vaccination.
We could show a decreased immune response in older employees after the first and second vaccination with BNT162/b2 against SARS- CoV-2. This may indicate that the efficacy of vaccination in the elderly should be monitored as it progresses and that individual intervals for booster vaccination may be necessary to ensure adequate protection. In general, the SARS-CoV-2 vaccination is associated with formation of an adequate level of antibodies.
Authors Contribution
Christoph Römmele and Elisabeth Schnoy contributed equally to the study.
References
- WHO (2020) Novel Coronavirus (2019-nCoV) - Situation Report.
- Institute RK (2021) Epidemiologischer Steckbrief zu SARS-CoV-2 und COVID-19.
- Sotgiu G, Barassi A, Miozzo M, Saderi L, Piana L, et al. (2020) SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med 20:203.
[Crossref] [Google Scholar] [PubMed]
- Sikkema RS, Pas SD, Nieuwenhuijse DF, Toole AO, Verweij J, et al. (2020) COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis 20:1273-1280.
[Crossref] [Google Scholar] [PubMed]
- Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, et al. (2020) SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol 128:104437.
[Crossref] [Google Scholar] [PubMed]
- Brehm TT, Schwinge D, Lampalzer S, Schlicker V, Kuchen J, et al. (2021) Seroprevalence of SARS-CoV-2 antibodies among hospital workers in a German tertiary care center: A sequential follow-up study. Int J Hyg Environ Health 232:113671.
[Crossref] [Google Scholar] [PubMed]
- Rudberg AS, Havervall S, Manberg A, Falk AJ, Aguilera K, et al. (2020) SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun 11:5064.
[Crossref] [Google Scholar] [PubMed]
- Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo C, et al. (2020) Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 5:e475-e483.
[Crossref] [Google Scholar] [PubMed]
- Lumley SF, Donnell DO, Stoesser NE, Matthews PC, Howarth A, et al. (2021) Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med 384:533-540.
[Crossref] [Google Scholar] [PubMed]
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, et al. (2021) Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med 385:1474-1484.
[Crossref] [Google Scholar] [PubMed]
- Melgaco JG, Azamor T, Ano Bom APD (2020) Protective immunity after COVID-19 has been questioned: What can we do without SARS-CoV-2-IgG detection? Cell Immunol 353:104114.
[Crossref] [Google Scholar] [PubMed]
- Pyhälä R, Alanko S, Forsten T, Haapa K, Kinnunen L, et al. (1994) Early kinetics of antibody response to inactivated influenza vaccine. Clin Diagn Virol 1:271-278.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, et al. (2020) Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586:589-593.
[Crossref] [Google Scholar] [PubMed]
- Deroux A, Marx A, Burkhardt O, Schweiger B, Borkowski A, et al. (2006) Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patients. Vaccine 24:1537-1542.
[Crossref] [Google Scholar] [PubMed]
- Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, et al. (2021) Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2. MedRxiv.
[Crossref] [Google Scholar] [PubMed]
- Lee H, Punt JA, Miller DC, Nagpal A, Smith CC, et al. (2021) Do Corticosteroid Injections for the Treatment of Pain Influence the Efficacy of mRNA COVID-19 Vaccines? Pain Med 22:994-1000.
[Crossref] [Google Scholar] [PubMed]
- Roux A, Marx A, Burkhardt O, Schweiger B, Borkowski A, et al. (2006) Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patients. Vaccine 24:1537-1542.
[Crossref] [Google Scholar] [PubMed]
- Kanda N, Tsuchida T, Tamaki K (1996) Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol 106:410-415.
[Crossref] [Google Scholar] [PubMed]
- Assen S, Holvast A, Benne CA, Posthumus MD, Leeuwen MA, et al. (2010) Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 62:75-81.
[Crossref] [Google Scholar] [PubMed]
- Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, et al. (2021) Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2 : A Prospective Cohort Study. Ann Intern Med 174:1572-1585.
[Crossref] [Google Scholar] [PubMed]
- Rahav G, Lustig Y, Lavee J, Benjamini O, Magen H, et al. (2021) BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine 41:101158.
- Embi PJ, Levy ME, Naleway AL, Patel P, Gaglani M, et al. (2021) Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults - Nine States, January-September 2021. MMWR Morb Mortal Wkly Rep 70:1553-1559.
[Crossref] [Google Scholar] [PubMed]
- Craven DE, Awdeh ZL, Kunches LM, Yunis EJ, Dienstag JL, et al. (1986) Nonresponsiveness to Hepatitis B Vaccine in Health Care Workers. Ann Intern Med 105:356-360.
[Google Scholar] [PubMed]
- Zimmermann P, Curtis N (2019) Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev 32:e00084-e00118.
[Crossref] [Google Scholar] [PubMed]
- Varla-Laftnerioti M, Papanicolaou M, Spyropoulou M, Vallindra H, Tsiroyianni P, et al. (1990) HLA-associated non-responsiveness to Hepatitis B vaccine. Tissue Antigens 35:60-63.
[Crossref] [Google Scholar] [PubMed]
- Chong-Shan Wang, Shan-Tair W, Chou P (2002) Efficacy and cost-effectiveness of influenza vaccination of the elderly in a densely populated and unvaccinated community. Vaccine 20:2494-2499.
[Crossref] [Google Scholar] [PubMed]
- Giefing-Kroll C, Berger P, Lepperdinger G, Loebenstein BG (2015) How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14:309-321.
[Crossref] [Google Scholar] [PubMed]
- Grubeck-Loebenstein B, Berger P, Teissi MS, Zisterer K, Wick G (1998) No immunity for the elderly. Nature Med 4:870-870.
- Gavazzi G, Krause KH (2002) Ageing and infection. Lancet Infect Dis 2:659-666.
[Crossref] [Google Scholar] [PubMed]
- Schwarz T, Lau PT, Hillus D, Helbig ET, Lippert LJ, et al. (2021) Delayed antibody and T-cell response to BNT162b2 vaccination in the elderly, Germany. Emerg Infect Dis 27:2174-2178.
[Crossref] [Google Scholar] [PubMed]
- Muller L, Andree M, Moskorz W, Ingo Drexler I, Walotka L, et al. (2021) Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis 73:2065-2072.
[Crossref] [Google Scholar] [PubMed]
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054-1062.
- Zheng Z, Peng F, Xu B, Zhao J, Liu H, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 81:e16-e25.
[Crossref] [Google Scholar] [PubMed]
- Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, et al. COVID-19 vaccine-associated anaphylaxis: A statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J 14:100517.
[Crossref] [Google Scholar] [PubMed]
- Kazama I (2021) Potential prophylactic efficacy of mast cell stabilizers against COVID-19 vaccine-induced anaphylaxis. Clin Mol Allergy 19:25.
[Crossref] [Google Scholar] [PubMed]
- Feldman RA, Fuhr R, Smolenov I, Ribeiro A, Panther L, et al. (2019) mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37:3326-3334.
[Crossref] [Google Scholar] [PubMed]
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, et al. (2020) COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586:594-599.
[Crossref] [Google Scholar] [PubMed]
- Turner JS, Halloran JA, Kalaidina E, Kim W, Schmitz AJ, et al. (2021) SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 596:109-113.
[Crossref] [Google Scholar] [PubMed]
- Plotkin SA (2010) Correlates of protection induced by vaccination. Clin Vaccine Immunol 17:1055-1065.
[Crossref] [Google Scholar] [PubMed]
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, et al. (2021) Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med 385:e84.
[Crossref] [Google Scholar] [PubMed]
- Naaber P, Tsrel L, Kangro K, Sepp E, Jurjenson V, et al. (2021) Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur 10:100208.
[Crossref] [Google Scholar] [PubMed]
Citation: Classen JM, Muzalyova A, Dhillon C, Kling E, Zellmer S, et al. (2022) Humoral Immune Response of Vaccinated Employees against SARS-CoV-2 in a Tertiary Hospital in Germany: CALM-UKA (COVID-19 Antibody Level Monitoring University Hospital Augsburg). J Infect Dis Ther 10:499. DOI: 10.4172/2332-0877.1000499
Copyright: © 2022 Classen JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2864
- [From(publication date): 0-2022 - Feb 21, 2025]
- Breakdown by view type
- HTML page views: 2493
- PDF downloads: 371