Research Article Open Access
Human Umbilical Vein Endothelial Cells Migration in Matrigel by the Concentration Gradient of Vascular Endothelial Growth Factor
Naoko Obi1,2, Hiroyuki Toda1 and Yasuhiko Tabata1*1Department of Biomaterials, Institute for Frontier Medical Sciences, Kyoto University, 53 Kawara-cho Shogoin, Sakyo-ku Kyoto 606-8507, Japan
2Corporate Technology Research and Development Nissha Printing Co.,Ltd. 3 Mibu Hanai-cho, Nakagyo-ku, Kyoto 604-8551, Japan
- Corresponding Author:
- Yasuhiko Tabata
Department of Biomaterials
Institute for Frontier Medical Sciences
Kyoto University, 53 Kawara-cho Shogoin
Sakyoku Kyoto 606-8507, Japan
Tel: +81 75 751 4121
Fax: +81 75 751 4646
E-mail: yasuhiko@frontier.kyoto-u.ac.jp
Received date: October 16, 2015; Accepted date: December 02, 2015; Published date: December 09, 2015
Citation: Obi N, Toda H, Tabata Y (2015) Human Umbilical Vein Endothelial Cells Migration in Matrigel by the Concentration Gradient of Vascular Endothelial Growth Factor. J Biotechnol Biomater 5:210. doi:10.4172/2155-952X.1000210
Copyright: © 2015 Obi N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Vascular endothelial growth factor (VEGF) has an ability to induce the migration of human umbilical vein endothelial cells (HUVEC). The objective of this study is to prepare several patterns of gelatin hydrogels for VEGF release and evaluate the 3-dimensional pattern of HUVEC migration in Matrigel by VEGF release. VEGF was incorporated into the gelatin hydrogel sheet to achieve the sustained release and generate the concentration gradient of VEGF. When Matrigel was put on the gelatin hydrogel sheet incorporating VEGF, the VEGF was released into the Matrigel to form a gradient pattern of VEGF concentration in the Matrigel with time and the area of VEGF released by the Matrigel depended upon the position of gelatin hydrogel sheet put on. In addition, HUVEC were seeded on the surface of Matrigel to evaluate the ability of VEGF released to enhance the cell migration into the Matrigel. HUVEC were migrated with time into the Matrigel to the direction and the position of VEGF released. It is concluded that the VEGF release induces the migration of HUVEC in Matrigel based on the concentration gradient and the position of VEGF formed in Matrigel.
Keywords
HUVEC; Migration; 3D; VEGF; Matrigel; Gelatin
Introduction
Tissue engineering is a biomaterial technology to assist the proliferation and differentiation of cells for cell-based tissue formation. For example, scaffolds with or without growth factor combinations allow cells to induce 3-dimensioned (3D) tissue regeneration [1-5]. The multi-layer of a cell sheet generated by temperature-responsive polymers [6-8] and the preparation of cell spheroids by non-adhesive cell substrates have been reported for the 3D tissue formation [9,10]. However, the insufficient supply of oxygen and nutrient to cells inside the 3D tissue often causes their reduced function and death [11,12]. In the body tissue, the vascular network permits the sufficient supply of oxygen and nutrients. Thus, the formation of blood vessel networks is critical to allow cells to survive and remain their functions in 3D cell structures.
Vascular network is generally formed in two ways, vasculogenesis in which meshwork-like structures are generated de novo, and angiogenesis in which new vessels emerge from existing ones [13]. In angiogenesis, which is considered to be particularly important for vascular formation in adult, the migration of vascular endothelial cells stimulated by VEGF is deeply involved [14-16]. Early angiogenesis begins with the detachment of pericytes from microvascular tissues consisting of vascular endothelial cells and pericytes. Endothelial cells at the site where the pericytes detachment has occurred are then stimulated by angiogenic growth factors, such as VEGF in turn producing various proteases that digest the basement membrane [13,16]. This process leads to the cells migration and proliferation to form new blood vessels. It is well known that the direction and position of new blood vessels are well regulated by controlling the type, concentration gradients, and secretion sites of angiogenic growth factors.
Several researches on in vitro experimental models for angiogenesis have been reported. For example, a boyden chamber assay is well known to determine the migratory response of endothelial cells to angiogenesis factors, such as VEGF and basic fibroblast growth factor (bFGF) [17-19]. A tube formation assay is one of the most specific tests for angiogenesis measurement of the ability of endothelial cells cultured on Matrigel, which is well known as a natural extracellular matrix (ECM) extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma [20,21]. In the case of vascular endothelial cells, Matrigel is commonly used not only as the substrate for angiogenesis assay both in vitro and in vivo, but also as the 3D substrate. A 3-D in vitro angiogenesis model by co-culturing HUVEC and GBM cells were established to study the role of VEGF in mediating capillary tubule formation [22]. However, the direction of vascular network in these models is controlled only in the 2D culture, but random in 3D one, because there is no 3D model system in which well-controlled direction and position of concentration gradients.
On the other hand, gelatin is used as the substrate to regulate the release pattern of proteins. For example, our previous studies have demonstrated that the feasibility of specifying the timing and location of VEGF release was achieved with a gelatin hydrogel [23,24]. Gelatin is suitable for regulating the location of the protein, because it is easy to control shapes and doses. This study is undertaken as one trial to control the direction and position of VEGF concentration gradient in the Matrigel of a 3D hydrogel and subsequently regulate the direction and position of HUVEC migration in the Matrigel.
After VEGF was incorporated into the several patterns of the gelatin hydrogel sheets, Matrigel was put onto the obtained gelatin hydrogel sheet. Then, HUVEC were seeded on the surface of the Matrigel. The direction of HUVEC migration in the Matrigel was evaluated in terms of incubation time and VEGF release site. We examine the time profile and pattern of VEGF released from the gelatin hydrogel sheet in the Matrigel.
Materials and Methods
Materials
A gelatin sample with an isoelectric point (IEP) of 5.0 was kindly supplied by Nitta Gelatin Inc. (Osaka, Japan). rhVEGF165 was obtained from R&D Systems, Inc., MI. Matrigel was purchased from Becton Dickinson & Co., Franklin Lakes, NJ. Other chemicals were obtained from Nacalai tesque Inc., Kyoto, Japan, and used without further purification.
Preparation of the several shapes of gelatin hydrogel sheets
Gelatin hydrogel sheets of 100 μm thickness were prepared on glass molds. Briefly 30 w/v % gelatin aqueous solution was preheated to 40°C, flown into the glass mold with 100 μm in depth, and kept at 4°C for gelation. Next, the gelatin hydrogel sheet was removed from the glass mold, and freeze-dried. The gelatin hydrogel sheet was punched out to prepare the 5 mm-diameter discs. The discs were cut by a blade to prepare the arcuate shape of 0.5, 1.5, and 2.5 mm in width. These samples were performed by dehydrothemal treatment for 48 hr at 140°C.
Evaluation of sorption and release of VEGF for gelatin hydrogel sheets
VEGF was radioiodinated by the conventional chloramine T method [25]. Briefly, 5 μl of Na 125I (740 MBq/ml in 0.1 N NaOH aqueous solution, NEN Research Products, Perkin Elmer, Inc., Waltham, MA) was added to 30 μl of an aqueous solution of VEGF (10 μg/ml). Then, 0.2 mg/ml chloramine T in 0.5 M potassium phosphatebuffered solution (pH 7.5) containing 0.5 M NaCl (100 μl) was added to the solution mixture. After agitation at room temperature for 2 min, 100 μl of phosphate-buffered saline solution (PBS, pH 7.4) containing 0.4 mg of sodium metabisulfate was added to the reaction mixture to stop the radioiodination. The reaction mixture was passed through PD-10 column (GE Healthcare UK Ltd., UK) to remove the uncoupled 125I molecules from the 125I-labeled VEGF. The protein amount in the 125I-labeled protein solution was quantified by using Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). The radioactivity of 125I-labeled VEGF was measured on the gamma counter (ARC-360, Aloka Co., Ltd., Japan) to calculate the specific radioactivity of the 125I-labeled VEGF.
5 mm-diameter discs of gelatin hydrogel sheet were immersed in 5 μl of PBS solution containing 125I-labeled VEGF, and then the amounts of VEGF incorporated into the gelatin hydrogel sheets were calculated by measuring the radioactivity. The VEGF-impregnated gelatin hydrogel sheets were left to stand in PBS at 37°C, and time-dependent changes in the amounts of VEGF diffused into PBS were monitored.
To evaluate in vitro VEGF release, the gelatin hydrogel sheet incorporating 125I-labeled VEGF was incubated in 1 ml of PBS solution with or without 10 μg/ml of collagenase (Sigma-Aldrich Co. LLC, MO) at 37°C. 24 hr after incubation in the PBS solution, the PBS solution was changed to fresh PBS with 10 μg/ml of collagenase. At different time intervals, the solution supernatant was removed and replaced with the same volume of fresh PBS solution with or without collagenase. The supernatant was sampled at scheduled times and the amount of 125I-labeled VEGF in each supernatant was determined by the radioactivity measurement described above. Experiments were performed for 3 specimens independently for each sample unless otherwise mentioned.
Evaluation of VEGF release from gelatin hydrogel sheets in matrigel
VEGF solution was put onto the discs and arcuate shapes of gelatin hydrogel sheet to incorporate 3 ng of VEGF. Moreover, a glass tube (inner diameter: 5 mm, height: 10 mm) was adhered onto a poly(ethylene terephthalate) (PET) film by a detachable silicon glue. The obtained gelatin hydrogel sheet was placed onto the PET film inside the glass tube, and then 50 μl of Matrigel was added into the glass tube to form the glatin-Matrigel layer (GML) (Figure 2A). After incubation for 0, 15, and 30 min, or 1, 3, and 8 hr, the GML were fixed with 4 wt% paraformaldehyde at 37°C for 20 min and removed from the glass tube. Next, the GML was embedded in an optimal cutting temperature compound (Sakura Finetek Japan Co. Ltd., Tokyo, Japan) and frozen by liquid nitrogen. The frozen sample was sectioned in the X-Y or Y-Z plane on a cryotome (CM3050S, Leica Microsystems, Wetzlar, Germany) to prepare each section of 500 or 100 μm in thickness, respectively. To dissolve the Matrigel, 150 μl of PBS containing 500 μg/ml dipase (Gibco®, Invitrogen, Carlsbad, CA) and 10 μg/ml collagenase was added into every section and incubated for 24 hr at 37°°C. The amount of VEGF in each solution was quantified with Human VEGF Quantikine ELISA Kit (R&D systems, Inc., MI) according to the manufacturer’s instructions.
Cell culture
HUVEC (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) were cultured in Medium 199 (M199, Gibco®, Invitrogen, Carlsbad, CA) supplemented with 20 vol% fetal bovine serum (FBS, Hyclone Laboratories, Kagan, Utah), 50 μg/ml endothelial mitogen (Biomedical technologies, Inc., MA), 292 μg/ml L-glutamin (Sigma- Aldrich Co. LLC, MO), 100 U/ml heparin (Sigma-Aldrich Co. LLC, MO), and 1 vol% penicillin-streptamycin (Sigma-Aldrich Co. LLC, MO) at 37°C in a 5% CO2-95% air atmospheric condition to expand. Cells between passages 3 and 4 were used for all experiments.
Migration assay
HUVEC were serum-starved in M199 containing 0.5 vol% FBS and 1 vol% penicillin-streptamycin (starvation medium) for 5-6 hr before testing. Then, cells were stained with PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich Co. LLC, MO) by the manufacturing protocol. After cell preparation, migration assays were performed in a 24-well cell culture insert fluoroblock (pore size 3.0 μm) and companion 24-well plates (BD Bioscience, San Jose, CA) (Figure 3A). Briefly, 50 μl of Matrigel was placed in the top well of the cell culture inserts, and then incubated for 30 min at 37°C. Next, the starvation medium containing 0, 1, 10, 50, 100, and 200 ng of VEGF was added to the bottom well of companion plates. After 1 × 105 cells suspended in 250 μl of starvation medium was added on the Matrigel, the cell culture inserts were put into the bottom well to start cell culture. After culturing for 8 hr, the number of cells migrated from the Matrigel surface of the top well to the opposite side of the well through the porous membrane was measured by a fluorescence spectrometer (Sectra Max Gemini EM, Molecular Devices, Sunnyvale, USA) at excitation and emission wavelengths of 551 and 567 nm, respectively.
Evaluation of HUVEC migration into Matrigel by VEGF incorporated into gelatin hydrogel sheets
The several shapes of the gelatin hydrogel sheet incorporating 670 pg of VEGF were placed into the glass tube as described above. While an arcuate shape gelatin hydrogel sheet of 0.5, 1.5, and 2.5 mm in width were placed on one side of the PET film, the arcuate shape gelatin hydrogel sheets of 0.5 mm in width were placed on both sides of the PET film. And then, 50 μl of Matrigel was added into each sample to form GML. After incubating at 37°C for 30 min, 1 × 105 cells suspended in 250 μl of starvation medium was added on the Matrigel and cells were cultured. After culturing for 1, 3, and 8 hr, the GML was fixed with 4 wt% paraformaldehyde at 37°C for 20 min and removed from the glass tube. The GML was put onto a cover glass to observe the cell migration by using a confocal microscope (LSM 410, Zeiss, Germany). The photographs were taken with a depth step of 20 μm for the 500 μm-thick GML. The number of cells migrated into the Matrigel was measured and counted from each photograph. The number ratio of cells migrated were calculated by the cell number of each step per total cell numbers.
Statistical analysis
All the data were expressed as the mean ± the standard deviation. The data were statistically analyzed by a one-way ANOVA combined with a post-hoc Tukey-Kramer multiple comparisons test to determine the significance at P<0.05.
Results
Release profiles of VEGF from gelatin hydrogel sheets in PBS
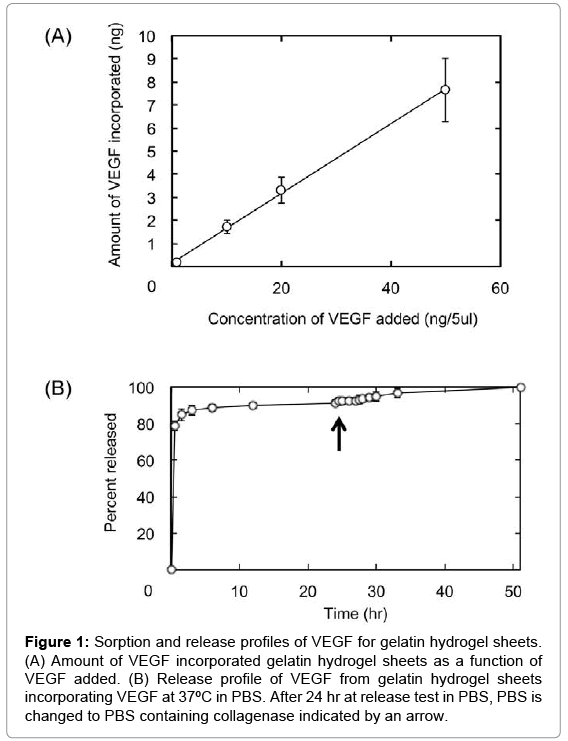
Figure 1 shows the amounts of VEGF incorporated and released from 5 mm-diameter discs of gelatin hydrogel sheets. The amount of VEGF incorporated into gelatin hydrogel sheet linearly increased with an increase in the concentration of VEGF solution (Figure 1A). Approximately 90% of VEGF was released from the gelatin hydrogel sheets in collagenase-free PBS within 10 hr, followed by the remaining VEGF release by collagenase addition.
Figure 1: Sorption and release profiles of VEGF for gelatin hydrogel sheets. (A) Amount of VEGF incorporated gelatin hydrogel sheets as a function of VEGF added. (B) Release profile of VEGF from gelatin hydrogel sheets incorporating VEGF at 37ºC in PBS. After 24 hr at release test in PBS, PBS is changed to PBS containing collagenase indicated by an arrow.
Distribution of VEGF release from gelatin hydrogel sheets in the matrigel
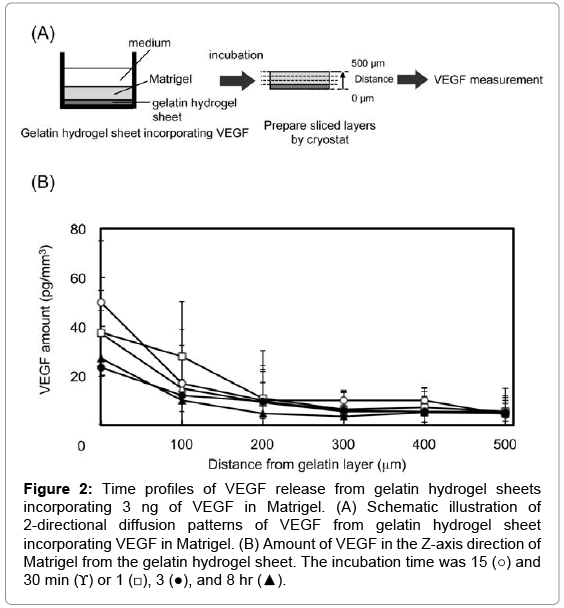
Figure 2 shows the time profiles of 2-directional diffusion patterns of VEGF in the Z-axis direction of Matrigel. VEGF was released from the gelatin hydrogel sheets into the Matrigel layer. The VEGF concentration gradually decreased at the site of 0 μm. VEGF was released from the gelatin hydrogel sheets over time, and the concentration differences in the VEGF concentration between the site of 0 and 500 μm of Matrigel layer become small.
Figure 2: Time profiles of VEGF release from gelatin hydrogel sheets incorporating 3 ng of VEGF in Matrigel. (A) Schematic illustration of 2-directional diffusion patterns of VEGF from gelatin hydrogel sheet incorporating VEGF in Matrigel. (B) Amount of VEGF in the Z-axis direction of Matrigel from the gelatin hydrogel sheet. The incubation time was 15 (○) and 30 min (ϒ) or 1 (□), 3 (●), and 8 hr (▲).
Effect of gelatin hydrogel sheet size on the release pattern of VEGF in the matrigel
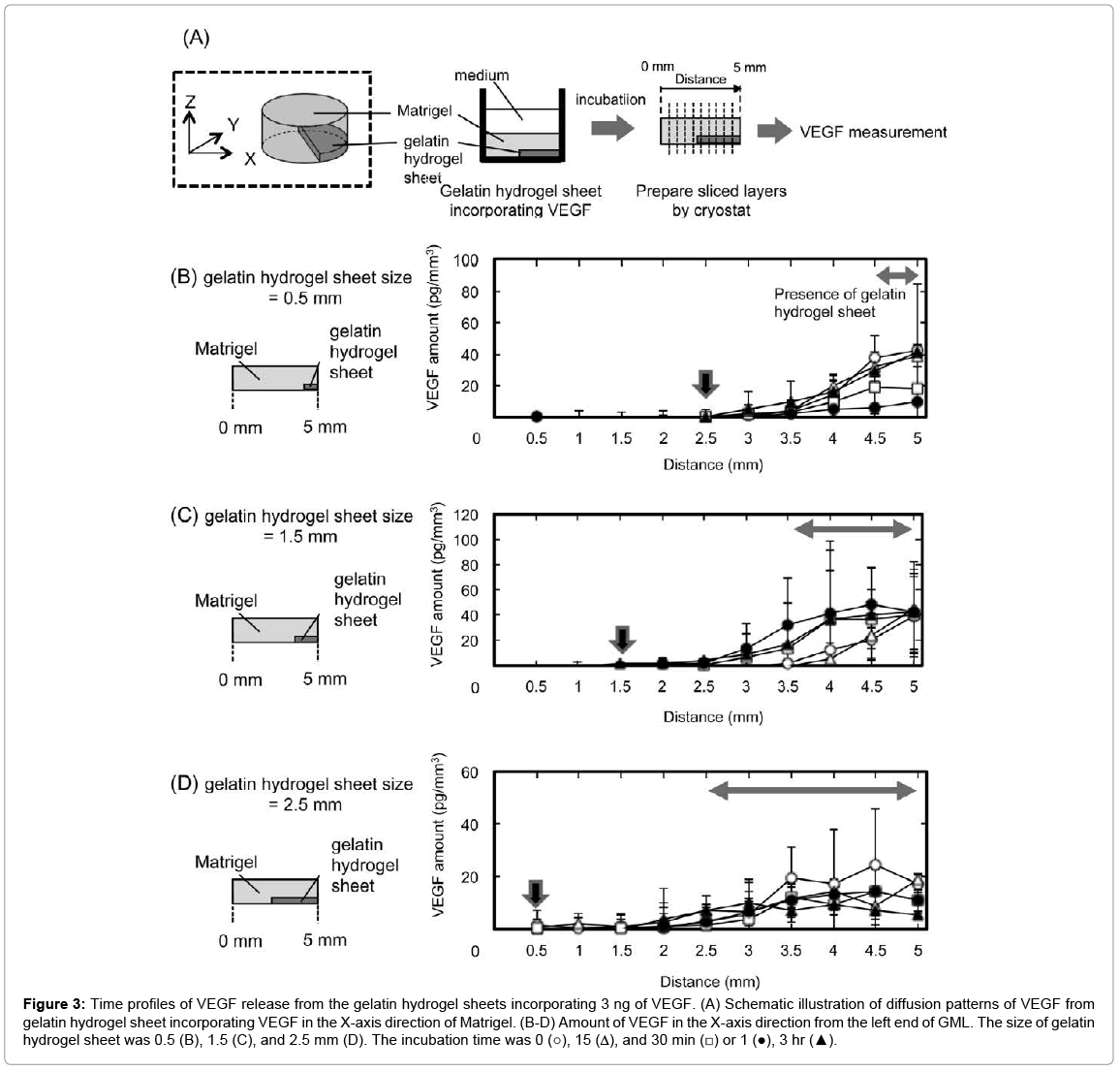
Figure 3 shows the diffusion patterns of VEGF from gelatin hydrogel sheet incorporating VEGF in the X-axis direction of Matrigel. VEGF was released from the gelatin hydrogel sheets into the Matrigel. Irrespective of the sheet size, the pattern of VEGF concentration changed with time, and reached the equilibrium within 3 hr. Irrespective of the sheet size, the VEGF concentration was almost same at the area where the gelatin hydrogel sheet was present. In addition, the VEGF concentration decreased with distance from the gelatin hydrogel sheets. The arrows in Figures 3B–3D indicate the distance where the amount of VEGF reached zero. This suggests that VEGF was no longer present at 2 mm from the edge of gelatin hydrogel sheets for any sizes.
Figure 3: Time profiles of VEGF release from the gelatin hydrogel sheets incorporating 3 ng of VEGF. (A) Schematic illustration of diffusion patterns of VEGF from gelatin hydrogel sheet incorporating VEGF in the X-axis direction of Matrigel. (B-D) Amount of VEGF in the X-axis direction from the left end of GML. The size of gelatin hydrogel sheet was 0.5 (B), 1.5 (C), and 2.5 mm (D). The incubation time was 0 (○), 15 (Δ), and 30 min (□) or 1 (●), 3 hr (▲).
Evaluation of HUVEC migration into the matrigel by VEGF
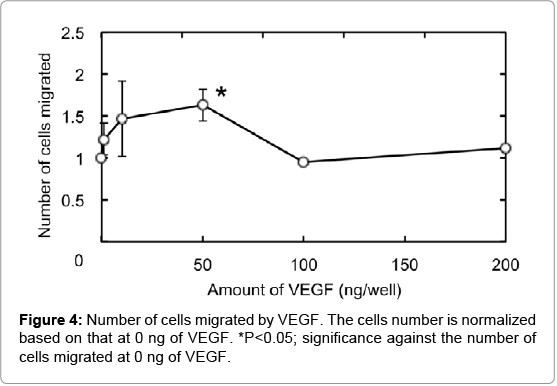
Figure 4 shows HUVEC migration by VEGF using the 24-well cell culture insert fluoroblock (pore size 3.0 μm). Compared with the VEGF-free group, the number of HUVEC migrated increased as the VEGF concentration became high. The number of HUVEC migrated was significantly higher for the VEGF dose of 50 ng than other doses. The number was decreased at the VEGF concentration of 100 ng or higher.
HUVEC migrations into the matrigel with VEGF incorporated into gelatin hydrogel sheet
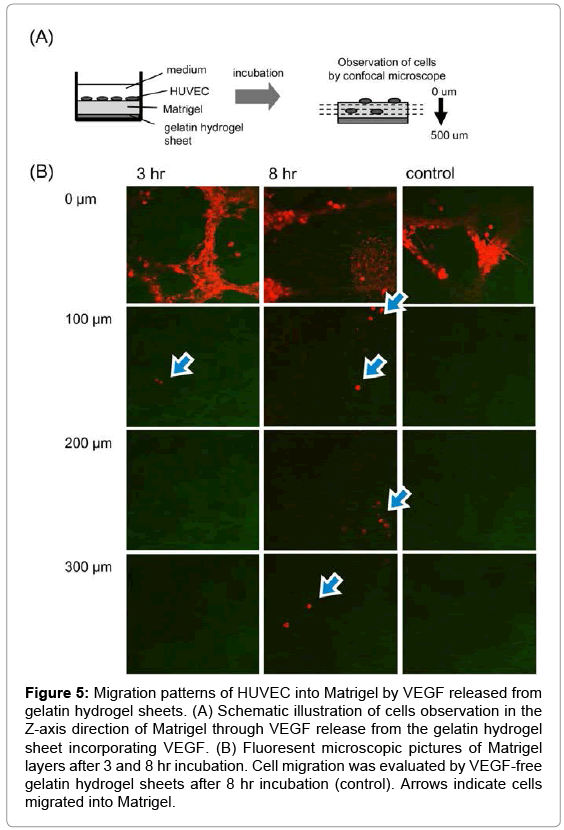
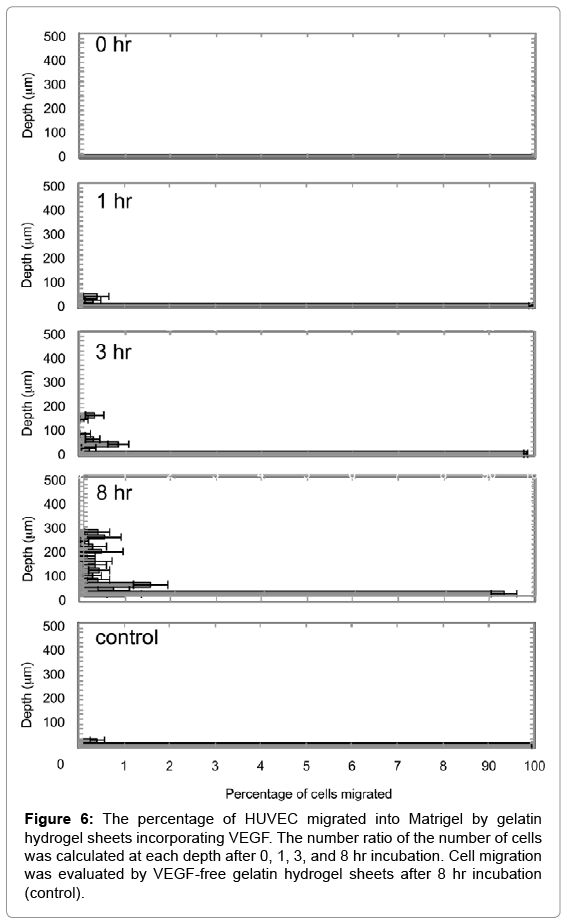
Figure 5 shows migration patterns of HUVEC into the Z-axis direction of the Matrigel by VEGF released from gelatin hydrogel sheet. HUVEC migrated into the Matrigel were deeply with incubation time. On the other hand, no cell migration was observed for VEGF-free gelatin hydrogel sheet. Figure 6 shows the number ratio of HUVEC migrated into the Matrigel by gelatin hydrogel sheets incorporating VEGF. HUVEC were migrated deeply over time to the depth of 300 μm at 8 hr.
Figure 5: Migration patterns of HUVEC into Matrigel by VEGF released from gelatin hydrogel sheets. (A) Schematic illustration of cells observation in the Z-axis direction of Matrigel through VEGF release from the gelatin hydrogel sheet incorporating VEGF. (B) Fluoresent microscopic pictures of Matrigel layers after 3 and 8 hr incubation. Cell migration was evaluated by VEGF-free gelatin hydrogel sheets after 8 hr incubation (control). Arrows indicate cells migrated into Matrigel.
Figure 6: The percentage of HUVEC migrated into Matrigel by gelatin hydrogel sheets incorporating VEGF. The number ratio of the number of cells was calculated at each depth after 0, 1, 3, and 8 hr incubation. Cell migration was evaluated by VEGF-free gelatin hydrogel sheets after 8 hr incubation (control).
Effect of gelatin hydrogel sheet size on the pattern of HUVEC migration into the matrigel
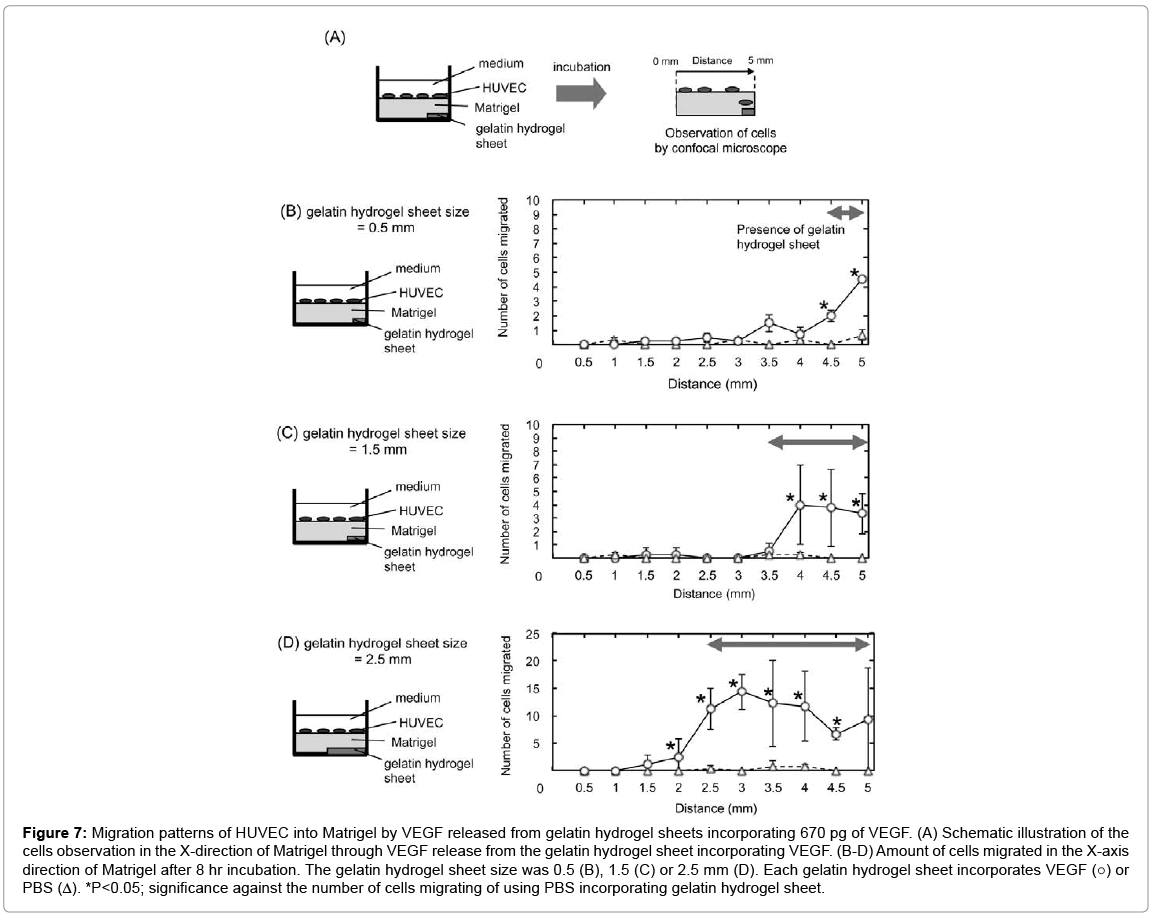
Figure 7 shows the migration patterns of HUVEC into the Matrigel by VEGF released from gelatin hydrogel sheets in the X-axis direction of Matrigel. HUVEC migrated in an area where the gelatin hydrogel sheet incorporating VEGF was present to a significantly great extent compared with gelatin hydrogel sheet-free area. More specifically significant migration was observed at X=4.5 mm and 5.0 mm incorporating VEGF, X=4.0–5.0 mm, and X=2.5–4.5 mm for 0.5 mm, 1.5 mm, and 2.5 mm gelatin hydrogel sheet, respectively.
Figure 7: Migration patterns of HUVEC into Matrigel by VEGF released from gelatin hydrogel sheets incorporating 670 pg of VEGF. (A) Schematic illustration of the cells observation in the X-direction of Matrigel through VEGF release from the gelatin hydrogel sheet incorporating VEGF. (B-D) Amount of cells migrated in the X-axis direction of Matrigel after 8 hr incubation. The gelatin hydrogel sheet size was 0.5 (B), 1.5 (C) or 2.5 mm (D). Each gelatin hydrogel sheet incorporates VEGF (○) or PBS (Δ). *P<0.05; significance against the number of cells migrating of using PBS incorporating gelatin hydrogel sheet.
HUVEC migration into matrigel by VEGF incorporated into patterned gelatin hydrogel sheet
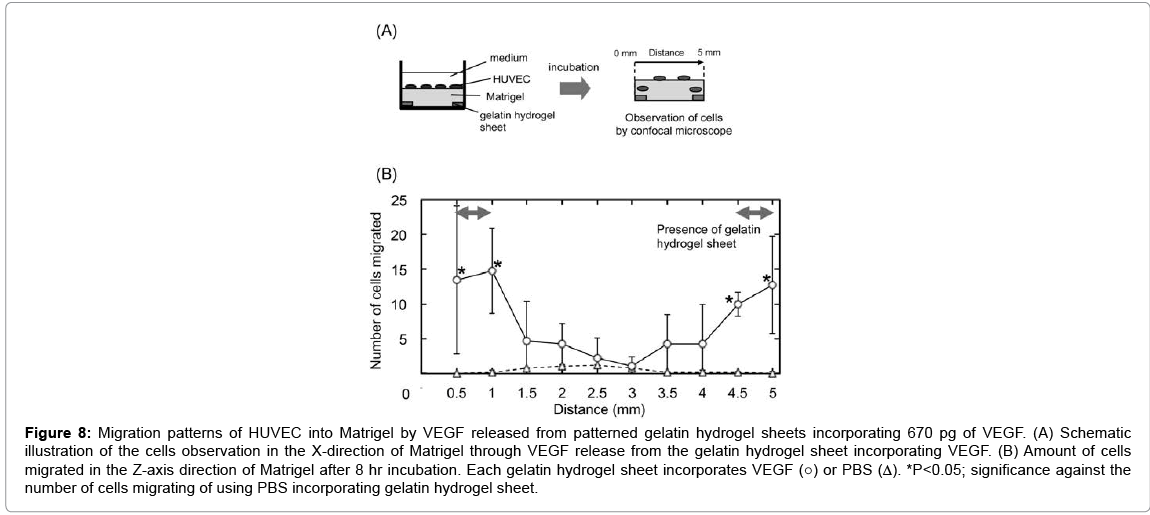
Figure 8 shows the migration patterns of HUVEC into the Matrigel by VEGF released from patterned gelatin hydrogel sheet in the X-axis direction of Matrigel. Significant cell migration was observed only at X=0.5–1.0 and 4.5–5.0 mm, where the gelatin hydrogel sheets were placed.
Figure 8: Migration patterns of HUVEC into Matrigel by VEGF released from patterned gelatin hydrogel sheets incorporating 670 pg of VEGF. (A) Schematic illustration of the cells observation in the X-direction of Matrigel through VEGF release from the gelatin hydrogel sheet incorporating VEGF. (B) Amount of cells migrated in the Z-axis direction of Matrigel after 8 hr incubation. Each gelatin hydrogel sheet incorporates VEGF (○) or PBS (Δ). *P<0.05; significance against the number of cells migrating of using PBS incorporating gelatin hydrogel sheet.
Discussion
The present study demonstrates that VEGF is released from the gelatin hydrogel sheet by a simple diffusion. Many researchers have been studied the angiogenesis assay by using VEGF and bFGF [17,22,26,27]. bFGF is an important factor for endothelial cell mitogen, migration, and capillary tube formation [28,29]. However, it is not endothelial specific and induces the differentiation and proliferation of many other cells. VEGF is one of the most well studied factors that control blood vessel formation and function. The effects of VEGF on endothelial cells have been studied for many years in an array of different model systems [17,30-32]. VEGF in humans has at least 3 major subtypes, which are 121, 165, and 189-amino acid proteins [33-36]. In this study, VEGF165 was selected because it is well known as an important factor that participates in migration of endothelial cells [37-39]. Previous studies reveal that the pattern of proteins release from the gelatin hydrogel is generated by the interaction force between gelatin and protein molecules [23,24,40]. The VEGF molecules have a weak interaction with gelatin, resulting in the diffusion-determined release.
It is apparent from Figure 2 that the Z-dimentional gradient of VEGF concentration in the Matrigel could be formed by the gelatin hydrogel sheet. The mechanism of VEGF concentration gradient formation in the Matrigel can be explained in terms of the simple diffusion and the VEGF Matrigel interaction. VEGF has a heparin-binding domain that can be biologically bound heparan sulfate proteoglycan of a Matrigel component [41-43]. When the VEGF solution was used in the cell migration assay, a certain amount of VEGF was detected in Matrigel (data not shown). It is likely that more VEGF molecules are present in the Matrigel sites closer to the VEGF-supplying gelatin hydrogel sheet and associated in the Matrigel, resulting in the formation and maintenance of VEGF gradation. The concentration profile reached a constant 3 hr later. Unfortunately, the error bars were slightly large because of the technical barriers in this study. To evaluate the amount of VEGF in the GML, GML was carefully removed from the glass tube with tweezers, followed by cutting the GML with the cryotome manually. However, the phenomena observed in the evaluation of the amount of VEGF in the GML are reproducible although the significant difference was not detected.
When the gelatin hydrogel sheet of different sizes was prepared and contact with the Matrigel, VEGF was not released uniformly throughout the Matrigel, but was localized around the hydrogel (Figure 3), indicating the formation of X-directional VEGF concentration gradient. Because of the interaction between Matrigel and VEGF, the VEGF concentration of X-direction might be similar to that of Z-direction (Figure 2). However, the VEGF concentration of X-direction varied widely and does not correlate to incubation time. When the GML was cut out to X-direction with the cryostat, it is difficult to determine strictly the edge and direction of GML. As the reason, some errors may be observed.
Compared with the VEGF-free group, the number of HUVEC migrated increased as more VEGF was added at the VEGF amount of 50 ng or less. However, the number of HUVEC decreased at higher concentrations. It is reported that the HUVEC migration is suppressed by an excessive amount of VEGF [17]. It is the practically assessed that when 50 ng VEGF was added to the bottom chamber, approximately 670 pg VEGF was sorbed to the Matrigel layer of the top chamber (data not shown). We accordingly adjusted the amount of VEGF in the Matrigel layer to 670 ng in the following HUVEC migration experiments.
HUVEC migrated more deeply over the time the range of 8 hr (Figure 5). Many researches have been reported on HUVEC migration along a VEGF concentration gradient. Barkefors et al. reported HUVEC migration by pouring VEGF solution of 2 different concentrations (0 ng/ml and 10-100 ng/ml) in a micro-flow channel (width, 400 μm) to create a concentration gradient [44]. Shamloo et al. demonstrated that HUVEC migration was induced by pouring VEGF solutions of 2 different concentrations (0 and 50 ng/ml) to create a concentration gradient in the cell culture chamber and HUVEC were migrated to higher VEGF concentrations [45]. On the contrary, HUVEC were randomly migrated when the distribution of VEGF was consistent in the chamber. The paper shows that the VEGF concentration gradient induces filopodia localization, cell polarization, and subsequent directed migration. Other studies show that the filopodia senses the VEGF concentration gradient, and then cells migrate toward the high concentrations of VEGF, because VEGF are binding the VEGF receptor type II, a tyrosine kinase-type receptor expressed on the cell surface [17,46]. In our experiment, VEGF was released in the Matrigel to form a concentration gradient in the Z-direction (Figure 2), while HUVEC migrated in response to the gradient. This result is consistent with the results of papers previously reported.
Previous studies showed that most of cells were randomly migrated by the stimulation of VEGF and bFGF in the 2D culture in the scratch assay and the migration assay with a microfluidics device [44,45]. On the contrary, a few cells in the 3D culture are migrated into the hydrogels accompanying with their degrading the matrix. For example, in the case of a monolayer of endothelial cells seeded at 1 × 104 cells onto the surface of a 3D collagen gel, only 10 cells were migrated into the collagen gel [47]. This phenomenon of cell migration in the aortic ring outgrowth and the sprouting assays, which are well performed as the 3D angiogenesis assay, show the same behavior [48,49]. It is suggested that the percentage of HUVEC migrated to total cells is quite small. Significant cells migration was observed at X=4.5 mm and 5.0 mm, X=4.0–5.0 mm, and X=2.5–4.5 mm (Figure 7). The space range of HUVEC migration corresponded well with that of VEGF diffused shown in Figure 3. The result strongly suggests that VEGF release from the gelatin hydrogel sheet can form the concentration gradient of VEGF in the Matrigel, which causes the HUVEC migration.
In this study, to construct the 3D model system where the direction of chemokine gradients and cell migration were controlled, 3D pattern of HUVEC migration in Matrigel were regulated by the VEGF release from gelatin hydrogel sheets. Several researches on in vitro experimental models for the migration of HUVEC have been reported. For example, boyden chamber and scratch assays are well known as traditional methods to evaluate angiogenesis [17,27]. In the assays, HUVEC were stimulated by the addition of chemokines in a 2D culture. On the other hand, since it is well known that blood vessels are grown by a gradient of chemokine secreted during angiogenesis, some microfluidics-based techniques are used to make the gradient of factors and evaluate the effect of gradient on the migration of HUVEC [44,45]. However, the gradients are not prepared for the 3D culture system. In this study, the VEGF gradients and Matrigel of 3D system were combined to mimic the in vivo growth of blood vessels. Several natural polymers, such as collagen and Matrigel, are used to evaluate the migration of dendritic cells by making a chemokine gradient [50-53]. On the contrary, for vascular endothelial cells, Matrigel is normally used as the substrate for angiogenesis assay both in vitro and in vivo. In this study, the VEGF gradients were prepared in the 3D Matrigel, and then the HUVEC migration into Matrigel was evaluated.
Irrespective of the gelatin hydrogel sheet size, VEGF was diffused over the range of 2 mm from the edge of gelatin hydrogel sheets. Thus, to prevent the overlapping of VEGF diffused from multiple gelatin hydrogel sheets, the gelatin hydrogel sheets must be placed at least 4 mm apart to each other. Based on this, 0.5 mm size of the gelatin hydrogel sheet incorporating VEGF was placed Matrigel at 4 mm intervals (Figure 8). In response to the VEGF concentration gradient, HUVEC migrated. We can say with certainly that the localization of gelatin hydrogel sheets of 0.5 mm-wide at 4 mm intervals enabled HUVEC to migrate in a 3D gel and form a steric configuration.
It is essential to study the migration of vascular endothelial cells stimulated by angiogenic growth factors such as VEGF165 to understand the process of angiogenesis. In in vivo angiogenesis, angiogenic growth factors are secreted from the cells present at the ischemic area where angiogenesis is required to occur. The growth factors then create directionally controlled concentration gradients, and vascular endothelial cells, in turn, migrate in response to the concentration gradients. However, conventional in vitro studies had the limitation that cells were allowed to migrate only in 2D environments with less cell-cell and cell-matrix interactions [17,22,26,27]. It is conceivable that cells environment in the 3D condition is different from that in the 2D condition. There have been reported on the HUVEC migration of in vitro angiogenesis in 3D conditions. However, most of them have been performed on the cells migration in the random direction. In this study, the VEGF release system is applied to form the VEGF concentration gradient in the 3D matrigel, which affects the direction of cell migration. We believe this system is one of the new approaches to experimentally control the direction and position of cell migration in the 3D matrix.
This study demonstrates that the 3-dimentional gradient of VEGF in the Matrigel could be formed by adjusting the size of the gelatin hydrogel sheets incorporating VEGF to control the place of HUVEC migration. This gradient allowed HUVEC to form the localization in the Matrigel in response to the VEGF concentration. This technology may be promising to form the 3D pattern of blood vessels in the Matrigel in vitro.
Conclusion
We have established the way to control the 3-dimensional pattern of HUVEC migration in Matrigel by VEGF. In this model, the VEGF was released into Matrigel from the gelatin hydrogel sheet to form a gradient pattern of VEGF concentration in the Matrigel. After GML were prepared, HUVEC were seeded on the surface of the Matrigel to evaluate the cells migration into Matrigel. HUVEC were migrated with time into Matrigel to the direction of VEGF released. It is indicated that the concentration gradient of VEGF formed in Matrigel induce the migration of HUVEC in Matrigel.
References
- Vacanti CA, Langer R, Schloo B, Vacanti JP (1991) Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plastic and reconstructive surgery 88: 753-759.
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260: 920-926.
- Isogai N, Landis W, Kim TH, Gerstenfeld LC, Upton J, et al. (1999) Formation of phalanges and small joints by tissue-engineering. J Bone Joint Surg Am 81: 306-316.
- Fuchs JR, Nasseri BA, Vacanti JP (2001) Tissue engineering: a 21st century solution to surgical reconstruction. Ann ThoracSurg 72: 577-591.
- Shin M, Yoshimoto H, Vacanti JP (2004) In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospunnanofibrous scaffold. Tissue Eng 10: 33-41.
- Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, et al. (2005) Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials 26: 6415-6422.
- Ito A, Hibino E, Kobayashi C, Terasaki H, Kagami H, et al. (2005) Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng 11: 489-496.
- Masuda S, Shimizu T, Yamato M, Okano T (2008) Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev 60: 277-285.
- Kunz-Schughart LA, Kreutz M, Knuechel R (1998) Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. International journal of experimental pathology 79: 1-23.
- Lin RZ, Chang HY (2008) Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 3: 1172-1184.
- Kellner K, Liebsch G, Klimant I, Wolfbeis OS, Blunk T, et al. (2002) Determination of oxygen gradients in engineered tissue using a fluorescent sensor. BiotechnolBioeng 80: 73-83.
- Malda J, Klein TJ, Upton Z (2007) The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng 13: 2153-2162.
- Bauer SM, Bauer RJ, Velazquez OC (2005) Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg 39: 293-306.
- Serini G, Ambrosi D, Giraudo E, Gamba A, Preziosi L, et al. (2003) Modeling the early stages of vascular network assembly. EMBO J 22: 1771-1779.
- Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6: 273-286.
- Clapp C, Thebault S, Jeziorski MC, Martinez De La Escalera G (2009) Peptide hormone regulation of angiogenesis. Physiological reviews 89: 1177-1215.
- Yoshida A, Anand-Apte B, Zetter BR (1996) Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth factors 13: 57-64.
- Schor AM, Ellis I, Schor SL (2001) Chemotaxis and Chemokinesis in 3D Macromolecular Matrices: Relevance to Angiogenesis. Methods in molecular medicine 46: 163-183.
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N (2003) Angiogenesis assays: a critical overview.ClinChem 49: 32-40.
- Ponce ML (2009) Tube formation: an in vitromatrigel angiogenesis assay. Methods MolBiol 467: 183-188.
- Arnaoutova I, Kleinman HK (2010) In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5: 628-635.
- Chen Z, Htay A, Dos Santos W, Gillies GT, Fillmore HL, et al. (2009) In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. Journal of neuro-oncology 92: 121-128.
- Yamamoto M, Ikada Y, Tabata Y (2001) Controlled release of growth factors based on biodegradation of gelatin hydrogel. J BiomaterSciPolym Ed 12: 77-88.
- Patel ZS, Ueda H, Yamamoto M, Tabata Y, Mikos AG (2008) In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res 25: 2370-2378.
- Greenwood FC, Hunter WM, Glover JS (1963) The preparation of i-131-labelled human growth hormone of high specific radioactivity. Biochem J 89: 114-123.
- Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, et al. (2000) A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell 6: 851-860.
- Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2: 329-333.
- Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, et al. (1984) Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science 223: 1296-1299.
- Rifkin DB, Moscatelli D (1989) Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol 109: 1-6.
- Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, et al. (1996) Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. The American journal of pathology 149: 293-305.
- Vernon RB, Sage EH (1999) A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvascular research 57: 118-133.
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 13: 9-22.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306-1309.
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, et al. (1989) Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 246: 1309-1312.
- Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, et al. (1991) The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J BiolChem 266: 11947-11954.
- Houck KA, Ferrara N, Winer J, Cachianes G, Li B, et al. (1991) The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Molecular endocrinology 5: 1806-1814.
- Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18: 4-25.
- Shibuya M (2001) Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell StructFunct 26: 25-35.
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, et al. (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56: 549-580.
- Kaur H, Yung LY (2012) Probing high affinity sequences of DNA aptamer against VEGF165. PLoS One 7: e31196.
- Ono K, Hattori H, Takeshita S, Kurita A, Ishihara M (1999) Structural features in heparin that interact with VEGF165 and modulate its biological activity. Glycobiology 9: 705-711.
- Robinson CJ, Stringer SE (2001) The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 114: 853-865.
- Krilleke D, Ng YS, Shima DT (2009) The heparin-binding domain confers diverse functions of VEGF-A in development and disease: a structure-function study. BiochemSoc Trans 37: 1201-1206.
- Barkefors I, Le Jan S, Jakobsson L, Hejll E, Carlson G, et al. (2008) Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J BiolChem 283: 13905-13912.
- Shamloo A, Ma N, Poo MM, Sohn LL, Heilshorn SC (2008) Endothelial cell polarization and chemotaxis in a microfluidic device. Lab Chip 8: 1292-1299.
- Lamalice L, Le Boeuf F, Huot J (2007) Endothelial cell migration during angiogenesis. Circ Res 100: 782-794.
- Bayless KJ, Kwak HI, Su SC (2009) Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat Protoc 4: 1888-1898.
- Aplin AC, Fogel E, Zorzi P, Nicosia RF (2008) The aortic ring model of angiogenesis. Methods Enzymol 443: 119-136.
- Stahl A, Wu X, Wenger A, Klagsbrun M, Kurschat P (2005) Endothelial progenitor cell sprouting in spheroid cultures is resistant to inhibition by osteoblasts: a model for bone replacement grafts. FEBS Lett 579: 5338-5342.
- Haessler U, Kalinin Y, Swartz MA, Wu M (2009) Anagarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed Microdevices 11: 827-835.
- Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, et al. (2010) Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32: 703-713.
- Tayalia P, Mazur E, Mooney DJ (2011) Controlled architectural and chemotactic studies of 3D cell migration. Biomaterials 32: 2634-2641.
- Haessler U, Pisano M, Wu M, Swartz MA (2011) Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. ProcNatlAcadSci U S A 108: 5614-5619.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 13533
- [From(publication date):
December-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12500
- PDF downloads : 1033