Human Papillomavirus Genotypes in Invasive Cervical Carcinoma in HIV Seropositive and Seronegative Women in Zimbabwe
Received: 14-Nov-2017 / Accepted Date: 24-Nov-2017 / Published Date: 27-Nov-2017 DOI: 10.4172/2332-0877.1000343
Abstract
Background: Invasive cervical carcinoma (ICC) accounts for 23% of all cancer-related deaths in Zimbabwean women. Trials for a national program of genotype-specific HPV vaccines are underway to prevent cervical carcinoma, but the distribution of HPV types among women with ICC according to HIV status is unknown.
Methods: To determine prevalence and distribution of high-risk HPV genotypes by HIV status in women with ICC, we performed a cross-sectional study on women referred for ICC testing at four urban referral hospitals in Zimbabwe from June 2014-December 2015. Cervical biopsies were obtained for histology and HPV genotyping. HIV serology testing was performed. HPV testing was performed using MY09/MY11 PCR followed by typing using dot blot hybridization.
Results: Of 107 participants with histologically-proven, HIV prevalence was 49.5% (53/107). HIV-positive women tended to be younger (median age 44 years) than HIV-negative women (median age 59 years). HPV prevalence was 94% (101/107), ranging from 1-5 genotypes per participant. HPV 16 (81.5%), 18 (24%), 33 (13%), 35 (11%), 56 (9%) and 45(7.4%) were the most prevalent genotypes amongst HIV-negative participants; HPV 16 (67.9%), 18 (43.4%), 56 (18.9%), 45 (15.1%), 33 (11.3%), and 58 (9.4%) were most prevalent amongst HIV-positive participants. 83% of women were infected with either HPV-16 or HPV-18.
Conclusion: Effective vaccination programs against HPV 16 and 18 could prevent up to 83% of cases of cervical cancer in Zimbabwe. HIV may influence distribution of some HPV genotypes given the significant increase in prevalence of HPV 18 amongst HIV positive participants.
Keywords: HPV 16; HPV 18; HIV; Cervical cancer; Zimbabwe; Dot blot hybridization; HPV vaccine
Background
Invasive cervical carcinoma (ICC) is the fourth most common cancer globally. The highest incidence and mortality are found in low to middle to income countries which account for 85% of the disease burden worldwide. In Zimbabwe [1], ICC is the leading cause of cancer and cancer-related deaths in women; the crude incidence is 34.5 per 100 000 women per year, which results in 2,270 new cases and 1,451 deaths annually [2].
Cervical cancer and its precursor lesions are caused by the human papillomavirus (HPV), a sexually transmitted virus that infects women mostly during their reproductive years [3]. Approximately 90% of infections are cleared or controlled by the immune system within two years without causing clinical disease. The remaining 10% persist as viral wart infection, but some progress to pre-invasive carcinoma and a small percentage ultimately to invasive carcinoma [4]. Cell transformation occurs through the actions of viral oncoproteins E6 and E7, which act in concert with co-carcinogens, cervical infections, and possibly a compromised immune system resulting in progressive dysplasia through to invasive carcinoma [5].
Sub-Saharan Africa is decades into the HIV pandemic [6]. The HIV prevalence rate in Zimbabwe stands at 16.7% in adults (15-49 years) [3] with a prevalence of 4.2% in women aged 15-24 years (UNAIDS). Although the CDC declared cervical cancer to be an AIDS-defining illness in 1993 [7] the exact role played by HIV in the pathogenesis of ICC remains poorly understood. Studies on the effects of concomitant HIV and HPV infection are scarce but most indicate that the vast majority of HIV-positive women are co-infected with HPV; ICC in HIV positive women shows up to a 22-fold increase in incidence [8] and presents 10 to 15 years earlier [9]. In addition, some studies have reported that HIV-positive women harbour more HPV genotypes and have a larger number of multiple infections than their HIV-negative counterparts [10].
Certain high-risk HPV types, i.e., those most likely to progress to ICC, notably HPV 16 and 18, have been detected in more than 70% of ICC worldwide [11]. Variations in the prevalence of specific HPV genotypes have been reported from various world regions including sub-Saharan Africa. Some of the earliest international studies suggested that there may be HPV type-specific differences according to host immune status [12]. It is postulated that HPV-16 in particular may account for the most cases of cervical cancer because of its innate ability to avoid immune surveillance. This may in part explain the differences seen in distribution of various genotypes and more significantly it may have implications on the currently available vaccines on the immunosuppressed. In an earlier study from Zimbabwe [13], 97% of the women with ICC also had HPV infection, of which types 16, 33, 18 and 31 were the most prevalent genotypes obtained from the cervical scrapings.
Currently, genotype-specific vaccines are being rolled out in many countries, including Zimbabwe, where a Gavi-funded pilot project aimed at vaccinating girls age 9-13 years is underway in Marondera and Beitbridge towns [14].
The present study determined the prevalence of HPV genotypes associated with ICC in both HIV-positive and HIV-negative women in Zimbabwe. These data will allow policymakers to assess the potential impact of the vaccination program on the prevalence of ICC, particularly in a setting of high HIV prevalence. This knowledge is crucial, given the projected increase in the incidence and mortality from cervical cancer in Sub-Saharan Africa over the next 20 years [15].
Materials and Methods
Study participants
One hundred and ninety eight women, age ≥ 18 years presenting with signs and symptoms suggestive of ICC were enrolled into the cross-sectional study between June and December 2014. Participants were referred from primary healthcare centres to the Gynaecology Out Patient Departments of 4 major urban hospitals in Zimbabwe (2 in Harare and 2 in Bulawayo). Senior nursing staff received training on the recruitment of participants. Participants provided signed informed consent for an HIV test, CD4 count and two cervical biopsies; the first was obtained for histological confirmation of cervical carcinoma and was placed immediately into 10% buffered formalin and sent to a consultant pathologist for routine histological assessment. The second, taken for HPV genotyping, was obtained from an area adjacent to the first. It was transported in normal saline and stored in an ultra-freeze at -81˚C within 2-3 h following biopsy. Histological assessment and storage facilities were available in both Harare and Bulawayo.
All the participants received pre- and post-HIV test counselling. On being informed of their HIV and biopsy results, participants were offered appropriate counselling and treatment options per standard of care.
Ethical approval
Permission to conduct the study was granted by the clinical directors at all study sites (Parirenyatwa, Harare Central, United Bulawayo and Mpilo Hospitals). Ethics clearance was granted by the Joint Research Committee of the University of Zimbabwe (JREC 27/14) and Medical Research Council of Zimbabwe (MRCZ/B/645). The project was reviewed by the Human Research Ethics Committee (Medical) of the University of Witwatersrand, Johannesburg, South Africa which issued an ethics waiver (W-CJ-1511271-1). The study adhered to and complied with the Helsinki Declaration of 2013.
Demographic, medical, sexual, and reproductive history
Participants completed an interviewer-administered questionnaire where demographic, medical, sexual and reproductive history data was collected. A crude assessment of literacy was performed by enquiring whether the participants could read and write, either in their vernacular or English language, and the highest level of education attained (primary school, secondary and university degree). Those who could read and write were described as literate.
HIV serology and CD4 count
HIV and CD4 testing were performed in the Department of Immunology, University of Zimbabwe. Rapid serum HIV antibody testing was performed using Alere Determine™ HIV Ag/Ab Combo kit (Inverness Medical, Massachusetts, USA). All positive cases were confirmed using First Response® HIV1/HIV2 test kit (WB Premier Medical Corporation, Darman, India). A CD4 count was performed on all cases that tested positive for HIV with both kits.
Histology
One hundred and ninety-eight women were recruited into the study. Haematoxylin and eosin-stained recut slides of the original cervical biopsies were obtained and the initial histological diagnosis was reassessed. Ninety-one participants were excluded either due to either normal histology, suboptimal tissue for diagnosis, incomplete patient data, in-situ squamous lesions, or due to poor DNA quality (2 cases had negative human β-globin). The final study sample meeting the inclusion criteria comprised 107 cases. Tumours were classified according to the World Health Organization’s criteria (WHO Classification of Tumours of Female Reproductive Organs, 2014).
DNA extraction and HPV genotyping
DNA was extracted from the stored frozen biopsies using QIAGEN DNeasy Blood and Tissue kit (QIAGEN Incorporated, Valencia, California, U.S.A) according to the manufacturer's instructions. The extracted DNA was first subjected to conventional PCR for human β- globin gene amplification as an internal control. Samples that were positive for human β-globin gene were subjected to conventional PCR for HPV DNA viral amplification using MY09/MY11 primers (Eurofins, Hamburg, Germany). Two samples negative for -globin were amongst cases excluded from the study.
Genotyping was performed using dot blot hybridization. Biotinlabelled probes for specific genotypes were mixed with the amplicon together with streptavidin-horseradish peroxidase (S-HRP). The presence of S-HRP was detected using the enhanced chemiluminescence (ECL) method. The maximum light emission at a wavelength of 428 nm was detected by exposing the reaction to a negative film. Short exposure to blue light on an X-Ray film allowed for the detection of specific reaction/s denoting wells in which hybridisation would have occurred. The selected probes were able to identify 14 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) as well as 23 low-risk HPV genotypes (6, 11, 26, 32, 40, 42, 53, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89).
Statistical analysis
Baseline description of study participants was presented using percentages for categorical variables such as sex, and mean (standard deviation) for continuous variables such as age, or median and range were the variable was not normally distributed. Prevalence and distribution of HPV genotypes were presented as percentages and histograms. The Mann-Whitney and Chi-square tests were used to test for probability. Multiple infections were determined with an HPV multiple infection being described as infection with two or more HPV types. Level of significance was set at p=0.05. All analyses were conducted using Statistica version 12 (Tulsa, Oklahoma USA).
Results
Demographic, medical, sexual and reproductive history
Age at first sexual intercourse ranged from 10 to 26 years, average 18.4 years (SD ± 3). The average reported number of lifetime sexual partners was 2 per participant. Only 8% (9/107) of the participants reported that their sexual partners had been circumcised. A previous history of sexually transmitted infection was noted in 25% of participants (8 HIV-negative and 19 HIV-positive participants). Eighteen percent (19/107) of the participants did not know what a Pap smear was, 30% (32/107) had been previously screened and 51% (54/107) knew what a Pap smear was (or had heard about the procedure) but had never undertaken the screening test. The median number of pregnancies was 4 (IQR=3-7). The literacy rate was 85% (91/107). Fifty eight percent (62/107) of the participants resided in the rural areas whilst 40% (43/107) resided in an urban setting.
Histology
Histological analysis revealed that 90.6% (97/107) of the participants had squamous cell carcinoma, 5.6% (6/107) had adenocarcinoma, 1.9% (2/107) showed neuroendocrine differentiation and the remaining 1.9% (2/107) were considered too necrotic to properly classify. Six participants showed squamous cell carcinoma together with coincidental presence Schistosoma ova. Table 1 shows HPV distribution by tumour subtype. Of the six cases showing adenocarcinoma, 4 were positive for HPV 18.
| ICC subtypes Total (%) | HPV Genotypes | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 18 | 31 | 33 | 35 | 39 | 45 | 51 | 52 | 56 | 58 | 59 | 66 | 73 | 82 | 6 | 26 | 40 | 16 only | 18 only | |
| Squamous cell carcinoma* 97 (90,6) | 72 | 31 | 6 | 11 | 8 | 4 | 11 | 6 | 2 | 15 | 4 | 2 | 1 | 2 | 3 | 1 | 1 | 1 | 30 | 3 |
| Adenocarcinoma# 6 (5,6) | 6 | 4 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Neuroendocrine 2 (1,9) | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Unclassified 2 (1,9) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Total 107 (100) | 80 | 36 | 6 | 13 | 8 | 4 | 12 | 6 | 2 | 15 | 6 | 3 | 3 | 2 | 3 | 1 | 1 | 1 | 34 | 4 |
| (75) | (34) | (6) | (12) | (8) | (4) | (11) | (6) | (2) | (14) | (6) | (3) | (3) | (2) | (3) | (1) | (1) | (1) | (32) | (4) | |
| *Includes one case of adenoid cystic carcinoma #Includes one case of adenosquamous carcinoma and one case of clear cell carcinoma |
||||||||||||||||||||
Table 1: HPV genotypes by ICC subtypes.
Age and HIV status
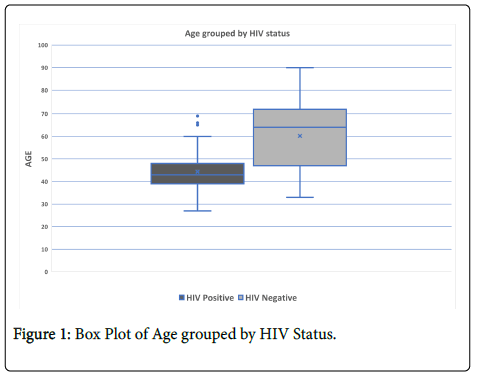
The study sample consisted of 107 participants with histologically-proven ICC. The median age for all participants was 48 years, (IQR=40-64). Half of the participants 49.5% (53/107) were HIV-positive and 50.5% (54/107) were HIV negative. HIV-positive women tended to be significantly younger, median 44 years (IQR=38-48), than HIV- negative women, median 59 years (IQR=46-70) (p=0.000001). Figure 1 shows a box plot of age distribution by HIV status. Sixty seven percent (36/53) of HIV-positive participants had been on HAART for a median of 4 years (Interquartile range, IQR=2-5). The median CD4 count amongst HIV positive participants was 337 cells/mm3 (IQR=232-486) with 26% (14/53) having a count ≤ 250 cells/mm3.
HPV genotypes
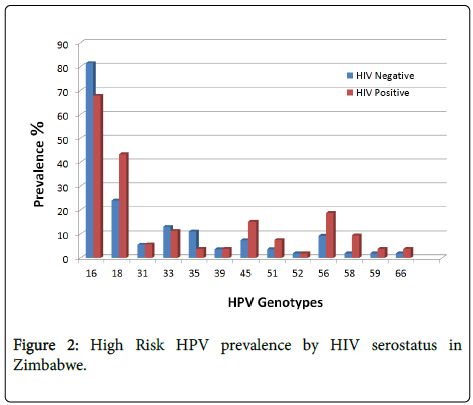
The HPV prevalence was 94.4% (101/107), ranging from 1-5 genotypes per participant. It increased slightly from 93% (50/54) amongst HIV-negative cases to 96% (51/53) amongst HIV-positive cases. This difference was not statistically significant. The 5 most common high-risk HPV genotypes were HPV 16 (74.8%), 18 (33.6%), 56 (14%), 33 (12.2%) and 45 (11.2%). These occurred as single or multiple infections. Amongst HIV-negative participants the most common HPV genotypes were HPV 16 (81.5%), 18 (24.1%), 33 (13.0%), 35 (11.1%), 56 (9.3%) and 45 (7.4%). Amongst HIV-positive participants the most common genotypes were HPV 16 (67.9%), 18 (43.4%), 56 (18.9%), 45 (15.1%), 33 (11.3%), and 58 (9.4%).
Eighty three percent (89/107) of women were infected with HPV 16 and/or 18. The combined prevalence of HPV 16 and/or 18 was 79% (42/53) amongst HIV positive women and 87% (47/54) amongst HIV negative women, this difference was not statistically significant (p=0.67). A greater number of HIV-negative participants were found to have HPV 16, 33, and 35 infection compared to HIV-positive patients. In contrast, HIV-positive patients more commonly had HPV 18, 45, 56 and 58 compared to HIV-negative participants (Figure 2). However, only the prevalence of HPV 18 differed significantly between HIV-positive participants (43.4%) and HIV-negative patients (24.1%) (p=0.03). Thirty two percent (34/107) of participants had infection with HPV 16 as a single infection.
Fifty-one percent (55/107) of participants showed infection with multiple HR-HPV genotypes. This distributed as over half, 54.5% (30/55), for HIV-positive participants and 45.5% (25/55) for HIV-negative participants, but this difference was not statistically significant.
Participants on HAART had a higher prevalence of multiple infections than those not on HAART (28/36 vs . 9/36). Twenty six percent (14/53) of HIV-positive participants had a CD4 count <250 cells/mm3. There was no association between low CD4 count (<250 cells/mm3) and increased prevalence of high-risk HPV genotypes (P=0.904). The distribution of the rest of the genotypes by HIV status is shown in Table 2.
| HPV Genotype | Total n (%) | HIV Status Total n (%) | |
|---|---|---|---|
| Negative 54 (50.5) | Positive 53 (49.5) | ||
| Any HPV | 101 (94.4) | 50 (93) | 51 (96) |
| High Risk HPV | |||
| 16 | 80 (74.8) | 44 (81.4) | 36 (67.9) |
| 18 | 36 (33.6) | 13 (24.1) | 23 (43.4) |
| 31 | 6 (5.6) | 3 (5.6) | 3 (5.7) |
| 33 | 13 (12.1) | 7 (13) | 6 (11.3) |
| 35 | 8 (7.5) | 6 (11.1) | 2 (3.8) |
| 39 | 4 (3.7) | 2 (3.7) | 2 (3.8) |
| 45 | 12 (11.2) | 4 (7.4) | 8 (15.1) |
| 51 | 6 (5.6) | 2 (3.7) | 4 (7.5) |
| 52 | 2 (1.9) | 1 (1.9) | 1 (1.9) |
| 56 | 15 (14) | 5 (9.3) | 10 (18.9) |
| 58 | 6 (5.6) | 1 (1.9) | 5 (9.4) |
| 59 | 3 (2.8) | 1 (1.9) | 2 (3.8) |
| 66 | 3 (2.8) | 1 (1.9) | 2 (3.8) |
| Low Risk HPV | |||
| 6 | 1 (0.9) | 0 | 1 (1.9) |
| 26 | 1 (0.9) | 0 | 1 (1.9) |
| 40 | 1 (0.9) | 1 (1.9) | 0 |
| 73 | 2 (1.9) | 1 (1.9) | 1 (1.9) |
| 82 | 3 (2.8) | 1 (1.9) | 2 (3.8) |
Table 2: Distribution of HPV Genotypes by HIV status.
Discussion
Invasive cervical carcinoma accounts for 23% of all cancer-related deaths in Zimbabwean women and is second only to Kaposi sarcoma for overall cancer related mortality in both sexes [2]. HPV infection is well established as the primary cause of ICC and there is growing evidence of HPV being an important factor in other anogenital cancers such as anus, vulva, vagina and penis [8]. What is less well established is the possible role of concomitant HIV infection especially on the prevalence and genotypes of HPV. Zimbabwe has about 1.4 million people living with HIV/AIDS, 56% of these are women aged 15 years and older [6]. Prevalence rates of HPV infection in HIV-positive women in the age group 15-44 years exceed 90% with the most frequent oncogenic high risk types being HPV 16 and 18 [3]. Using MY09/11 primers, the current study assessed the prevalence of HPV genotypes and the effect of concomitant HIV infection on the distribution of HPV genotypes in women with histologically-proven ICC.
Almost half (49.5%) of the study participants of women with ICC in Zimbabwe were HIV-positive. Among HIV-positive participants ICC was diagnosed in much younger women and occurred on average 16 years earlier than among their HIV-negative counterparts. The overall HPV prevalence of 94% was in keeping with rates found elsewhere in literature [11,16]. HPV 16 and 18 remained the commonest HPV genotypes isolated in patients with ICC regardless of HIV status, being isolated in 83% of cases. The combined prevalence rates of HPV 16 and 18 were comparable in both HIV-positive (79%) and HIV-negative participants (87%). The spectrum of HPV genotypes seen in participants with ICC differed among HIV-positive and HIV-negative women. HIV-positive participants showed a slight reduction in cases infected with HPV 16, an almost doubling of HPV 18 and HPV 56 and quadrupling of HPV 58 positive cases. However, only the difference in the prevalence of HPV 18 by HIV status was found to be statistically significant (Table 2). Similar findings were also reported in other studies [15,17].
HPV genotypes ranged from 1-5 genotypes per participant with 51% of the participants showing the presence of multiple (two or more) HR-HPV infections. Unlike in previous reports [18,19], we found no significant difference in the prevalence of multiple genotypes by HIV status. While this could be attributed to our small sample size, recent studies have also shown that advances in technology and improved DNA extraction protocols have resulted in increased detection of HPV genotypes [20], and therefore the unmasking of the role played by previously underestimated genotypes. We used fresh frozen cervical tissue as our DNA source as some studies have proven this offers an improved DNA yield which may also explain our higher HPV prevalence compared to, for instance, formalin fixed paraffin embedded tissue [21]. The increased rate of multiple genotypes was also associated with a decrease in the cases attributed to HPV 16 alone (32%) when compared to previous studies. The presence of a high proportion of concomitant in-situ/CIN3 lesions within a biopsy, as seen in cases of early/micro invasive ICC, is also associated with detection of a wider variety of HPV genotypes regardless of HIV status [22,23]. In this study, emphasis was only placed on the presence or absence of invasive malignancy. Further studies using laser capture microscopy on H and E slides can be used to assess the presence of hr- HPV genotypes in cases with a micro-invasive component and a large background CIN3 component.
There was no association between the prevalence of multiple genotypes with any particular histological type. Although we only had 6 cases of adenocarcinoma, four of them were positive for HPV 18, prevalence of 66.7%, in keeping with this association seen in other studies [17,24].
Participants on HAART had a higher incidence of multiple infections than those not on ARVs. HAART increases the life expectancy of women living with HIV and AIDS and therefore not only allows for increased potential exposure time to HR-HPV but also permits for accumulation of cellular genetic changes that ultimately lead to cervical cancer [15]. While some studies have shown HAART to promote clearance of HR-HPV, its effect on the incidence and progression of high-grade cytological squamous intraepithelial lesions remains controversial [25]. The introduction of HAART more than 10 years ago in Zimbabwe has not been matched with a decline in the incidence of ICC [1,26]. This may be due to lack of access to prevention and appropriate management services-only 30% of women in this study had ever received a pap smear-incorrect and inconsistent use of HAART, and possibly due to differences in the molecular pathogenesis of ICC in HIV-positive and HIV-negative individuals [27]. The median CD4 count amongst HIV-positive participants was 337 cells/mm3 (IQR=232-486) and we found no association between low CD4 count (less than 250 cells/mm3) and increased prevalence of high-risk HPV genotypes.
In an earlier study from Zimbabwe in women with ICC [13], Stanczuk et al. found HPV types 16 (61%), 33(39%), 18 (18%) and 31(4%) as the most common genotypes in cervical cytobrush scrapings with a multiple HPV infection rate of 24%. In a recent meta-analysis of HPV genotypes in ICC in Sub-Saharan Africa [16], HPV16, 18, 45, 35, 33, 52, 31, 58, 51 and 68 were the top ten most frequently detected genotypes amongst women with ICC. These studies, however did not take into consideration the patients’ HIV status.
Two vaccines, Cervarix™, a bivalent vaccine offering protection against HPV16 and HPV18 and a quadrivalent vaccine, Gardasil®™ offering additional protection against HPV6 and HPV11, both non– oncogenic types are available in most countries. The U.S. Food and Drug Administration has approved Gardasil® 9™, a nonavalent vaccine which protects against HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58, but it is yet to be widely distributed in Sub-Saharan Africa. It has been calculated that a vaccine against the 7 most common HPV genotypes would prevent 87% of cases of ICC worldwide, whereas one effective against types 16 and 18 only, could prevent of 71% of cases [28]. The vaccines directed against HPV 16 and 18 have the potential to reduce up to 83% of cancer occurring in our population according to this study, this is however complicated by the presence of a high number of multiple high-risk HPV genotypes associated with HIV infection and the limited knowledge of the contribution made by each genotype in the pathogenesis of ICC. Evidence regarding cross-protection of the vaccines is still scarce; therefore the nonavalent vaccine may be the most effective option in our population given the high rate of multiple HPV infections (51%) noted regardless of HIV status. Further studies, including cross-protection and cost-benefit ratios, will be required to determine the most advantageous type of HPV vaccination in the Zimbabwean setting.
We recognize several limitations to our study. There are no standardized laboratory protocols for HPV DNA extraction and amplification methods and hence it is difficult to compare results even those from the same population. Another limitation to the study was lack of data as to when participants acquired HIV. This prevents us from estimating the potential impact that the period of infection may have had on the distribution of HPV genotypes.
The current policy regarding HIV-positive women and cervical screening allows for annual Pap-smears for these women as they have higher risk of developing ICC. Evidence presented in this paper, and the earlier presentation of ICC in HIV positive woman, justifies this policy. There should also be shorter screening intervals to allow for the early detection of the quickly advancing lesions in HIV-positive women. Concomitant HR-HPV testing has been recommended [29] but the high prevalence of HR-HPV makes this exercise costly and of possibly limited benefit in a resource-poor setting like Zimbabwe. Current cervical cancer screening coverage of this target population is less than 10% [3] due to lack of facilities and human resources including cytopathologists and screeners. Fifty one percent of the participants recalled hearing about a Pap-smear and a third had one performed previously. Male circumcision was introduced in Zimbabwe in 2009 and we found its coverage to be only 8% amongst our participants. Identification and control of such factors may also help contribute to the reduction of cervical cancer. HIV and HPV have each been shown to facilitate infection with the other [28]; given our findings therefore, it is recommended that programs aimed at controlling one infection should also target the other.
Conclusion
In conclusion, this study confirms that women living with HIV in sub-Saharan Africa have extraordinarily high rates of HR-HPV infection. Among women with ICC, the proportion with multiple genotypes is high and almost equal in both HIV-positive and HIV-negative participants. This has important implications on the choice and anticipated benefits of each HPV vaccine as effective vaccines should be polyvalent and active against the most common oncogenic HPV genotypes in that geographic region. The nonavalent vaccine appears most ideal for our population regardless of HIV status. HIV does seem to have an impact on the distribution of some HPV genotypes as shown by the significant difference in the prevalence of HPV 18 by HIV serostatus. Larger studies are needed to further investigate the effect and significance of HIV on other genotypes. Further studies are needed to understand the role of multiple HPV infections in the pathogenesis of ICC.
The data underscores the importance of cervical cancer screening and access to treatment in these highly exposed and vulnerable populations, every woman should be screened at least once (ideally more often) and results should be made available timeously. There is need for close monitoring of both HIV-related and HPV/CIN parameters before and during ART treatment as part of a comprehensive cervical cancer prevention programme and vaccination programmes. We should aim to vaccinate those HIV-positive and HIV-negative women who fall within the local age guidelines.
References
- Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Makunike-Mutasa R (2014) Pattern of Cancer in Zimbabwe 2012 Annual Report.
- Fact Sheets by Cancer (2014) International agency for research on cancer.
- Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, et al. (2016) Human Papillomavirus and Related Diseases Report. ICO Inf Cent HPV Cancer (HPV Inf Centre) Hum Papillomavirus Relat Dis World Summ Rep.
- Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD (1998) Natural History of Cervicovaginal Papillomavirus Infection in Young Women. N Engl J Med 338: 423-428.
- Matovelo D, Magoma M, Rambau P, Massinde A, Masalu N (2012) HIV serostatus and tumor differentiation among patients with cervical cancer at Bugando Medical Centre. BMC Res Notes 5: 1.
- Government Printers (2014) Global AIDS Response Country Progress Report Zimbabwe.
- Castro KG, Ward JW, Slutsker L, Buehler JW, Jaffe HW, et al. (1993) Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults.
- Denny LA, Franceschi S, Sanjosé S De, Heard I, Barbara A, et al. (2012) Human Papillomavirus, Human Immunodeficiency Virus and Immunosuppression. Vaccine 30: F168-F174.
- Moodley M, Moodley J, Kleinschmidt I (2001) Invasive cervical cancer and human immunodeficiency virus (HIV) infection: a South African perspective. Int J Gynecol Cancer 5: 194-197.
- McDonald AC, Tergas AI, Kuhn L, Denny L, Wright TC (2014) Distribution of human papillomavirus genotypes among HIV-positive and HIV-negative women in Cape Town, South Africa. Front Oncol 14: 48.
- Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, et al. (2014) Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer 134: 1389-1398.
- Strickler HD, Palefsky JM, Shah KV, Anastos K, Klein RS, et al. (2003) Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst 95: 1062-1071.
- Stanczuk GA, Kay P, Sibanda E, Allan B, Chirara M, et al. (2003) Typing of human papillomavirus in Zimbabwean patients with invasive cancer of the uterine cervix. Acta Obstet Gynecol Scand 82: 762-766.
- Chin N, Sebata NL, Ruhanya V, Matarira HT (2014) Human papillomavirus genotypes in cervical cancer and vaccination challenges in Zimbabwe Human papillomavirus genotypes in cervical cancer and vaccination challenges in Zimbabwe. Infect Agent Cancer.
- De Vuyst H, Alemany L, Lacey C, Chibwesha CJ, Sahasrabuddhe V, et al. (2013) The burden of human papillomavirus infections and related diseases in sub-saharan Africa. Vaccine 31: F32-F46.
- Ogembo RK, Gona PN, Seymour AJ, Park HS, Bain PA, et al. (2015) Prevalence of Human Papillomavirus Genotypes among African Women with Normal Cervical Cytology and Neoplasia: A Systematic Review and Meta-Analysis. PLoS One 10: e0122488.
- Sanjose S De, Quint WG V, Alemany L, Geraets DT, Klaustermeier JE, et al. (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11: 7-11.
- Singh DK, Anastos K, Hoover DR, Burk RD, Shi Q, et al. (2009) Human Papillomavirus Infection and Cervical Cytology in HIV-Infected and HIV-Uninfected Rwandan Women. J Infect Dis 199: 1851-1861.
- Harris TG, Burk RD, Palefsky JM, et al. (2005) Incidence of Cervical Squamous Intraepithelial Lesions Associated with HIV serostatus, CD4 counts and HPV test results. JAMA 293: 1471-1476.
- Li N, Franceschi S, Howell-Jones R, Snijders PJF, Clifford GM (2011) Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer 128: 927-935.
- Li N, Yang L, Zhang Y, Zhao P, Zheng T, et al. (2011) Human Papillomavirus Infection and Bladder Cancer Risk: A Meta-analysis. J Infect Dis 204: 217-223.
- Ndiaye C, Alemany L, Ndiaye N, Kamaté B, Diop Y, et al. (2012) Human papillomavirus distribution in invasive cervical carcinoma in sub-Saharan Africa: could HIV explain the differences? Trop Med Int Health 17: 1432-1440.
- Castellsagué X (2008) Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol 110: 4-7.
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, et al. (2007) Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 121: 621-632.
- Firnhaber C, Van Le H, Pettifor A, Schulze D, Michelow P, et al. (2009) Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control 21: 433-443.
- Chirenje ZM (2005) HIV and cancer of the cervix. Best Pract Res Clin Obstet Gynaecol 19: 269-276.
- Kapur U, Wojcik EM (2007) Follicular neoplasm of the thyroid--vanishing cytologic diagnosis? Diagn Cytopathol 35: 525-528.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348: 518-527.
- Womack SD, Chirenje ZM, Blumenthal PD, Gaffikin L, McGrath JA, et al. (2000) Evaluation of a human papillomavirus assay in cervical screening in Zimbabwe. BJOG 107: 33-38.
Citation: Mudini W, Palefsky JM, Hale MJ, Chirenje MZ, Makunike-Mutasa RT, et al. (2017) Human Papillomavirus Genotypes in Invasive Cervical Carcinoma in HIV Seropositive and Seronegative Women in Zimbabwe. J Infect Dis Ther 5: 343. DOI: 10.4172/2332-0877.1000343
Copyright: © 2017 Mudini W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5133
- [From(publication date): 0-2017 - Apr 27, 2025]
- Breakdown by view type
- HTML page views: 4289
- PDF downloads: 844