Research Article Open Access
Improving the Understanding of the Properties and Retention Behavior of Chemically Bonded Stationary Phases Employing Suspended-state HR/MAS NMR Spectroscopy
Klaus Albert*, Volker Friebolin, Silvia Marten, and Helen YemanInstitute of Organic Chemistry, University of Tuebingen, Germany
- *Corresponding Author:
- Klaus Albert
University of Tuebingen, Institute of Organic Chemistry
Auf der Morgenstelle 18, D-72076 Tuebingen, Germany
Tel: 07071-29-75335
Fax: 07071-29-5875
E-mail: klaus.albert@uni-tuebingen.de
Received date: November 12, 2013; Accepted date: December 12, 2013; Published date: December 16, 2013
Citation: Albert K, Friebolin V, Marten S, Yeman H (2013) Improving the Understanding of the Properties and Retention Behavior of Chemically Bonded Stationary Phases Employing Suspended-state HR/MAS NMR Spectroscopy. J Anal Bioanal Tech S12: 001. doi: 10.4172/2155-9872.S12-001
Copyright: © 2013 Albert K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Molecular recognition processes are predicted to become the basis of all advanced separation techniques. In solution and recently also in suspension ligand-receptor interactions can be studied using methods of high-resolution (HR) suspended-state NMR spectroscopy, for example by employing the saturation transfer difference (STD) NMR technique.
In this approach molecular recognition processes of (R) and (S)-flurbiprofen on different derivatized polysaccharide-based silica chiral stationary phases (CSP) typically used in high performance liquid chromatography (HPLC) have been investigated. In addition to chromatographic investigations suspended-state high-resolution/ magic angle spinning (HR/MAS) NMR measurement were performed. A separation of racemic flurbiprofen was obtained on two out of three CSP column materials under identical chromatographic conditions. Hence, we expected significant different binding affinities resulting in spatial interactions stabilizing one transient diastereomeric complex relative to the other. Due to these differences we used for screening purposes T1 relaxation time measurements and the saturation transfer difference (STD) NMR methodology to study the interaction strength between (R) and (S) enantiomers and the three CSP materials used. Thus, saturation transfer difference (STD) NMR experiments can provide valuable information for the design and implementation of novel, task-specific or tailored stationary phases.
Keywords
HR/MAS NMR Spectroscopy; HPLC method; Racemic flurbiprofen
Introduction
Chirality is crucial for living organisms because the enantiomers of a racemic mixture very often exhibit a different physiological behavior [1]. For the development of optical active drug candidates, the analysis of chiral compounds by high performance liquid chromatography (HPLC) has become an important field in the pharmaceutical industry. Basically, improvements in e.g. the selectivity of the chiral stationary phase (CSP) materials used enabled considerable advances in chiral separation science during the past decades. In particular silicabased CSP derivatives of amylose and cellulose, first investigated by Okamoto et al. [2,3] can be used to separate chiral compounds. Among them, amylose tris (3,5-dimethylphenylcarbamate) and its cellulose derivative as well as cellulose tris (4-methylphenylcarbamate) attached to a macroporous silica gel are very attractive due to their ability to separate a wide range of racemic compounds [2-5].
An enantioseparation achieved by those CSPs can be realized due to the different interaction behavior of the two enantiomers towards the stationary phase. While the substrate binds during the separation process to a receptor molecule (host-guest relationship) a transient macromolecular diastereomeric complex is formed which is described by its thermodynamic and kinetic stability [4]. For this binding process the dedicated and released energy is characteristic and determines the binding selectivity. However, mainly the separation mechanism of Pirkle-type or cyclodextrin CSPs had been investigated during the last years [2,3,6].
To achieve a further insight into the chiral recognition mechanism of a racemic separation process NMR spectroscopy can be used as a powerful tool to elucidate differences in the enantiomeric discrimination of the chiral receptors. However, reasonable resolution cannot be achieved for such heterogeneous systems used in chromatography by solution- or solid-state NMR. Thus, a direct comparison between chromatography and solution NMR is hardly worthwhile, because under these conditions the whole complexity of the two-phase system consisting of a dissolved analyte, a solid support material and a mobile phase is not fully considered. Hence, a hybrid technique based upon solution- and solid-state NMR, called High-Resolution combined with Magic Angle Spinning (HR/MAS) NMR spectroscopy has the potential to extract valuable information in the suspended-state about the chromatographic behavior [7-9]. The mechanical sample rotation around the “magic angle” at 54.74° (typical in the range of 4-6 kHz for suspended samples) influences the spectrum of the swollen CSP, since it modulates suszeptibility distortions thus leading to an increased signal resolution due to a decreased signal line width [7-9]. With this methodology an alternating adjustment of various parameters for optimization of the sepa-ration process can be achieved. Several one and multidimensional NMR techniques can be used which are based on the detection of dynamic parameters like the chemical shift due to chiral additives, relaxation time measurements or the Nuclear-Overhauser- Effect (NOE) and provide an elegant tool to gain information about the host-guest complex structure and its interaction forces [7,8,10-15].
For screening purposes and to elucidate the specific interaction behavior of a ligand toward a receptor on a molecular level, Meyer et al. [10] investigated for ligand-protein interactions the Saturation Transfer Difference (STD) NMR spectroscopy which we adopted to HR/MAS [7-9,16,17]. This technique utilizes cross relaxation processes to detect interactions between low molecular weight analytes and macromolecular receptors [17,18]. Principally, ligands adopt magnetization in dependence of their distance to a selective saturated receptor. The closer the proximity to the macromolecule, the stronger the transfer of saturation resulting in a decreased ligand signal intensity. In particular, two spectra were recorded alternately, one with (ON resonance spectrum) and the other without (OFF resonance spectrum) selective receptor saturation resulting after spectrum subtraction in the STD spectrum. Hence, in the difference spectrum only ligand resonances with binding affinity to the receptor are visible.
In the current approach three polysaccharide-based chiral stationary phase materials were tested for their separation ability of racemic flurbiprofen. Hence, suspended-state HR/MAS NMR including spin-lattice (T1) relaxation time measurements and the saturation transfer difference (STD) technique were used to elucidate molecular recognition processes and ligand-receptor interactions. The results obtained were directly correlated to the chromatographic behavior and can be used to predict the elution order in the case of inefficient enantiomer resolution.
Experimental Section
Materials
(R) and (S)-flurbiprofen (2-(3-fluoro-4-phenyl-phenyl)propanoic acid) enantiomers (Figure 1) and 1,3,5-tris tert. butylbenzene as dead volume marker in HPLC were obtained from Aldrich, Milwaukee, USA. Trifluoroacetic acid was received from VWR, Darmstadt, Germany. Methanol was provided from Merck, Darmstadt, Germany. The derivatized polysaccharide-based silica chiral stationary phases (CSP) had a particle size of 3 μm and a pore diameter of 1000 Å with an approx. surface area of 25 m2/g donated by Knauer GmbH, Berlin, Germany. The polysaccharide coverage on the silica surface was about 20%.
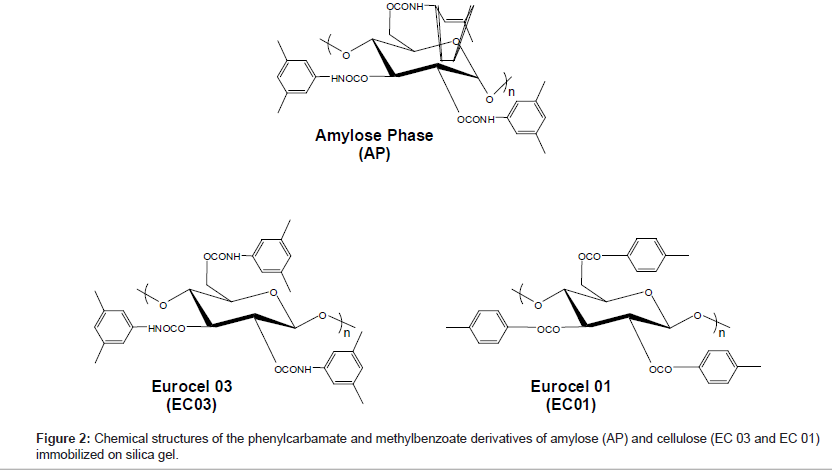
The glycosidic bonding is different for the polysaccharide-based CSPs. For the amylose phase it is α-1,4 and for the Eurocel phases it is β-1,4. The polysaccharide of Eurocel 01 (EC01) is derivatized with 4-methylbenzoate and with 3,5-dimethylphenylcarbamate for Eurocel 03 (EC 03). The amylose phase (AP) is similar to EC 03 derivatized with 3,5-dimethylphenylcarbamate. The chemical structures of the coated CSPs surface selectors are presented in Figure 2.
For the NMR experiments, methanol-d4 with a HDO content of less than 0.03% was purchased from Euriso-Top, France. Deuterated trifluoroacetic acid (TFA-d1) with a purity of more than 99.5% was obtained from Fluka AG, Switzerland.
HPLC method
The HPLC separations of racemic flurbiprofen on the three CSP columns were carried out with a Knauer Smartline System equipped with a Smartline Manager 5000 with degasser and LPG unit, a Pump 1000, UV Detector 2800 and a Smartline Column Oven. The samples were introduced via an auto sampler 3950. The chromatograms obtained were analyzed with the help of ChromGate Software. Racemic flurbiprofen was preliminary dissolved in methanol and separated isocratically in methanol acidified with 0.1% TFA at a flow rate of 0.5 ml/min by the chiral stationary phases (CSPs). The separations were monitored by UV at 210 nm at 25°C. 10 μl of the sample were injected. The peak identification in the chromatogram was performed by single analyte injection. The retention factor k’ for the CSPs was determined by k’=(tr-t0)/t0, wherein tr represents the retention time of the analyte and t0 the elution time of the dead volume marker. The enantioselectivity α of the CSP towards both enantiomers was obtained by α=k’(S)/k’(R) [4]. Due to the α value, the differences in the Gibbs free energy (ΔΔG≠) at a given tem-perature can be calculated by ΔΔG≠=-RT ln α. For baseline separation of the two enantiomers, an α-value of app. 1.2 is necessary which corresponds to a ΔΔG≠ between the CSP and the enantiomers of only -0.11 kcal mol-1 [2,3].
HR/MAS NMR Spectroscopy in the suspended state
Suspended-state 1H High Resolution/Magic Angle Spinning (HR/ MAS) NMR spectra of the heterogeneous system were recorded on a Bruker ARX 400 MHz spectrometer. The spectrometer was equipped with a deuterium lock setup which was set on the resonance frequency of methanol-d4. The samples were recorded in 4 mm double bearing ZrO2 rotors at a spinning rate of 6 kHz. The rotor contained for each experiment 80 μl of a 0.1 M single enantiomer solution in methanol-d4 (with 0.1% TFA-d1) with or without 10 mg of the chiral stationary phase (CSP) material. The spectra were recorded at 25°C and multiplied by an exponential line broadening function of lb=0.5 Hz prior to Fourier transformation. Spectra processing was performed using Bruker TOPSPIN 2.0 software. Signal assignment was accomplished using a two dimensional 1H-1H-COSY-NMR spectrum. To reduce esterfication reactions with the solvent under TFA acidic conditions, short experimental times were chosen thus avoiding occurring signals of the flurbiprofen methyl ester.
Spin-lattice relaxation time measurements (T1) were performed by the inversion recovery experiment. The time interval τ between the 90° and 180° pulses was varied within 16 experiments from 0.01-20 s with a relaxation delay of at least 60 s. The reproducibility of relaxation times is estimated to be within ± 5%.
Saturation transfer difference (STD) NMR spectra were obtained by selective saturation of the chiral stationary phase material by a series of 40 shaped 270° Eburb-1 pulses of 50 ms length, separated from each other by a 30 ms delay which yielded to a total saturation time of about 2 s [7,18-20]. The magnetization spreads due to intramolecular spin diffusion processes all over the receptor molecule. Broad stationary phase background signals were reduced additionally by using a spin lock pulse (T1ρ filter) with duration of 30 ms and an attenuation of 8 dB to destroy both transverse and longitudinal magnetization of the receptor macromolecule. Signals of the free analyte were not affected considerably. The irradiation frequency was set more than 1700 Hz away from any analyte resonances leading to a direct magnetization of ligand resonances of less than 0.2%. Spectrum subtraction to obtain the STD spectrum was achieved internally via appropriate phase cycling after each scan. In the binding case of a ligand during the receptor saturation time, intermolecular spin diffusion from the irradiated receptor leads to a partially saturation of the enantiomer resulting in decreased signal intensities after dissociation into solution. The OFF-resonance spectrum remains unchanged, since no receptor signal is saturated [12,17]. The ON-resonance irradiation frequency was set on broad stationary phase signals at 340 Hz (~1 ppm). The OFF experiment uses the same saturation pulses leading to equal energy contents to avoid e.g. temperature interferences between the ON and OFF experiment. Therefore, its irradiation frequency was set to 32 kHz (80 ppm) far away from any analyte signal. In total 4 k scans were recorded. The STD data [%] arise from the individual proton signal intensities of the STD spectrum in comparison to the OFF resonance 1H HR/MAS NMR spectrum referenced to the methyl signal at 1.53 ppm to an integral of 3. The ratios of intensities ISTD/IOFF-Res. are normalized to the strongest signal (proton HC, 100%) to obtain absolute saturation values.
Results and Discussion
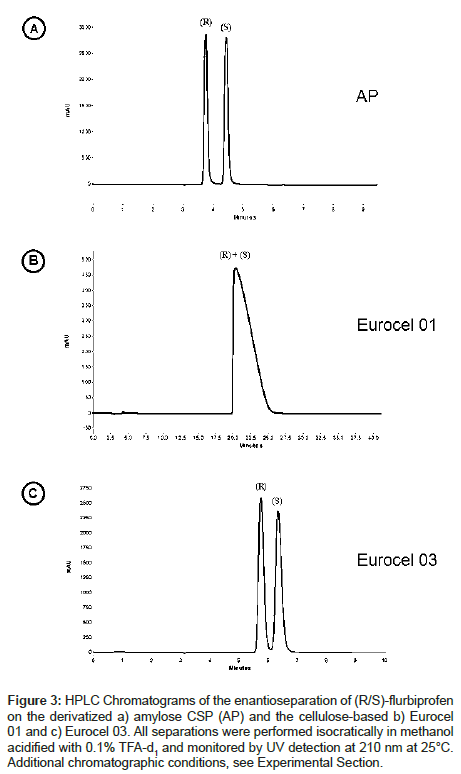
The separation of racemic flurbiprofen (Figure 1) into its enantiomers by HPLC was performed on three derivatized polysaccharide-based chiral stationary phases with a mobile phase consisting of methanol acidified with 0.1% trifluoracetic acid (TFA). Racemic flurbiprofen could be separated on the amylose phase (AP) and on Eurocel 03 (EC 03) but not with Eurocel 01 (EC 01). Figure 3 shows the HPLC chromatograms recorded in terms of resolution per time unit. Peak tailing was reduced due to the addition of trifluoroacetic acid (TFA) to the mobile phase.
Figure 3: HPLC Chromatograms of the enantioseparation of (R/S)-flurbiprofen on the derivatized a) amylose CSP (AP) and the cellulose-based b) Eurocel 01 and c) Eurocel 03. All separations were performed isocratically in methanol acidified with 0.1% TFA-d1 and monitored by UV detection at 210 nm at 25°C. Additional chromatographic conditions, see Experimental Section.
The retention order on AP and EC 03 is identical, the (R)- enantiomer elutes prior to the (S)-enantiomer. This indicates that the binding affinity of (S) is stronger towards the corresponding stationary phase than (R). The retention factor k’ characterizing the separation efficiency of the column material as well as the column selectivity α are shown in Table 1. AP separates (R)- and (S)-flurbiprofen with a higher α-value than EC 03 indicating that AP is obviously more suited for the flurbiprofen separation than EC 03. The differences in the Gibbs free energy, which provides an insight into the order of magnitude of the interaction energies, between AP and the enantiomers is ca. -1.37 kcal mol-1, for EC 03 the energy difference is app. -0.49 kcal mol-1. A structural comparison of AP and EC 01 shows that with the same substituents the ordered left-handed helical structure of the polysaccharide is apparently crucial for the separation process on the stationary phase. In addition, a comparison of EC 01 with EC 03 indicates that probably the derivatization of cellulose is important for the separation mechanism, because the phenylcarbamate CSP EC 03 is able to separate racemic flurbiprofen whereas the benzoate derivative EC 01 could not resolve the enantiomers. Obviously, the electron pushing methyl group in para-position as well as the polar ester group of EC 03 influences the molecular recognition ability of the stationary phase. Due to the inductive effect of the methyl group the electron density of the carbonyl group of the benzoate/carbamate CSPs should be increased. Thus, flurbiprofen should interact more effectively via electrostatic interaction forces like hydrogen bonds or dipole-dipole interactions.
| AP (Amylose-phase) | Eurocel 01 (Cellulose-based) | Eurocel 03 (Cellulose-based) | ||||
|---|---|---|---|---|---|---|
| Analyte | k’ | α | k’ | α | k’ | α |
| (R)-Flurbiprofen | 0.23 | 1.74 | No Separation | 0.84 | 1.22 | |
| (S)-Flurbiprofen | 0.4 | 1.02 | ||||
Table 1: Retention factor k’ and selectivity α of the separation of racemic flurbiprofen on the three different CSPs.
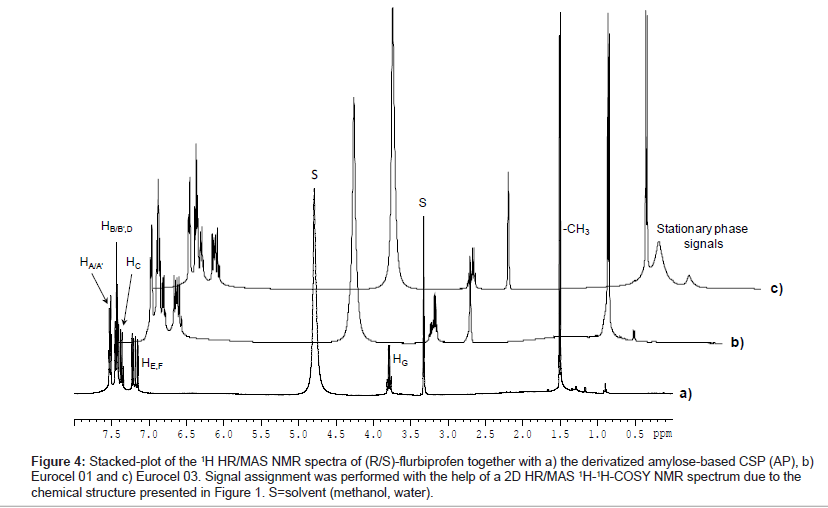
Figure 4 shows the stacked-plot of 1H HR/MAS NMR spectra of (R/S)-flurbiprofen in suspension with AP, EC 01 and EC03. For the single enantiomers no chemical shift differences of analyte resonances in the racemic mixture in presence of any chiral stationary phase could be detected. The signals were assigned with the help of a HR/MAS COSY NMR spectrum.
Figure 4: Stacked-plot of the 1H HR/MAS NMR spectra of (R/S)-flurbiprofen together with a) the derivatized amylose-based CSP (AP), b) Eurocel 01 and c) Eurocel 03. Signal assignment was performed with the help of a 2D HR/MAS 1H-1H-COSY NMR spectrum due to the chemical structure presented in Figure 1. S=solvent (methanol, water).
Binding enantiomers that are in exchange between the free sate and in the diastereomeric complex exhibit different relaxation properties promoted by dipolar interactions which represent the populationweighted average of the free and bound state. Therefore, complex formation of the enantiomer leads to changes in the tumbling behavior of bound ligand fractions, whereas no changes of the host behavior are expected [12]. Thus, measurements of the spin-lattice relaxation time (T1) for the (R)- and (S)-proton resonances in solution and in the diastereomeric complex were performed in order to elucidate the dynamic behavior of the two enantiomers towards the chiral stationary phases (Table 2). All T1 times of (R)- and (S)-flurbiprofen protons were decreased in suspension with the CSP material compared to those free in solution. These results suggest that probably the mobility of all protons of the enantiomers in presence of AP, EC 01 and EC 03, especially of the aromatic protons HA-F, are more restricted due to the ligand-receptor complex formation than free in solution.
| Signal Assignment | Chemical Shift [ppm] | T1 [S] | ||||||
| (R/S) in solution | AB | EC01 | EC03 | |||||
| R | S | R | S | R | S | |||
| HA/A’ | 7.56 | 2,28 | 2,07 | 1,71 | 1,90 | 2,22 | 2,02 | 1,96 |
| HB/B’,D | 7.47 | 2,29 | 2,07 | 1,66 | 1,79 | 2,20 | 2,03 | 1,93 |
| HC | 7.39 | 3,13 | 2,45 | 1,95 | 1,99 | 2,52 | 2,2 | 2,24 |
| HE,F | 7.25 | 2,31 | 2,09 | 1,80 | 1,85 | 2,21 | 2,02 | 2,02 |
| HG | 3.83 | 2,21 | 1,99 | 1,69 | 1,83 | 2,15 | 1,94 | 1,86 |
| -CH3 | 1.52 | 0,93 | 1,14 | 1,30 | 1,21 | 1,24 | 0,98 | 0,95 |
Table 2: Spin-lattice relaxation times (T1) for the (R)- and (S)-flurbiprofen enantiomers in solution and together with the CSP materials used. The estimated errors are approximately within ± 5%. The signal assignment in this table was performed in reference to the analyte structures presented in Figure 1.
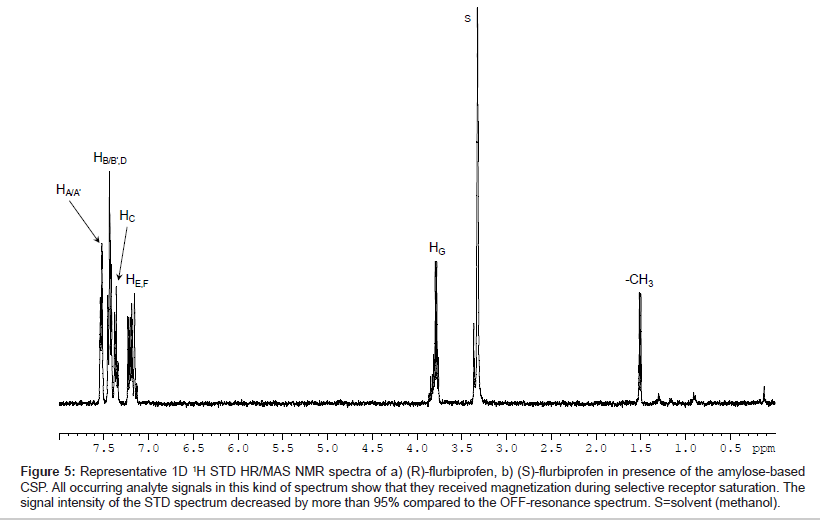
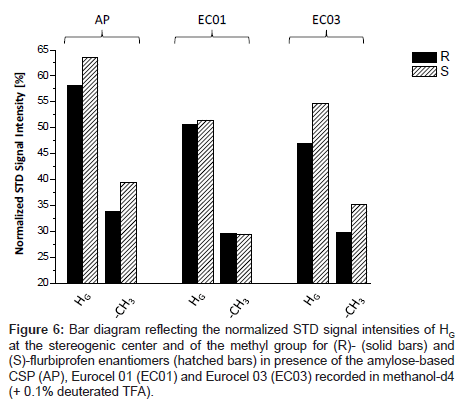
Representative, Figure 5 shows the T1ρ-filtered HR/MAS STD NMR spectrum of (S)-flurbiprofen in presence of the amylose-based CSP (AP). All signals of the analyte were detectable indicating that all protons of flurbiprofen are important for the binding process although the total intensity of the signals is only approximately 5% compared to the signals in the OFF-resonance spectrum. For a comparison of the three chiral stationary phases used, the signals were integrated in reference to the analyte methyl group at δ=1.53 ppm. Since both enantiomers exhibit only small saturation effects of about 2-6%, the signal intensities were normalized to the strongest resonance (HC, 100%). Hence, STD differences between (R) and (S)-flurbiprofen were only found for HG and to a lesser extent for the methyl group towards the different CSP materials. The values for those groups are listed as a bar diagram in Figure 6. The integral values of the aromatic proton signals HA-F in suspension with the three CSP materials are in the same range (74-87%) and are significantly stronger compared to HG (47-64%) and to the methyl group (29-40%). Probably, binding affinities due to hydrophobic and/or π-π interactions between the phenyl residues of the CSPs and the aromatic system of flurbiprofen are important for the different molecular recognition of the analytes. A comparison between the three CSPs of the signal intensities of HG and the methyl group showed, that the highest STD intensity, thus the strongest effect and therefore the closest proximity toward the CSP material, was found for the (S)-enantiomer in presence of the amylose phase. Weaker interactions of these groups were recorded with EC 03. Even EC 01 showed interactions with flurbiprofen although the strength and selectivity of this CSP material is not appropriate to separate both enantiomers from each other. On the basis of X-ray structure analysis, the glucose residues of the left-handed helical structure of the derivatized polysaccharides are ordered along the helix axis. The polar carbamoyl groups are situated in the inner part of the helix whereas the hydrophobic phenyl groups are on the outside of the polymer chain [2,3]. Thus, additional important factors in the recognition of flurbiprofen are probably steric features of the stationary phases.
Figure 5: Representative 1D 1H STD HR/MAS NMR spectra of a) (R)-flurbiprofen, b) (S)-flurbiprofen in presence of the amylose-based CSP. All occurring analyte signals in this kind of spectrum show that they received magnetization during selective receptor saturation. The signal intensity of the STD spectrum decreased by more than 95% compared to the OFF-resonance spectrum. S=solvent (methanol).
Figure 6: Bar diagram reflecting the normalized STD signal intensities of HG at the stereogenic center and of the methyl group for (R)- (solid bars) and (S)-flurbiprofen enantiomers (hatched bars) in presence of the amylose-based CSP (AP), Eurocel 01 (EC01) and Eurocel 03 (EC03) recorded in methanol-d4 (+ 0.1% deuterated TFA).
However, the stronger NMR effects were measured for the (S)- enantiomer compared to (R)-flurbiprofen each in suspension with the CSP materials used which match the information obtained by HPLC.
Conclusion
To characterize chiral molecular recognition processes of racemic compounds, a successful separation of flurbiprofen enantiomers was performed on the amylose-based CSP (AP) as well as on Eurocel 03 (EC 03). The enantiomers could not be separated under the chosen chromatographic conditions by Eurocel 01 (EC 01). Hence, the strength of different recognition forces against both enantiomers in the transient diastereomeric complex is responsible for the chiral discrimination of AP and EC 03 resulting in a different retention in HPLC.
Due to the heterogeneous system consisting of a CSP and an analyte solution, suspended-state HR/MAS NMR was used to obtain reasonable spectra as it approximates a chromatographic like environment. In order to study in more detail any occurring interactions, spin-lattice (T1) measurements and the saturation transfer difference (STD) NMR spectroscopy was applied to the suspensions yielding to more precise information upon binding affinities during the recognition processes.
Shorter T1 relaxation times of both enantiomers compared to solution-state indicate any interaction force toward the chiral stationary phases. Probably, restricted molecular mobility of the analytes e.g. due to electrostatic forces like hydrogen bonding or dipoldipol interactions between the hydroxy groups of the enantiomers and the carbonyl/carbamoyl groups of the CSPs is responsible for the different molecular recognition of both enantiomers.
In addition, all analyte signals were detectable by STD NMR which means that the whole flurbiprofen molecule interacts with the CSPs used. The aromatic flurbiprofen protons showed the strongest STD signal intensities and are probably based upon π-π interactions of the aromatic analyte system with the phenyl groups of the CSP material. STD NMR differences in their recognition ability between (R)- and (S)- flurbiprofen could only be detected for the stereogenic center as well as for the methyl group of the analyte suggesting electrostatic interaction forces like hydrogen bonding for example to the carbamoyl moiety of the column material. The higher STD signal intensities obtained with the amylose-based CSP give evidence to stronger binding forces with the enantiomers compared to Eurocel 03. Even slight differences between both enantiomers were recorded on Eurocel 01, but the interaction strength is obviously not sufficient to separate both enantiomers in chromatography on EC 01.
However, the NMR results obtained are in accordance to the chromatographic separation behavior and can be correlated to the retention order obtained in HPLC thus allowing a further insight into the chromatographic separation process. Results obtained out of such interaction studies performed by suspended-state HR/MAS NMR spectroscopy have the ability to take a key role allowing to increase the HPLC sample throughput in industry by preliminary performed short term molecular recognition studies leading to an optimized ligandreceptor system in chromatography.
Acknowledgement
The authors acknowledge the helpful discussions with Paul Schuler and his technical assistance concerning suspended-state NMR spectroscopy. We gratefully thank Dr. Ing. Herbert Knauer GmbH for the donation of the HPLCSystem and the CSPs used. This work was financially supported through the DFG (AL 298/14-1).
References
- Okamoto Y, Ikai T (2008) Chiral HPLC for efficient resolution of enantiomers. Chem Soc Rev 37: 2593-2608.
- Okamoto Y, Yashima E (1998) Polysaccharide derivatives for chromatographic separation of enantiomers. Angew Chem Int Ed 37: 1020-1043.
- Okamoto Y, Yashima E (1998) Chromatographische Enantiomerentrennung an Polysaccharidderivaten. Angew Chem 110: 1072-1095.
- Kromidas S (2006) HPLC Made to Measure: A Practical Handbook for Optimization. Wiley-VCH: Weinheim.
- Yamamoto C, Okamoto Y (2004) Optically active polymers for chiral separation. Bull Chem Soc Jpn 77: 227-257.
- Schurig V (1996) Terms for the quantitation of a mixture of stereoisomers. Enantiomer 1: 139-143.
- Friebolin V, Bayer MP, Matyska MT, Pesek JJ, Albert K (2009) 1H HR/MAS NMR in the suspended state: molecular recognition processes in liquid chromatography between steroids and a silica hydride-based cholesterol phase. J Sep Sci 32: 1722-1728.
- Hellriegel C, Skogsberg U, Albert K, Lämmerhofer M, Maier NM, et al. (2004) Characterization of a chiral stationary phase by HR/MAS NMR spectroscopy and investigation of enantioselective interaction with chiral ligates by transferred NOE. J Am Chem Soc 126: 3809-3816.
- Schauff S, Friebolin V, Grynbaum MD, Meyer C, Albert K (2007) Monitoring the interactions of tocopherol homologues with reversed-phase stationary HPLC phases by 1H suspended-state saturation transfer difference high-resolution/magic angle spinning NMR spectroscopy. Anal Chem 79: 8323-8326.
- Mayer M, Meyer B (1999) Characterization of Ligand Binding by Saturation Transfer Difference NMR Spectroscopy. Angew Chem Int Ed Engl 38: 1784-1788.
- Zerbe O (2003) BioNMR in Drug Research. Volume 16, Wiley-VCH: Weinheim.
- Vogtherr M, Peters T (2000) Application of NMR based binding assays to identify key hydroxy groups for intermolecular recognition. J Am Chem Soc 122: 6093-6099.
- Neuhaus D, Williamson MP (2000) The Nuclear Overhauser Effect in Structural and Conformational Analysis. 2nd edition, Wiley-VCH: Toronto.
- Oguni K, Ito M, Isokawa A, Matsumoto A (1996) 13C nuclear spin-spin relaxation times (T2Cs) of enantiomers in the presence of column packing material and solvents for chiral discrimination HPLC. Chirality 8: 372-376.
- Yashima E, Yamamoto C, Okamoto Y (1996) NMR Studies of Chiral Discrimination Relevant to the Liquid Chromatographic Enantioseparation by a Cellulose Phenylcarbamate Derivative. J Am Chem Soc 118: 4036-4048.
- Händel H, Gesele E, Gottschall K, Albert K (2003) Application of HRMAS 1H NMR spectroscopy to investigate interactions between ligands and synthetic receptors. Angew Chem Int Ed Engl 42: 438-442.
- Meyer B, Peters T (2003) NMR spectroscopy techniques for screening and identifying ligand binding to protein receptors. Angew Chem Int Ed Engl 42: 864-890.
- Claasen B, Axmann M, Meinecke R, Meyer B (2005) Direct observation of ligand binding to membrane proteins in living cells by a saturation transfer double difference (STDD) NMR spectroscopy method shows a significantly higher affinity of integrin alpha(IIb)beta3 in native platelets than in liposomes. J Am Chem Soc 127: 916-919.
- Cutting B, Shelke SV, Dragic Z, Wagner B, Gathje H, et al. (2007) Sensitivity enhancement in saturation transfer difference (STD) experiments through optimized excitation schemes. Magn Reson Chem 45: 720-724.
- Scherf T, Anglister J (1993) A T1 rho-filtered two-dimensional transferred NOE spectrum for studying antibody interactions with peptide antigens. Biophys J 64: 754-761.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14924
- [From(publication date):
specialissue-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10323
- PDF downloads : 4601