Research Article Open Access
HPLC Determination of the Levels of 6-Mercaptopurine Metabolites Suitable for the Clinical Risk Assessment of its Toxicity among Egyptian Children with Acute Lymphocytic Leukemia
Eman Abdelsayed M1, Sahar Maksoud A2, Iman Sidhom3,4, Mohamed Gad Z2 and Rasha Hanafi S1*
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy and Biotechnology, German University in Cairo, Cairo 11835, Egypt
2Department of Biochemistry, Faculty of Pharmacy and Biotechnology, German University in Cairo, Cairo 11835, Egypt
3Department of Pediatric Oncology, Faculty of Medicine, NCI - Cairo University, Cairo 11562, Egypt
4Children Cancer Hospital Egypt - 57357, Cairo, Egypt
- *Corresponding Author:
- Rasha Hanafi S
Department of Pharmaceutical Chemistry
Faculty of Pharmacy and Biotechnology
German University in Cairo, Cairo 11835, Egypt
Tel: 002-01005322522
E-mail: rasha.hanafi@guc.edu.eg
Received Date: March 30, 2017; Accepted Date: April 04, 2017; Published Date: April 08, 2017
Citation: Abdelsayed ME, Maksoud AS, Sidhom I, Gad ZM, Hanafi SR (2017) HPLC Determination of the Levels of 6-Mercaptopurine Metabolites Suitable for the Clinical Risk Assessment of its Toxicity among Egyptian Children with Acute Lymphocytic Leukemia. J Anal Bioanal Tech 8: 358. doi: 10.4172/2155-9872.1000358
Copyright: © 2017 Abdelsayed ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The need of a robust, sensitive HPLC method for the quantitation of 6-thioguaninenucleotides (6-TG) and 6-methylmercaptopurine (6-MMP) is indispensable to relate levels of these metabolites with emergence of signs of toxicity in patients undergoing treatment with 6-mercaptopurine (6-MP), paving the road to accurate dose calculations and thus providing a cost-effective treatment approach. Previously reported methods were either laborious, required special types of C18 columns, or had long run times. A Design of Experiments (DoE) approach targeting the shortest run time with greatest selectivity was adopted using a user friendly HPLC method development simulation software (DryLab®). Analytes eluted within 10 min, at 3.8, 4.2, 5.6 and 7.5 min for 6-TG, 6-MP, 6-MMP and Dithiothreitol (DTT) respectively. Excellent recovery percentages of 90.9 ± 14.4, 87.8 ± 6.7 and 92.1 ± 9.08, respectively were obtained. The method proved its validity and robustness according to the International Conference on Harmonization (ICH) guidelines. The LOD of 6-MP, 6-TG and 6-MMP were 6, 9 and 24 pmol/8 × 108 RBCs, respectively. Twenty-Two Acute Lymphocytic Leukemia (ALL) children recruited from 57357 Cancer Hospital (Cairo, Egypt) had their 6-MP metabolites measured using the developed method. A strong negative correlation was manifested between TG and RBCs count and hemoglobin (p=0.009 and 0.002 respectively). WBC and neutrophils showed a negative correlation to TG at Continuation 1 phase of treatment, confirming the association of TG with myelotoxicity. The significant correlation between MMP and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (p=0.030, 0.004) explained its potential hepatotoxicity.
Keywords
Mercaptopurine; Thioguanine-Nucleotides; Methylmercaptopurine; HPLC; DryLab®; Toxicity
Introduction
Acute Lymphocytic Leukemia (ALL) is a widespread disease, mostly affecting childhood. Treatment is divided into 3 major phases: induction, consolidation and maintenance, each having its own regimens. 6-Mercaptopurine (6-MP) is an analog of hypoxanthine nucleoside. It is an antimetabolite anticancer drug used in the treatment of ALL in consolidation and remission phases. The drug belongs to the thiopurines family of anticancer drugs, which includes thioguanine (TG) and azathioprine (AZA). These drugs are also used for the treatment of inflammatory bowel disease (IBD) and as immune-suppressants in organ transplants, severe rheumatoid arthritis, and autoimmune hepatitis, since more than 5 decades [1]. Measuring plasma levels of 6-MP is of no clinical value, since only 16% of the drug remains in the systemic circulation, with the rest being extensively metabolized [2].
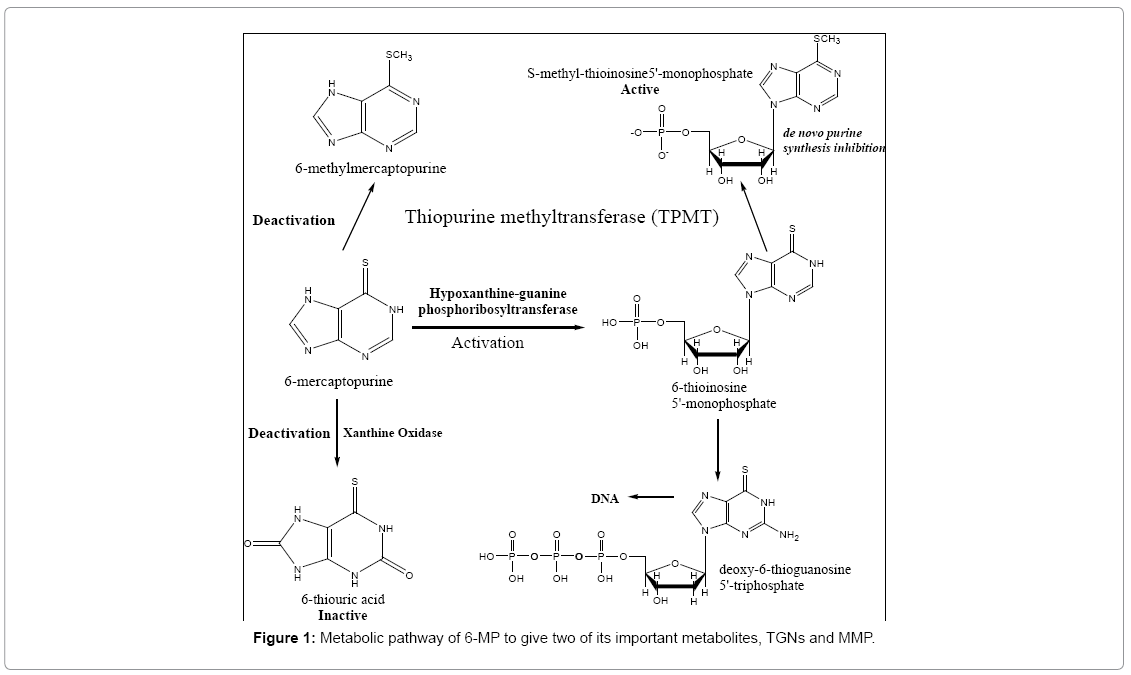
6-MP undergoes some routes for activation (Figure 1). These routes start with the generation of the active metabolite 6-thioinosine monophosphate (TIMP) by the action of the enzyme hypoxanthineguanine phosphoribosyltransferase (HGPRT). This transformation results in the formation of active metabolites including nucleotides of 6-thioguanine (6-Thioguaninenucleotides (6-TGN) or 6-Thioguaninemonophosphate (6-TGMP)), through the consecutive actions of inosine monophosphate dehydrogenase and guanosine monophosphate synthetase. The incorporation of 6-TGN as false nucleotides (mimicking those of normal guanine base) into DNA and RNA of cancer cells is considered to be the principal mechanism of action of 6-MP [3,4]. Back in the 1960s, it was discovered that a specific methylase is able to transfer a methyl group from S-adenosyl methionine (SAM) to the sulfur atom of thiopurines. Charles Remy was the first to describe specific thiopurine S-methyltransferase (TPMT) activity in several species [5]. This is the main route which competes with the activation pathway of the drug. Hence, any defect in this pathway will lead to toxicity due to accumulation of active TGNs. The other minor inactivation route occurs both in the intestinal mucosa and in the liver and involves the enzyme xanthine oxidase, which transforms 6-MP into thioxanthine and subsequently into thiouric acid [6,7]. It is important to note that TPMT is not only involved in the pathway which lowers the TGNs production, but also methylates TIMP to give MethylTIMP (or 6-Methylmercaptopurinenucleotides (6-MMPNs)) which inhibit the de novo purine synthesis. Both metabolites, TGNs and MMP have reported long t1/2 in red blood cells (RBCs), unlike the parent drug, of about 5 days, concentrations of which can be used as an index for the drug's cytotoxicity [8-10]. It was reported that upon administration of standard doses of 6-MP, some children showed signs of toxicity, which were related to genetic autosomal co-dominant polymorphism of the TPMT enzyme [3]. Therefore, patients who are heterozygous for the non-functional allele need reduced doses of 6-MP while those homozygous need a drastically reduced dose/alternative therapy [4] and unfortunately, dose reduction clinically is done based only on the TPMT genotype empirically, with limited accuracy and no individualisation. This raises the need of a reproducible, automated, fast process which quantitates the metabolites levels and aids in tailoring drug doses.
LC-MS/MS and HPLC-UV are the two chromatographic techniques reported to measure 6-MP and its metabolites levels in blood [1,11- 22]. Each of the reported methods had its advantages and drawbacks, many of which were overcome by the present method, which measures 6-MP, 6-TG and 6-MMP by HPLC-UV in one sample using a single wavelength and a conventional C18 column. Moreover, our method is novel by being the only one implemented using a systemic design of experiments approach, with the aid of DryLab® 2000 software, with exact determination of design space for the first time. This is in addition to resolution map generation which shows how temperature and gradient time affect the resolution and where the robust area of the method is.
The ease of the procedures employed in this method and low costs make it a good candidate for routine clinical use and research studies.
Materials and Methods
Subjects
Twenty-two children diagnosed with ALL, aged from 2-10 years, undergoing treatment with 6-MP according to two protocols: Low Risk (LR) and Very Low Risk (VLR), were recruited from 57357 Children's Cancer Hospital in Egypt. A written consent was obtained from the children's guardians, after fully explaining the purpose and the plan of the study. Also, an approval was obtained from the Ethics Committee at our institute and the hospital's board. Only children who had been administered 6-MP for at least 4 consecutive weeks (doses ranged from 28.5 to 57 mg/day) were enrolled in the study and blood samples were obtained over a 3-period follow-up (Consolidation (Cons) 3 and 2 Continuation Weeks (Cont W) 1 and 7) in order to assess the change in metabolites levels along the course of treatment. In addition, clinical data such as hemoglobin level, RBCs and leukocytes count, as well as liver function tests, such as ALT, AST, Alkaline Phosphatase and bilirubin levels were collected for each child at the time of blood sampling.
Reagents
6-Mercaptopurine (6MP), 6-Thioguanine (TG), 6-Methylmercaptopurine (6 MMP) and dithiothreitol (DTT) were purchased from Alfa Aesar GmbH (Karlsruhe, Germany). Sodium dihydrogen orthophosphate-1-hydrate and sodium hydroxide were purchased from El Nasr Pharmaceutical Chemical Co. (Egypt). Methanol (HPLC grade) and phosphoric acid were obtained from Sigma-Aldrich Chemie GmbH (Steinheim, Germany), perchloric acid 70% from Analytical Rasayan, s.d fine Chem limited (Mumbai), and normal saline from Otsuka Pharmaceutical Co., S.A.E. (Egypt). Water (HPLC grade) was obtained by Purelab UHQ water, ELGA (UK) purification system.
HPLC
The HPLC system (Thermo Finnigan Spectrasystem®, UK) consisted of a binary pump (Spectrasystem P2000), the variable UV-Vis detector (Spectrasystem UV3000), the autosampler (Spectrasystem AS3000) with Microvials (100 μl) and acquisition software (Chromquest 4.2 data system). The column used was a Hypersil Gold C18 (3 μm, 150 mm × 4.6 mm, Thermo Scientific), and was protected with a guard cartridge of the same type and particle size (10 mm × 4 mm).
The optimum HPLC conditions used included a mobile phase aqueous system consisting of 0.025M phosphate buffer (pH 2.6), while the concentration of the organic modifier, methanol in the elution solvent was varied from 5% to 80% within 10 min using a linear gradient profile. The total run time, including the equilibration time for the following run was 16 min. The flow rate was set at 1 ml/min, T at 25°C and the detection wavelength at 320 nm for all analytes.
The previous optimal conditions were obtained using a systematic approach of full factorial design for two factors (F) (gradient time, tG and temperature, T) at two levels (l) each (tG =10 min and 30 min, T= 25 and 45°C), requiring Fl = 22 experiments to establish the response surface (the resolution map) that relates resolution to retention for the studied parameters.
Preparation of samples
Erythrocytes preparation: Three to five ml venous blood samples were obtained in violet top (EDTA) tubes from each child. Preparation was based on the protocol used by the St. Jude Children's Research Hospital [23], where samples were kept at 4°C until processed within 24 hours and then centrifuged at 170 g, for 10 minutes at 4°C. Plasma and buffy coat were removed while the remaining RBC volume was measured with a pipette, recorded and left in the tube sample was drawn in. Next, cells were washed in approximately 2 volumes normal saline, vortexed on setting 1 (low speed vortexing) for 15 seconds and centrifuged at 200 g, for 10 min at 4°C. The supernatant was discarded and the washing step was repeated. The sample was centrifuged at about 750 g for 10 min at 4°C. Finally, the supernatant was discarded, and cells were resuspended in 1 volume normal saline. The RBC count was obtained and recorded by a Beckmann CoulterTM Counter (USA). Samples were stored in 1.5- ml Eppendorfs at -80°C until further analysis.
Sample extraction and hydrolysis: The extraction procedure was based on Dervieux and Boulieu [17]. 200 μl of suspended erythrocytes were mixed with 100 μl of Dithiothreitol (DTT) (75 mg/ml, final concentration of 120 mM), 50 μl of water and 50 μl of perchloric acid (700 ml/L) by vortex mixing for 30 s in a 1.5 ml Eppendorf tube (Sarstedt, Germany). The tube was then centrifuged for 15 min at 13,000 × g at room temperature. The supernatant (300 μl) was transferred to another Eppendorf tube and heated in a compact thermomixer (Hamburg, Germany) for 45 min at 100°C to hydrolyse thiopurine nucleotides into their corresponding bases. During hydrolysis, 6-MMP is converted completely into 4-amino-5-(methylthio) carbonyl imidazole (AMTCI). The latter product is easily quantified under the same chromatographic conditions used for other bases and is what is eventually detected by the described method. After cooling, a 25 μl aliquot was injected onto the column.
Preparation of calibrators
Working standard solutions were prepared at six different concentrations for 6-MP, 6-TG and 6-MMP by dissolving required amounts of each compound in 0.1 M NaOH and then diluting with water. These were then used to prepare erythrocyte calibrators by spiking erythrocyte aliquots (blood obtained from healthy individuals and processed as described in section 2.4.1) with 50 μl of the working standards instead of adding 50 μl water and hydrolyzing them as described in the above section. These erythrocyte calibrators were used to construct calibration curves, with 6-MP, 6-TG and 6-MMP concentrations of 20-800, 30-6000, 200-60000 pmol/8 × 108 RBCs, respectively. These concentrations were chosen to be within the predicted range of those in-patient samples.
Assay validation
Validation parameters such as selectivity, linearity, extraction yield, accuracy and precision, limit of detection and lower limit of quantification were determined according to the International Conference on Harmonization (ICH) guideline for validation of analytical procedures. The intra-assay variation was calculated from triplicate measurements on the same day at different concentrations of erythrocyte calibrators, while the inter-assay variation was obtained from experiments completed on 3 different days.
Stability
Bench-top stability of the analytes of interest (6-MP and its major metabolites) in human RBCs was evaluated at room temperature. Two concentrations per analyte were tested (low and high) at t=1 h and t=24 h. The ratio of the results at these 2 occasions was then calculated. Stability of analytes after storage at -80°C for 3 months was also evaluated for one of the concentrations, by analyzing the samples 15 days apart.
Statistical methods
Means, standard deviations and linearity (based on linear regression analysis) were calculated using Microsoft™ Excel software (Microsoft Corporation, USA). Correlations between measured metabolites levels and manifested toxicity signs were demonstrated by scatter plots generated by Minitab 17® (Minitab Ltd, UK).
Results and Discussion
Optimization of chromatographic separation
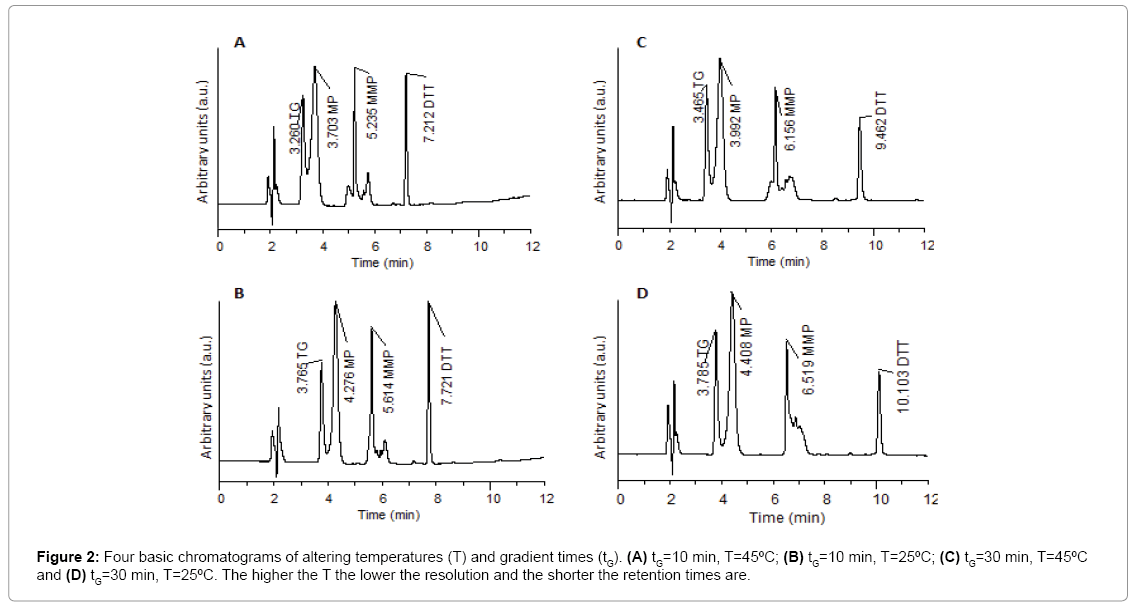
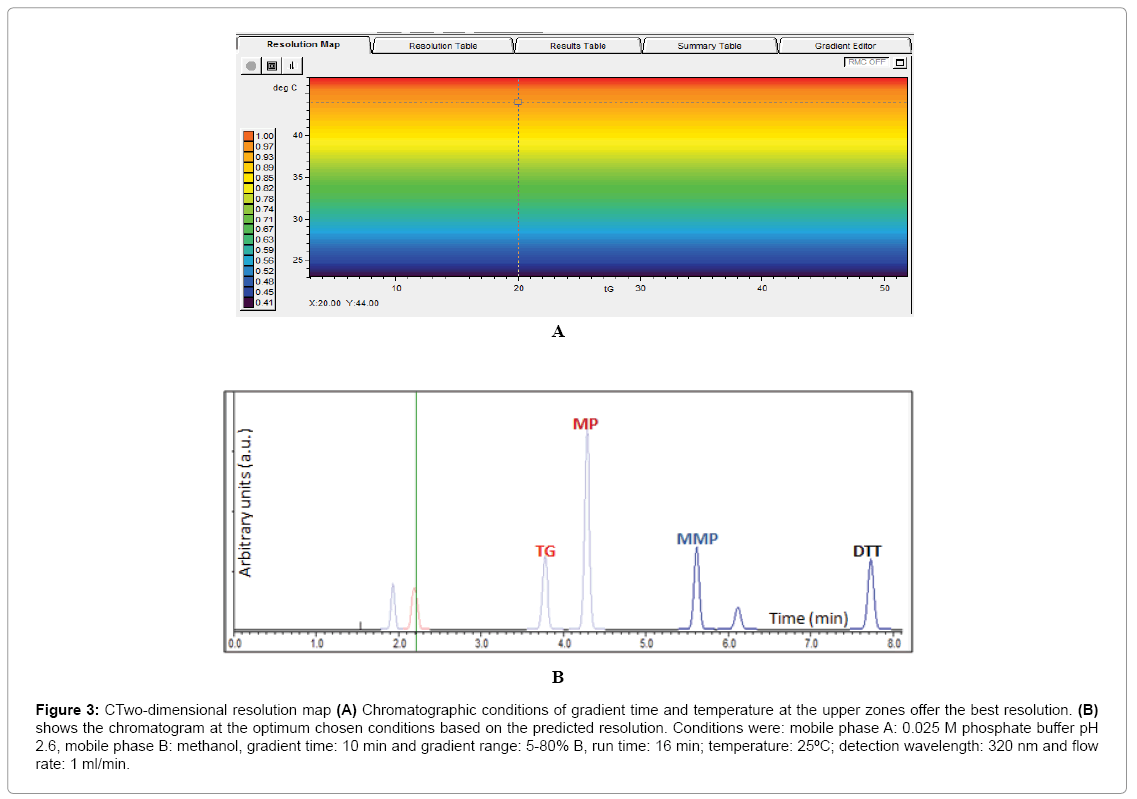
Results obtained from the four basic runs of the full factorial design (Figure 2) were fed into the modeling software (DryLab®2000) to generate the resolution map (Figure 3A) that shows the effect of change of gradient times and temperatures on the resolution and retention factors. The map (Figure 3A) proved that column temperature-rather than gradient time-is the major factor controlling resolution of 6-MP, 6-TG and 6-MMP; where reddish areas (Rs>1.16) in the map were attributed to temperatures >50°C, regardless of the gradient time. This aided in the selection of the optimum HPLC conditions used in the development of our method as previously described in (section 2.3. HPLC).
Figure 3: CTwo-dimensional resolution map (A) Chromatographic conditions of gradient time and temperature at the upper zones offer the best resolution. (B) shows the chromatogram at the optimum chosen conditions based on the predicted resolution. Conditions were: mobile phase A: 0.025 M phosphate buffer pH 2.6, mobile phase B: methanol, gradient time: 10 min and gradient range: 5-80% B, run time: 16 min; temperature: 25ºC; detection wavelength: 320 nm and flow rate: 1 ml/min.
The chromatogram obtained using the predicted optimum conditions shows base line resolution of the analytes both virtually (Figure 3B) and practically (Figure 2A) with retention factors k>2. Error in prediction of retention times between the predicted and experimental chromatograms was <5%, which proved validity of the resolution map regarding other possibly predicted chromatograms at different conditions.
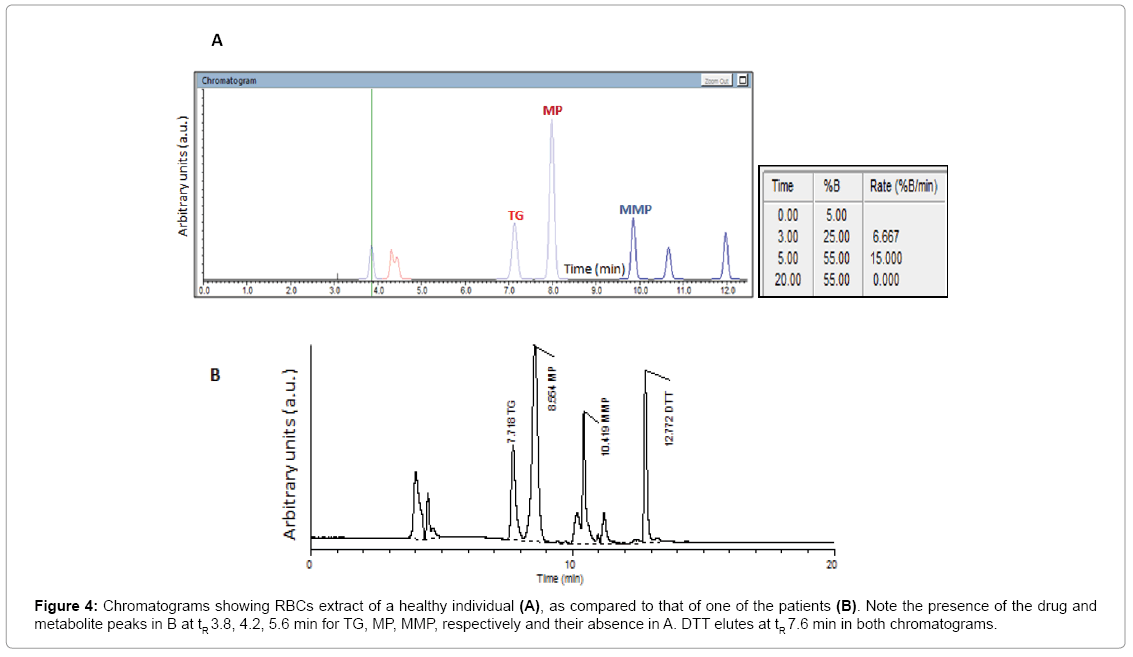
The method proved to be selective for the compounds of interest, with no interfering substances present in the processed erythrocytes obtained from healthy subjects or patients with ALL (Figure 4). Moreover, no interference with the commonly co-administered drugs in ALL such as methotrexate, vincristine, prednisone or dexamethasone was found.
Figure 4: Chromatograms showing RBCs extract of a healthy individual (A), as compared to that of one of the patients (B). Note the presence of the drug and metabolite peaks in B at tR 3.8, 4.2, 5.6 min for TG, MP, MMP, respectively and their absence in A. DTT elutes at tR 7.6 min in both chromatograms.
Method Validation
Limit of detection and lower limit of quantification: The described method was validated at the optimum conditions by determining the limit of detection (LOD) and lower limit of quantitation (LLOQ), having a signal/noise ratio of 3/1 and 10/1, respectively. According to ICH guidelines, determination of the signal-to-noise ratio is performed by comparing measured signals from samples with known low concentrations of analyte with those of blank samples and establishing the minimum concentration at which the analyte can be reliably detected/ quantified. The LOD of 6-MP, 6-TG and 6-MMP were found to be 6, 9 and 24 pmol/8 × 108 RBCs, respectively. The LOQ of 6-MP, 6-TG and 6-MMP were found to be 20, 30 and 80 pmol/8 × 108 RBCs, respectively.
Linearity: Five calibration curves on three different days were constructed, for each metabolite, to show the direct proportionality between the response ratio (peak area of each metabolite divided by the peak area of the internal standard) and the concentration of each in the sample. Slopes, intercepts and coefficients of correlation (r2) are shown in Table 1. Linear relationships were observed between the relative peak areas and concentrations of 6-MP, 6-TG and 6-MMP over the ranges previously specified in section.
| A | |||||||
|---|---|---|---|---|---|---|---|
| Day | 1 | 2 | 3 | 3'' | 3''' | Mean | sdv |
| Intercept | 0.0010 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0004 |
| Slope | 0.000025 | 0.000030 | 0.000025 | 0.000020 | 0.000025 | 0.000025 | 0.000003 |
| r2 | 0.989 | 0.994 | 0.989 | 0.985 | 0.992 | 0.982 | 0.003 |
| B | |||||||
| Day | 1 | 2 | 3 | 3'' | 3''' | Mean | sdv |
| Intercept | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.0008 | 0.0004 |
| Slope | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.00000 |
| r2 | 0.999 | 0.997 | 0.999 | 0.997 | 0.998 | 0.998 | 0.001 |
| C | |||||||
| Day | 1 | 2 | 3 | 3'' | 3''' | Mean | sdv |
| Intercept | 0.025 | 0.008 | 0.011 | 0.012 | 0.012 | 0.013 | 0.006 |
| Slope | 0.000009 | 0.00001 | 0.000008 | 0.000008 | 0.000008 | 0.000008 | 0.0000008 |
| r2 | 0.998 | 0.999 | 0.999 | 0.997 | 0.993 | 0.997 | 0.002 |
Table 1: Pre-study validation: regression parameters for the calibration curves on three different days. One calibration curve was obtained on the first two days, and three on the third day (n=5) [A: 6-MP; B: 6-TG; C: 6-MMP].
Preparation of calibrators: The equation obtained from standard curves was y=2.5 × 10-5x+0.0002 (r2=0.982, P<0.001) for 6-MP, y=1 × 10-5x+0.0008 (r2=0.998, P<0.001) for 6-TG and y=8 × 10-6x+0.013 (r2=0.997, P<0.001) for 6-MMP.
Precision and accuracy: In order to demonstrate the repeatability (precision) and accuracy of the results as part of pre-study validation, healthy RBCs spiked with the three analytes at six different concentrations representing the low, medium and high portions of the calibration curve, were studied by replicate analyses (n=3) on 3 different days. Precision was expressed as relative standard deviation or coefficient of variation (CV%), where CV%=standard deviation/mean × 100. Accuracy was determined by comparing the measured concentrations of these spiked samples with expected concentrations, and was expressed as % Bias, which represents the mean deviation from the nominal content of the sample (%). It is reported as % Bias=[(measured value - true value)/ true value] × 100. For the intra-day precision, CV% ranged from 2.89% to 8.17%, 6.17% to 9.43% and 4.46% to 13.96% for 6-MP, 6-TG and 6-MMP respectively. For the inter-day precision, CV% ranged from 2.64% to 8.68%, 3.75% to 5.19% and 1.15% to 9.51% for 6-MP, 6-TG and 6-MMP respectively. Accuracy (%Bias) for the calibration curves were in the range of -4.5% to 4%, -3.79% to 7.72% and -2% to 4.54% for 6-MP, 6-TG and 6-MMP respectively. Quality control samples were used for each analyte as part of validation during routine analysis, yielding precision and accuracy percentages of 2.64% to 8.68% and -1.92% to 0.22%, respectively for 6-MP, whereas 3.75% to 4.73% and -0.85% to 1.09%, respectively for 6-TG, and 1.15% to 9.51% and 0.6% to 4.38%, respectively for 6-MMP. Results are shown below in Table 2. As %RSD is fairly constant throughout the calibration curves and the precision and accuracy are within ≤ ± 15% at all concentrations, so no special weighting factor is used.
| A | ||||
|---|---|---|---|---|
| Concentration | Mean | s.dv | Precision | Accuracy |
| (pmol/8×108 RBCs) | (pmol/8×108 RBCs) | (C.V. %) | (% Bias) | |
| 40 | 39.70 | 3.45 | 8.68 | -0.73 |
| 80 | 80.17 | 2.12 | 2.64 | 0.22 |
| 120 | 117.69 | 3.26 | 2.77 | -1.92 |
| B | ||||
| Concentration(pmol/8×108 RBCs) | Mean(pmol/8×108 RBCs) | s.dv | Precision(C.V. %) | (% Bias) |
| 300 | 303.28 | 14.34 | 4.73 | 1.095 |
| 600 | 594.88 | 30.89 | 5.19 | -0.85 |
| 1200 | 1211.38 | 45.54 | 3.75 | 0.94 |
| C | ||||
| Concentration | Mean | s.dv | Precision | Accuracy |
| (pmol/8×108 RBCs) | (pmol/8×108 RBCs) | (C.V.%) | (% Bias) | |
| 1200 | 1252.60 | 14.43 | 1.15 | 4.38 |
| 6000 | 6154.17 | 171.166 | 2.78 | 2.56 |
| 18000 | 18109.29 | 1722.37 | 9.51 | 0.60 |
Table 2: Validation during routine assays / Summary of intraday precision and accuracy data for quantification of 6-MP [A], 6-TG [B], 6-MMP [C] obtained from quality control samples during routine analysis.
Recovery: Recovery percent or extraction yield was obtained for each analyte as follows (Table 3), by comparing the relative area of each standard to DTT in water and the respective relative area in RBCs preparation, at 3 different standard concentrations. The mean extraction efficiency was 87.8% for 6-MP, 90.9% for 6-TG and 92.1% for 6-MMP. Such recoveries are higher than that obtained by previous methods [1,12-17], due to applying some modifications to the extraction process as advised by Hawwa et al. [11]. Recovery seemed to increase with increasing drug concentrations, and performing a oneway ANOVA test revealed a statistically significant difference (p<0.05) between recovery % of low and high concentrations of the 3 analytes, however with no significant difference between the low/intermediate/ high concentrations. A possible explanation lies in the extraction process and the chemical properties of the analytes, where the analytes seem to exhibit possible binding to macromolecules (like proteins of RBCs) or glass or plastic (of the used disposables) and this process maybe saturable. Nevertheless, further experiments need to be carried out to exactly determine the causative factor. It is expected that such differences do not affect method performance as even lower recoveries are acceptable and mean values are better than other reported methods [1,12-17].
| Concentration (pmol/8×108 RBCs) |
40 | 160 | 320 | 200 | 600 | 1200 | 1200 | 12000 | 24000 |
|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | |||||||||
| 6-MP | 80.9 | 88.2 | 94.3 | - | - | ||||
| 6-TG | - | 74.3 | 98.7 | 99.8 | |||||
| 6-MMP | - | 82.1 | 94.4 | 99.9 | |||||
| Mean Recovery% | 87.8 ± 6.7 | 90.9 ± 14.4 | 92.1 ± 9.08 | ||||||
Table 3: Recovery% for 6-MP, 6-TG and 6-MMP at 3 different concentrations.
Stability: The mean ratios and standard deviation from the 6 measurements at room temperature for each analyte were determined and were as follows: 0.99 ± 0.02, 0.99 ± 0.01, 1.02 ± 0.02, for 6-MP, 6-MMP and 6-TG respectively. Results after storage at -80ºC showed a 3% decrease in the concentration of 6-MP, no significant decrease in 6-MMP concentrations, and a 5% decrease in the concentration of 6-TG (Table 4). Thus, all three analytes were therefore stable for at least one day at room temperature, and for at least 3 months when stored at -80°C.
| Days at -80ºC | 6-MP | 6-MMP | 6-TG |
|---|---|---|---|
| (pmol/8×108 RBCs) | (pmol/8×108 RBCs) | (pmol/8×108 RBCs) | |
| 0 | 32 ± 2 | 308 ± 7 | 55 ± 1 |
| 15 | 30 ± 1 | 310 ± 6 | 54 ± 2 |
| 30 | 31 ± 3 | 312 ± 8 | 50 ± 2 |
| 45 | 29 ± 1 | 308 ± 9 | 51 ± 3 |
| 60 | 30 ± 5 | 305 ± 10 | 53 ± 1 |
| 75 | 29 ± 4 | 302 ± 12 | 52 ± 5 |
| 90 | 31 ± 3 | 305 ± 9 | 52 ± 4 |
Table 4: Stability of 6-MP, 6-MMP and 6-TGN in blood at -80ºC. Quality control samples having expected concentrations of 30, 300 and 50 pmol/8 × 108 RBCs, respectively, were used for the test. Values are the mean ± SD, n=3.
Analysis of patient samples
A total of 55 measurements from 22 ALL patients were possible (for some patients, a sample at Continuation Week 10 or 12 was sometimes also obtained, depending on the feasibility of the conditions, such as being able to reach the patient, if his/her blood veins are readily accessible and the availability of the nursing team. The children took their 6-MP dose at night (50 mg/m2 during Consolidation and 75 mg/ m2 during Continuation), and after 8 hours (in the next morning), samples were withdrawn. During sample extraction, it is assumed that no further degradation of the parent drug occurs, as metabolism occurs enzymatically by TPMT and HGPRT -not chemically- and the majority of the prepared samples were stored at -80°C until analysis, ceasing any possible enzymatic activity. Furthermore, the parent drug has a relatively short half-life (1.64 h) in comparison to its metabolites (about 5 days), so by the time of analysis, it is expected that the majority of the drug has been converted into its metabolites, and any further metabolism (if occurred) would be negligible and should not affect the accuracy of the results. Furthermore, metabolism of 6-MP follows steady state kinetics [3].
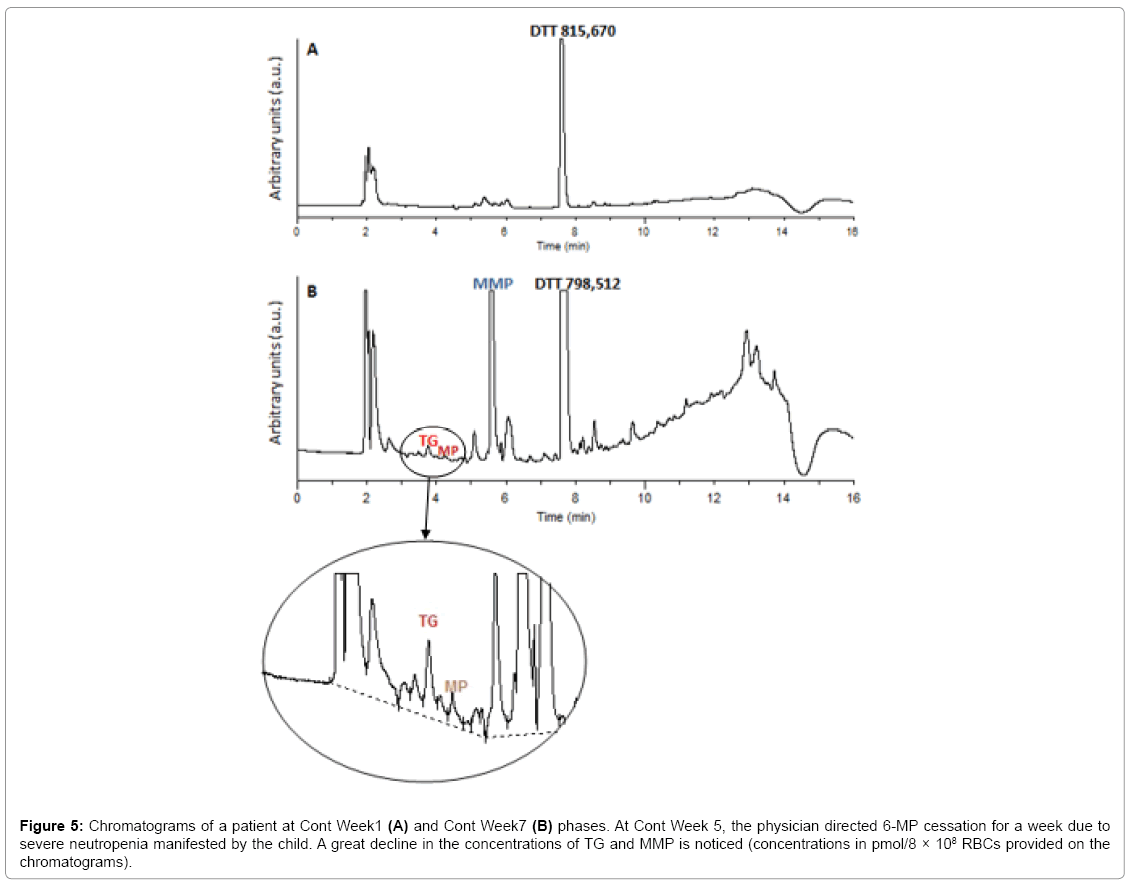
Statistical analysis revealed that only 16% of the children had therapeutic TGN levels, 24% had supratherapeutic levels and 60% had highly elevated levels of the metabolite (>800 pmol/8 × 108 RBCs), with mean TGN levels of 1257.64 and a median of 879 pmol/8 × 108 RBCs. Leucopenia was encountered in 80% of the children and 62% were anemic. For MMP, 64% had levels >5700 pmol/8 × 108 RBCs, with a mean of 21505 and a median of 10319.5 pmol/8 × 108 RBCs. ALT/ AST levels were shown to be elevated in 66% of the patients (Table A1). Figure 5A illustrates the HPLC chromatogram of a sample obtained from a patient undergoing treatment with 6-MP at a dose of 57 mg/ day. Figure 5B shows the HPLC chromatogram of the same patient, having stopped 6-MP intake for a week due to manifested toxicity. The great difference in the areas of the metabolites shows that the developed method could also be used to track non-adherence, or 6-MP cessation.
Figure 5: Chromatograms of a patient at Cont Week1 (A) and Cont Week7 (B) phases. At Cont Week 5, the physician directed 6-MP cessation for a week due to severe neutropenia manifested by the child. A great decline in the concentrations of TG and MMP is noticed (concentrations in pmol/8 × 108 RBCs provided on the chromatograms).
Correlations between metabolites levels and drug toxicity
TGN and MMP: A positive correlation was found between TGNs and MMP levels (r=0.501, p<0.0001), in a similar finding to Dubinsky et al. [2] but contradicting with Chrzanowska et al. [24] who found no relation, although theoretically the 2 metabolites should be negatively correlated, being part of competing pathways (Figure 1). A possible explanation could be that the majority of the recruited children have wild type TPMT genotype, yielding proportional amounts of both metabolites in a dose dependent manner.
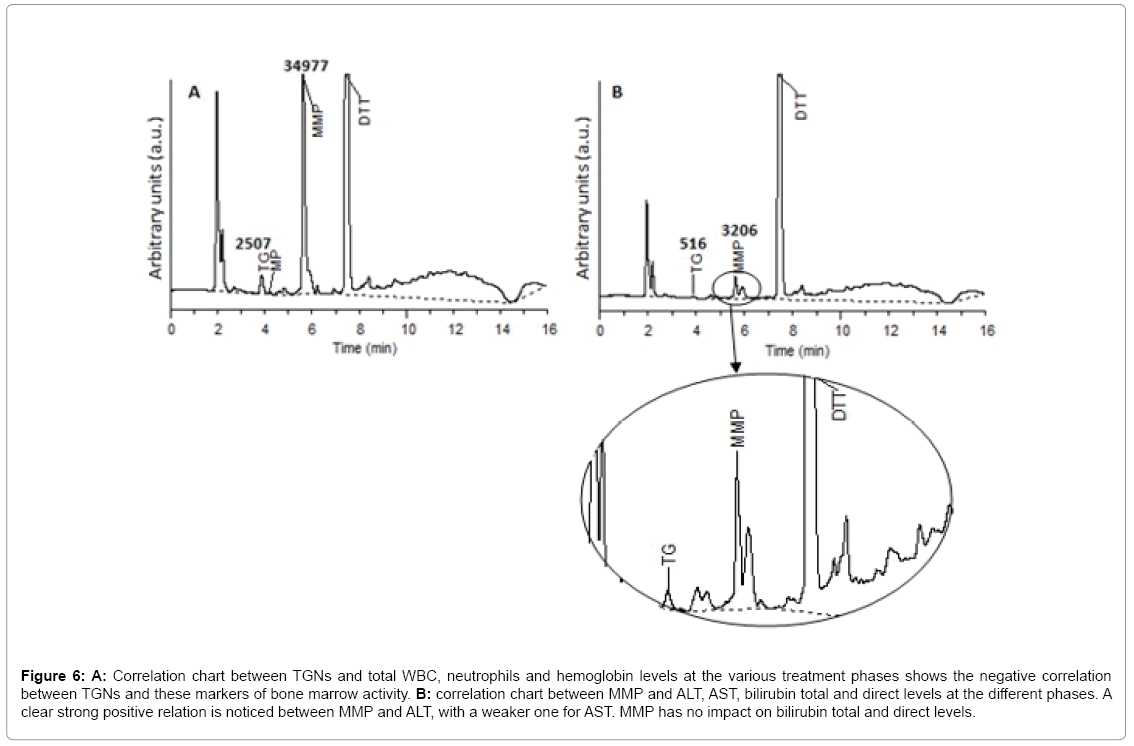
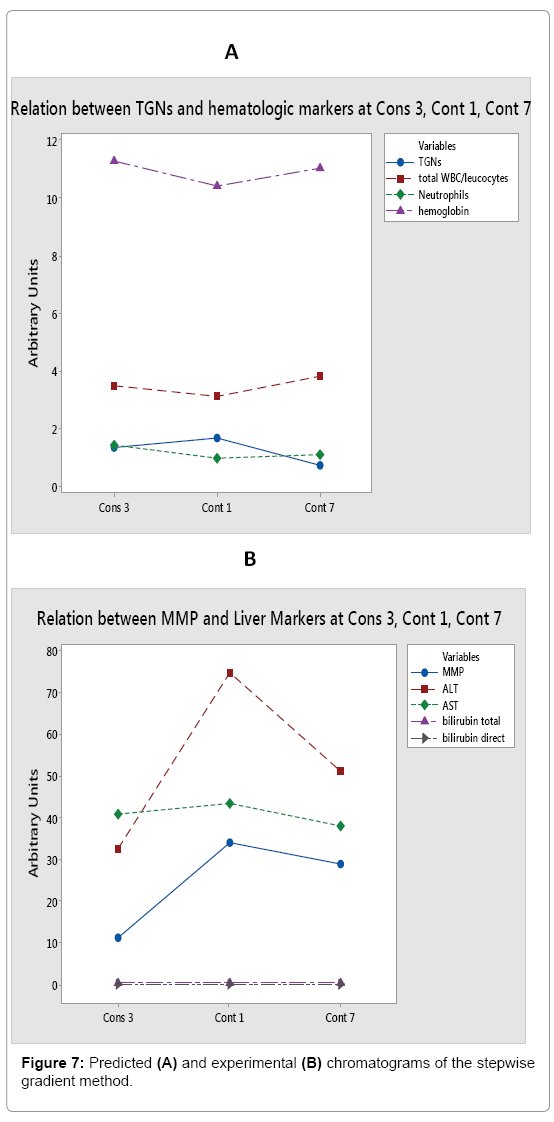
TGN and hematological toxicity: Generally, a weak negative correlation was found between TGNs levels and RBCs count (r=- 0.367, p=0.009). However, zooming in at Cons 3 and Cont W1 phases, a stronger correlation was revealed (r=-0.642, p=0.002 and r=-0.520, p=0.068 respectively). Generally, a weak negative correlation was obtained between TGNs levels and hemoglobin concentration (r=- 0.397, p=0.005). At Cont W1, however, a much stronger correlation was noticed (r=-0.798, p=0.001). This is explained with regards to the bone marrow toxic effect of elevated TGNs, in agreement with the literature [2,25-27]. The better correlation as treatment proceeds may be attributed to the generally increased doses of the drug administered at Cont W1, according to the treatment protocol. In our study, no general correlation was found between TGN levels and total WBC counts or neutrophils (r=-0.107, p=0.463 and r=0.044, p=0.761 respectively). These results are similar to those found by Ref. [8,28]. However, it is interesting to find that by Cont W1 phase, a negative correlation was attained between TGNs and WBCs and neutrophils (r=-0.485, p=0.093 and r=-0.455, p=0.118 respectively), in a similar finding to Lennard et al. [10] (Figure 6A). This can be inferred as the delay required for TGNs to achieve a cytotoxic effect on leukocytes, and due to accumulation of such nucleotides along the treatment process. As predicted, no correlation was concluded between TGNs and ALT, AST, total and direct bilirubin, confirming the theory that such metabolite is not associated with hepatotoxicity, but rather 6-MMP [2,25,29].
Figure 6: A: Correlation chart between TGNs and total WBC, neutrophils and hemoglobin levels at the various treatment phases shows the negative correlation between TGNs and these markers of bone marrow activity. B: correlation chart between MMP and ALT, AST, bilirubin total and direct levels at the different phases. A clear strong positive relation is noticed between MMP and ALT, with a weaker one for AST. MMP has no impact on bilirubin total and direct levels.
MMP and hepatotoxicity: A general positive correlation was found between MMP and ALT levels (r=0.454, p=0.001). Weaker correlations were noticed between the other liver markers, such as AST, total and direct bilirubin (r=0.206, p=0.155; r=0.234, p=0.106; r=0.218, p=0.133 respectively). A stronger correlation was observed between the metabolite and ALT, AST and total/direct bilirubin in Cont W1 phase (r=0.601, p=0.030; r=0.736, p=0.004; r=0.632, p=0.021; r=0.606, p=0.028 respectively) (Figure 6B). Moreover, it was noticed that in most cases where MMP levels exceeded 25,000 pmol/8 × 108 RBCs, ALT and/or AST levels were highly elevated by three to four folds. Another interesting finding is the slight negative correlation between MMP and WBC, RBCs count and hemoglobin (r=-0.226, p=0.119; r=-0.214, p=0.140; r=-0.248, p=0.086). This can be contributed to the slight myelosuppressive effect of the 6-MMPNs (or Me-TIMP) in the measured 6-MMP pool. No correlation however was found to the neutrophil count.
General discussion
6-MP has a short half-life in plasma following oral administration, which makes the measurement of its plasma levels only of value for pharmacokinetic and pharmacodynamic studies, but not for long-term therapeutic monitoring of patients receiving oral thiopurines [12]. Therefore, the concentrations of 6-TGN and 6-MMP are better measured in washed erythrocytes as a surrogate to the intracellular levels of these metabolites in the target tissues [13]. The first two methods were developed by Lennard and Singleton [1], and Dervieux and Boulieu [17]. Being developed earlier, the procedure of Lennard and Singleton has been used exclusively in clinical studies to establish therapeutic ranges for 6-TG or toxic levels for 6-MMP, however the procedure was tedious, reagent consuming, and mercury-based waste generator. An anion exchange method for the determination of intracellular mono-, di-, and triphosphate thiopurine and methylthiopurine nucleotides in RBCs was also described by Keuzenkamp-Jansen et al. [30]. Dervieux et al. later worked on implementing a Liquid Chromatography-Tandem Mass Spectrometry (LC-MS) method [19]. However, drawbacks of this method include the necessity to use deuterated internal standards, and the fact that some chemical laboratories in developing countries still miss that sophisticated instrumentation, especially in hospitals where the determination is most needed. A recent method by Vikingsson et al. aimed at measuring thiopurine mono-, di-, and triphosphates separately in red blood cells, where analytes were separated by ionpairing liquid chromatography using tetrabutylammonium ions [21], however this method requires a laborious protocol, with sampling and pre-analytical handling difficulties due to the rapid interconversion between the phosphorylated forms.
The undertaken approach for systematic computer-assisted HPLC method development was estimated necessary to figure out the response surface of the HPLC method proposed. It is based on two sets of facts: the first being that stationary phase temperature is a major determinant of both retention of analytes and their resolution [31]. Secondly, elution of analytes from a reversed phase column massively depends on the water composition of the mobile phase [32]. The aim of this approach was therefore to study the effects of varying temperatures and gradient times on the separation of the three analytes of interest. DryLab® software could be considered as a good tool to “control” peak purity; as it allows generation of an infinite number of chromatograms by changing different chromatographic parameters with accurate determination of the parameters that would lead to coelution of peaks. Hence, it is very easy to “see” the conditions that would lead to peak overlap. The best chromatographic conditions were fulfilled with a stepwise gradient method (Figure 7), achieving the best resolution in the shortest possible tG (12 min). Nevertheless, it was decided to validate the best of the linear gradient runs (T 25°C and tG 10 min), to be used for patients' samples analysis for more reproducible chromatograms upon method transfer to the hospital laboratory. The reason behind this choice is that the main objective of this work is to develop an analytical method that could be easily implemented in every clinical lab equipped with the necessary instruments. In addition, it has been planned and agreed upon with the administrative and technical authorities at 57357 Children Cancer Hospital to transfer the developed and validated HPLC method to the analytical laboratory of the hospital, to be used for quantifying 6-MP levels in ALL children at various stages of the treatment process, in order to optimize and individualize the drug's dose. Implementation of a stepwise gradient in a different laboratory may produce practical inconveniences to the analysts there. This is due to the differences in the HPLC instrument that would be used for routine analysis at the hospital, and it is not guaranteed that the system would be able to cope with the increased equilibration and conditioning demands by the proposed stepwise method. Furthermore, large inter-individual variations between blood samples from different patients are expected, hence a linear gradient would easily expose the whole sample peaks more efficiently. Therefore, a linear gradient is more robust than a stepwise one under these 2 critical conditions. To sum up, for the sake of simplicity, reproducibility and least emergence of troubleshooting, the linear gradient was preferred especially that the chromatograms of both gradients (Figures 3B and 7A) have no major difference in outcome to sacrifice the convenience of the settings.
In light of previously reported HPLC methods for 6-MP and its metabolites determination, some shortcomings were investigated, such as the lack of internal standardization, except for a few [12,13,19,20]. Sulfamethoxazole and 6-MP were used by Pike et al. [13] and Stefan et al. [12] respectively, while Dervieux et al. [19] and Kirchherr et al. [20] used 8-bromoadenine and isotope labeled standards, necessary for LC-MS/MS. Our method is superior in this respect, as it overcame the lack of internal standard addition, and the need of expensive reagents or sophisticated chromatographs, through nominating one of the used reagents in the sample preparation, DTT, to be the internal standard.
Dithiothreitol (DTT), also known as Cleland's reagent, is a strong reducing agent, owing to its high conformational proprensity to form a six member ring with an internal disulfide bond. DTT is frequently used to reduce the disulfide bonds of proteins and, more generally, to prevent intramolecular and intermolecular disulfide bonds from forming between cysteine residues of proteins [33]. 6-MP and 6-TG have free thiol groups that DTT protects from oxidation during sample deproteinization step, which improves the percentage recovery of these 2 compounds. However, DTT doesn't contribute to 6-MMP recovery, since its methylated SH group is already protected from oxidation by the added perchloric acid. Since DTT is already used in the extraction process and is detectable at the same wavelength (320 nm) as the drug and its metabolites, it was chosen as an internal standard (IS). DTT being both a reagent and an IS raises the controversy that the consumption of the chemical in the reaction would result in a decrease in the chemical's concentration and thus detector's response, failing to meet the required criteria of a suitable IS. This was defeated by agreement of the areas of DTT in a blank sample and that of a patient's containing the analytes of interest as shown by Figure 4. This was further supported by the slight, acceptable variations obtained in the areas of DTT with increasing standards concentrations (6-TG, 6-MP and 6-MMP) upon calibration curves construction (Table A2). A possible explanation could be that the loss of hydrogens in the oxidation process does not negatively influence the UV absorbance of the compound at the selected wavelength. In fact, it is reported that the oxidized form of DTT shows strong absorbance starting at 260 nm [33]. DTT also showed a mean acceptable recovery of 90.5% ± 2, determined in the same way as discussed before for the other analytes.
The developed method offers the shortest retention times for analytes elution, compared to all methods measuring the drug and its metabolites simultaneously [1,11,15,16], with all peaks of interest eluting within 8 minutes. On top of that, the use of a derivatized (capped with polar groups) C18 column is inevitable if isocratic elution mode was to be performed for the separation of these analytes, as shown in previous methods [1,11,12,14,15]. This is probably explained as thiopurine compounds are highly polar and tend to co-elute with the use of a conventional C18 column. Thus, the use of an isocratic elution was sacrificed with a gradient one (despite being theoretically applicable according to Δt/tG rule), to keep the benefit of using a cheaper, readily available column, and to obtain narrow peak widths with highest sample capacity in the least run time.
The method's limitation was its inability to quantify the mono-, di-, and tri-phosphates of the methylated and non-methylated metabolites of 6-MP solely (but rather as a pool), in which technical sample handling difficulties hinders its success as previously discussed. Measuring the tri-phosphate form of TGNs is however important because it is estimated to be a better predictor for the response to treatment, yet clinical studies are lacking [21].
Conclusions
The described method permits accurate and efficient measurement of 6-MP and two of its clinically essential metabolites, 6-TGNs and 6-MMP, in erythrocytes, independently and in a timely fashion, making its extrapolation to service-providing laboratories possible. The method has a short run time as well as utilizes a cheap, readily available, conventional C18 column, and implements an already used reagent in the procedure as an internal standard. Such analysis is essential to monitor patients' progress in the course of therapy and identify interand intra- variability in the drug's metabolism among children. High MMP levels correlated with hepatotoxicity (high ALT and AST), while high TGN levels correlated with myelosuppression (low neutrophil counts and hemoglobin). Thus, determination of the concentrations of such metabolites has a great impact on the optimization of the child's needed 6-MP dose, due to the metabolites' contribution to the drug's therapeutic and toxic events in ALL. The developed method could also be used to track non-adherence, or 6-MP cessation. The high frequency of elevated TGNs and MMP levels in the tested population gives an indication that the administered 6-MP doses are not individualized, and it is clear from our data that each patient has a different tolerability. Thus, physicians need to seriously consider the incorporation of such HPLC methods to accurately determine the needed doses and tailor treatment, thus reducing leukemia relapse, myleosuppressive and hepatotoxic events, and providing a cost effective treatment approach. To our present knowledge, none of the previous publications did evaluate the clinical risk assessment of 6-MP in Egyptian ALL children by measuring the drug and its metabolites levels. This is the first study to be done in Egypt regarding this aspect, as recent studies on Egyptian children either just studied the possible relation between TPMT polymorphisms and 6-MP with short-term complications [6,9,34,35] or discussed the adherence of children to treatment [10,36].
Acknowledgements
Our deep thanks to 57357 Hospital for doing part of the lab work at their premises, especially the pharmacokinetics lab, and for providing us with the patient samples. We also acknowledge the support of pharmacist Ahmed El-Zeiny (Children Cancer Hospital, Egypt), who managed the patients' appointments and took note of the sampling dates of each patient. His effort in this study is greatly appreciated.
Conflict of Interest
We declare that all authors have seen and approved the final version of the manuscript being submitted, and that the article is our original work, hasn't received prior publication and isn't under consideration for publication elsewhere. In addition, no conflicts of interest are declared.
References
- Len nard L, Singleton HJ (1992) High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6 mercaptopurine: quantification of red blood cell 6 thioguanine nucleotide, 6 thioinosinic acid and methylmercaptopurine metabolites in a single sample. Journal of Chromatography B: Biomedical Sciences and Applications 583:83-90.
- Dubinsky MC, Lamothe SÑ, Yang HY, Targan SR, Sinnett D, et al. (2000) Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 118:705-713.
- Weinshilboum RM,Sladek SL (1980) Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. American journal of human genetics 32:651.
- Krynetski E, Evans WE (2003) Drug methylation in cancer therapy: lessons from the TPMT polymorphism. Oncogene 22:7403-7413.
- Remy CN (1963) Metabolism of thiopyrimidines and thiopurines S-Methylation with S-adenosylmethioninetransmethylase and catabolism in mammalian tissues. Journal of Biological Chemistry 238:1078-1084.
- Lennard LJSJ, Lilleyman JS, Van Loon J, Weinshilboum RM (1990) Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. The Lancet 336:225-229.
- Lancaster DL, Patel N, Lennard L, Lilleyman JS (2001) 6-Thioguanine in children with acute lymphoblastic leukaemia: influence of food on parent drug pharmacokinetics and 6-thioguanine nucleotide concentrations. British journal of clinical pharmacology 51:531-539.
- Cuffari C, Theoret Y, Latour S, Seidman G (1996) 6-Mercaptopurine metabolism in Crohn's disease: correlation with efficacy and toxicity. Gut 39:401-406.
- Lennard LBET, Gibson BE, Nicole T, Lilleyman JS (1993) Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Archives of disease in childhood 69:577-579.
- Lennard L, Rees CA, Lilleyman JS, Maddocks JL (1983) Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. British journal of clinical pharmacology 16:359-363.
- Hawwa AF, Millership JS, Collier PS, McElnay JC (2009) Development and validation of an HPLC method for the rapid and simultaneous determination of 6-mercaptopurine and four of its metabolites in plasma and red blood cells. Journal of Pharmaceutical and Biomedical Analysis 49:401-409.
- Stefan C, Walsh W, Banka T, Adeli K, Verjee Z (2004) Improved HPLC methodology for monitoring thiopurine metabolites in patients on thiopurine therapy. Clinical biochemistry 37:764-771.
- Pike MG, Franklin CL, Mays DC, Lipsky JJ, Lowry PW, et al. (2001) Improved methods for determining the concentration of 6-thioguanine nucleotides and 6-methylmercaptopurine nucleotides in blood. Journal of Chromatography B: Biomedical Sciences and Applications 757:1-9.
- Erb N, Haverland U, Harms DrO, Escherich G, Janka-Schaub G (2003) High-performance liquid chromatographic assay of metabolites of thioguanine and mercaptopurine in capillary blood. Journal of Chromatography B 796:87-94.
- Oliveira BM, Romanha AJ, Alves TMA, Viana MB, Zani CL (2004) An improved HPLC method for the quantitation of 6-mercaptopurine and its metabolites in red blood cells. Brazilian journal of medical and biological research 37:649-658.
- Mawatari H, Kato Y, Nishimura Si, Sakura N, Ueda K (1998) Reversed-phase high-performance liquid chromatographic assay method for quantitating 6-mercaptopurine and its methylated and non-methylated metabolites in a single sample. Journal of Chromatography B: Biomedical Sciences and Applications 716:392-396.
- Dervieux T, Boulieu R (1998) Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clinical chemistry 44:551-555.
- Boulieu R,Dervieux T (1999) High-performance liquid chromotographic determination of methyl 6-mercaptopurine nucleotides (Me6-MPN) in red blood cells: analysis of Me6-MPN per se or Me6-MPN derivative? Journal of Chromatography B: Biomedical Sciences and Applications 730:273-274.
- Dervieux T, Meyer G, Barham R, Matsutani M, Barry M, et al. (2005) Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clinical chemistry 51:2074-2084.
- Kirchherr H, Shipkova M, von Ahsen N (2013) Improved method for therapeutic drug monitoring of 6-thioguanine nucleotides and 6-methylmercaptopurine in whole-blood by LC/MSMS using isotope-labeled internal standards. Therapeutic drug monitoring 35:313-321.
- Vikingsson S, Almer S, Peterson C, Carlsson Br, Josefsson M (2013) Monitoring of thiopurine metabolites - A high-performance liquid chromatography method for clinical use. Journal of Pharmaceutical and Biomedical Analysis 75:145-152.
- Dervieux T, Chu Y, Su Y, Pui CH, Evans WE, et al. (2002) HPLC determination of thiopurine nucleosides and nucleotides in vivo in lymphoblasts following mercaptopurine therapy. Clinical chemistry 48:61-68.
- Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, et al. (1999) Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. Journal of the National Cancer Institute 91:2001-2008.
- Chrzanowska M, Kolecki P, Duczmal-Cichocka B, Fiet J (1999) Metabolites of mercaptopurine in red blood cells: a relationship between 6-thioguanine nucleotides and 6-methylmercaptopurine metabolite concentrations in children with lymphoblastic leukemia. European journal of pharmaceutical sciences 8:329-334.
- Dubinsky MC, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA (2002) 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology 122:904-915.
- Tassaneeyakul W, Srimarthpirom S, Reungjui S, Chansung K, Romphruk A, et al (2003) Azathioprine-Induced Fatal Myelosuppression in A Renal-Transplant Recipient Who Carried Heterozygous Tpmt *1/*3C. Transplantation 76:265-266.
- Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM (2004) Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clinical Gastroenterology and Hepatology 2:410-417.
- Lowry PW, Franklin CL, Weaver AL, Pike MG, Mays DC, et al. (2001) Measurement of thiopurine methyltransferase activity and azathioprine metabolites in patients with inflammatory bowel disease. Gut 49:665-670.
- Nygaard U, Toft N, Schmiegelow K (2004) Methylated Metabolites of 6-mercaptopurine are Associated with Hepatotoxicity. Clinical Pharmacology & Therapeutics 75:274-281.
- Keuzenkamp-Jansen CW, De Abreu RA, Bkkerink JPM, Trijbels JMF (1995) Determination of extracellular and intracellular thiopurines and methylthiopurines by high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications 672:53-61.
- Snyder LR, Kirkland JJ, Glajch JL (2012) Practical HPLC method development. John Wiley & Sons.
- HorvÉth C, Melander W, MolnÉr I (1976) Solvophobic interactions in liquid chromatography with nonpolar stationary phases. Journal of Chromatography A 125:129-156.
- Cleland WW (1964) Dithiothreitol, a new protective reagent for SH groups. Biochemistry 3:480-482.
- El-Rashedy FH, Ragab SM, Dawood AA, Temraz SA (2015) Clinical implication of thiopurine methyltransferase polymorphism in children with acute lymphoblastic leukemia: A preliminary Egyptian study. Indian Journal of Medical and Paediatric Oncology 36:265.
- Rashidy FH, Ragab SM, Dawood AA, Temraz SA (2015) Toxic complications of treatment with 6-mercaptopurine in pediatric acute lymphoblastic leukemia. Menoufia Medical Journal 28:411.
- Khalek ERA, Sherif LM, Kamal NM, Gharib AF, Shawky HM (2015) Acute lymphoblastic leukemia: Are Egyptian children adherent to maintenance therapy? Journal of cancer research and therapeutics 11:54.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 5902
- [From(publication date):
April-2017 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 4905
- PDF downloads : 997