Case Report Open Access
Host-oriented Inherent Measures and Eukaryotic Parasite Countermeasures
El-Ashram S1,2,*, Al Nasr I3,4, Hu M5, Suo X1,*1National Animal Protozoa Laboratory, College of Veterinary Medicine, China Agricultural University, Beijing 100193, China
2Faculty of Science, Kafr El-Sheikh University, Kafr El-Sheikh, Egypt
3College of Science and Arts in Unaizah, Qassim University, Unaizah, Saudi Arabia; College of Applied Health Sciences in Ar Rass, Qassim University, Ar Rass 51921, Saudi Arabia
4College of Applied Health Sciences in Ar Rass, Qassim University, Ar Rass 51921, Saudi Arabia
5State Key Laboratory of Agricultural Microbiology, Key Laboratory of Development of Veterinary Products, Ministry of Agriculture, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, Hubei, China
- *Corresponding Author:
- Saeed El-Ashram
National Animal Protozoa Laboratory &College of Veterinary Medicine, China Agricultural University
Beijing 100193, China and Faculty of Science, Kafr El-Sheikh University
Kafr El-Sheikh, Egypt
Tel: +86-13121120565
Email: saeed_elashram@yahoo.com
Xun Suo
National Animal Protozoa Laboratory & College of Veterinary Medicine
China Agricultural University, Beijing 100193, China
Tel: +86-13121120565
Email: suoxun@cau.edu.cn
Received date: February 02, 2017; Accepted date: February 03, 2017; Published date: February 09, 2017
Citation: El-Ashram S, Al Nasr I, Hu M, Suo X (2017) Host-oriented Inherent Measures and Eukaryotic Parasite Countermeasures. J Mol Immunol 2:106.
Copyright: © 2017 El-Ashram S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Molecular Immunology
Abstract
The recognition and elimination of eukaryotic parasites appear to be a hardwired prerequisite for host survival. However, eukaryotic parasites, such as Toxoplasma gondii, Leishmania sp., Trypanosoma cruzi, Giardia sp., and Schistosoma sp. employ the innate system of the host for their growth, replication, and continuation of their life cycle. So far, however, there has been little discussion about the interaction of the eukaryotic parasites and the innate immune system. Driven by this need, this review provides an overview of the host-oriented inherent measures and eukaryotic parasite countermeasures for evading host defences. Additionally, this review discusses control of parasite and its evasion strategy at innate and adaptive arms of the immune system. Taken together, this information could be exploited to discover novel therapies, vaccine strategies and prophylactic intervention points for broad-spectrum host-oriented inherent measures and eukaryotic parasite counter-measures, and to understand the parasitic disease progression and the infection consequence.
Keywords
Eukaryotic parasites; Innate immune system; Therapeutic and prophylactic intervention
Fighting / Evasion at the Front Lines (First and Second Defense Lines)
The role of the innate immune response in parasitic infections is to confront infections by producing non-specific immune responses. In addition to the physical barriers, such as skin and mucous membranes, which represent the first defense line, the innate immunity consists of cellular and humoral defense mechanisms (second defense line). The humoral molecules of innate immunity include fibronectin, complement, Tumor necrosis factor (TNF)-α, lysozyme, C-type lectins, lactoferin, and tranferin. Examples of the cellular branch include phagocytes, natural killer (NK) cells, γδ T-cells, and natural killer T (NKT) cells.
NK cells play a pivotal role in the immune response controlling Plasmodium infection. Moreover, NK cells contribute to the control of parasitemia via Th1 cytokines in the erythrocytic phase [1-3]. Filtjens et al. demonstrated that Ly49E expression has a transitory role in the immune control of Plasmodium pathogenesis [4].
Breaching the first defense line
The skin membrane barrier is one of the most important parts of innate immunity and first line of defense against invading organisms. The skin consists of thick epidermis (i.e. stratified squamous epithelial cells) and dermis. The thick outer layer acts as a physical barrier to the infection. Furthermore, the presence of fatty acids and sweat can trap and kill the small attackers. Though these strong anatomical barriers, the parasites use different ways to invade the skin and the mucosal membranes. They either actively insult the skin as in the case of cercariae of Schistosoma using serine and cysteine proteases or passively invade the skin (i.e. injected directly into the bloodstream, such as Plasmodium sporozoites, Brugia and wuchereria L3 larva or migrate from vector bite, for example, L3 of Onchocerca volvulus and trypomastigote of Trypanosoma cruzi [5]. Biting mosquito salivary glands have a lot of different soluble components, such as platelet aggregation inhibitors, anticoagulants (thrombin), and antihistamines. These components provide Plasmodium sporozoite survival and facilitate their inoculation [6,7]. The mucosal membranes can protect non-specifically against invaders by two effector mechanisms: luminal defense mechanism (for example, antimicrobial peptides, mucous, acidic pH, peristalsis and microbiota) and epithelial defense mechanism: single or multiple layers of cells depending upon the location and the animal species, tight junction between the cells, and Toll-like receptors (TLRs) on the cell surface and within the cytosol, and cytosolic nucleotide binding oligormerization domain (NOD) [8].
Most of the parasitic infections (eggs, oocysts, larvae, and metacercariae) occur through oral route. Apicomplexan parasites can actively deal with the first defense line by gliding motility. Other parasites invade by the bite of certain type of flies, and then spread through phagocytosis as in Leishmania and Trypanosoma cruzi. The newly emerged juvenile of trematodes can actively penetrate the intestinal mucosa through the secretion of Cathepsin B during invasion/migration processes. Some parasites reside in the intestine for the rest of their life as Giardia, which avoid peristalsis by attachment/re-attachment mechanisms [9]. However, cestodes move up- stream in a snake-like motion to avoid peristalsis.
Another Chance (Second Defense Line): Waves of the Inflammatory Response
First Wave of the Inflammatory Response
Toll-like receptors (TLRs) are most important primary innate immune receptors [10]. Thirteen mammalian TLRs have been described; 10 and 13 TLRs are present in humans, and mice respectively [11]. The role of TLR was first shown in tissue lesions in the context of T. gondii and Plasmodium berghei infections [12,13]. For examples, TLRs play an important role in adaptive/innate immune responses against helminth infections [14-16]. The first wave of immunological falls after evasion of the first line is the early inflammatory response in which TLRs employ a crucial role as the initiator of the innate immunity. TLRs are an emerging family of innate receptors that belong to pattern recognition receptors (PRRs). Immune and nonimmune cells use TLRs to trigger the innate immune response and to orchestrate adaptive immunity. Tabulated below, a brief summary of pathogen-associated molecular patterns (PAMPs) of protozoan parasite [17]. PAMPs of protozoan parasite (Table 1) [18].
| PAMP | Parasite | Expression stage | Structure | TLR |
|---|---|---|---|---|
| GPI anchors | L. major | Promastigotes | LPG | TLR2 |
| L. donovani | ||||
| T. cruzi | Trypomastigotes | Contains unsaturated alkylacylglycerol | TLR2 | |
| Epimastigotes | GIPLs containing ceramide | TLR4 | ||
| T. brucei | Trypomastigotes | GPI anchors of VSGs | ND | |
| P. falciparum | Merozites | GIPLs and GPI anchors of the MSP | TLR2 | |
| T. gondii | Tachyzoites | GIPLs and GPI anchors | TLR2 | |
| Genomic DNA | T. brucei | All stages | Contains unmethylated CpG motifs | TLR9 |
| T. cruzi | All stages | Contains unmethylated CpG motifs | TLR9 | |
| Haemozoin | P. falciparum | Merozoites | β-Haematin crystal made from haemin | TLR9 |
| PFTG | T. gondii | Tachyzoites | Profilin-like protein | TLR1 |
Table 1: GIPL, glycoinositolphospholipid; GPI, glycosylphosphatidylinositol; L. donovani, Leishmania donovani; L. major, Leishmania major; LPG, lipophosphoglycan; MSP, merozoite surface antigen; ND, not determined; P. falciparum, Plasmodium falciparum; PFTG, profilin-like protein; T. brucei, Trypanosoma brucei; T. cruzi, Trypanosoma cruzi; T. gondii, Toxoplasma gondii; TLR, Toll-like receptor; VSGs, variant surface glycoproteins.
It has been shown that TLR signalling pathway plays a pivotal role in host resistance and pathogenesis during protozoan infection (Table 2). Roles of TLR signalling pathway during protozoan infection [18].
| Parasite | Knockout | Phenotype |
|---|---|---|
| L. major | Myd88–/– | TH2 phenotype and increased susceptibility |
| Tlr4 mutant | No major effects on immune response | |
| Myd88–/– | Decreased pro-inflammatory cytokines and increased susceptibility | |
| Tlr1–/–, Tlr2–/–, Tlr4–/–, Tlr6–/– or Cd14–/– | Normal cytokine responses, parasitaemia and survival | |
| Tlr2–/– and Tlr4–/– or Tlr9–/– | Impaired IL-12 and IFNγ production and increased susceptibility | |
| T. cruzi | Myd88–/– | Impaired pro-inflammatory cytokines and increased susceptibility |
| Tlr2–/– | Increased IL-12 and IFNγ, and unaffected parasitaemia and survival | |
| Tlr4–/–, Tlr6–/–or Cd14–/– | Normal cytokine responses, parasitaemia and survival | |
| Tlr9–/– | Impaired IL-12 and IFNγ production, increased parasitaemia and accelerated mortality | |
| Tlr2–/– and Tlr9–/– | More pronounced effects than just Tlr9–/–mice | |
| P. berghei | Myd88–/– | Impaired pro-inflammatory cytokines and decreased pathology |
| Tlr2–/–, Tlr4–/– or Tlr6–/ | Normal cytokine responses, parasitaemia and pathology | |
| T. gondii | Myd88–/– | Impaired IL-12 and IFNγ production, and increased susceptibility |
| Tlr2–/– | High-dose inocula—increased susceptibility to infection; low or intermediate dose—normal cytokine responses, parasitaemia and survival | |
| Tlr4–/– | Normal cytokine responses, parasitaemia and survival | |
| Tlr11–/– | Impaired production of IL-12 and IFNγ, and increased susceptibility |
Table 2: IFN, interferon; IL, interleukin; L. major, Leishmania major; Myd88, myeloid differentiation primary-response gene 88; P. berghei, Plasmodium berghei; T. brucei, Trypanosoma brucei; T. cruzi, Trypanosoma cruzi; T. gondii, Toxoplasma gondii; TH, T helper; Tlr, Toll-like receptor.
Several recent studies have reported that TLR11, TLR12 (tachyzoitederived profilin-like protein detection) and the diverse IFN γ-inducible mouse GTPase (IRG) proteins are species-specific while TLR7 and TLR9 (parasite RNA and DNA detection) are widely distributed in different animal species [19-21]. Importantly, TLR11 expression is induced in neurons and glial cells in the immune response to T. gondii infection [22].
TLRs, in particular, TLR 2, TLR 3, TLR 4 and TLR 9, have been demonstrated to play an important role in Leishmaniasis [23] via upregulating the pro-inflammatory responses in Leishmania-infected macrophages. TLR 2 played a protective role in Leishmaniasis, and in addition both TLR 2 and TLR 3 were contributed to phagocytosis of L. donovani promastigotes [24,25]. Hosein et al. demonstrated that Th17 cytokines played an important pathological and protective role in L. infantum experimentally infected dogs [26]. Evasion of the innate signature; TLRs-PAMPS by protozoan parasites, for example, T. gondii enhances the production of IL-10, which exerts its antiinflammatory signal. The expression of amastigote of Leishmania mexicana – specific cysteine peptidase suppresses the production of IL-12 by macrophages. The dendritic cells (DCs) become unresponsive to lipopolysaccharides (LPS), and unable to activate T cells in the presence of malaria hemozoin, which is a TLR9 agonist [17]. The downregulation of TLR signaling pathway by these organisms leads to the long-term persistence in their hosts. Helminths and TLRs: Helminths (worms) include roundworms (nematode) and flatworms (trematode and cestode). The interfering with TLR expression and function may contribute to infection outcome as illustrated in the (Table 3). TLR activation and regulation of by two systemic helminth infections [18].
| Filarial infections | Schistosome infections | |||||||
|---|---|---|---|---|---|---|---|---|
| Murine models | Human infections | Murine models | Human infections | |||||
| in vitro | in vivo | in vitro | ex vivo | in vitro | in vivo | in vitro | ex vivo | |
| Direct Activation of TLR by parasite products | + | + | + | ND | + | + | + | ND |
| Alteration of TLR expression on APCs | + | ND | + | + | ND | ND | ND | ND |
| Inhibition of signaling through TLR | + | + | + | + | + | + | + | + |
| ND=Not Determined | ||||||||
Table 3: In addition to TLRs, the alternative and lectin pathway play an important role. The mediator like C5a of this pathway starts to recruit neutrophils and eosinophils to the site of inciting agents to amplify the inflammatory event. Macrophage has many receptors such as mannose-fucose receptors, complement receptors (CR3 for C3bi), Toll-like receptors, etc. Some parasites as we will mention to start to gain access to the intracellular milieu via these receptors as Leishmania and Trypanosoma cruzi.
Second Wave of the Inflammatory Response
second amplification wave of the inflammatory response comes from the secretion of mediators released by activated macrophages and epithelial cells interleukin (IL)-1, IL-6 and TNF-α. These mediators exacerbate the acute inflammatory responses, which starts locally (by increasing vascular permeability, recruitment of leukocytes, and coagulation) and spreads systemically causing fever and the production of acute phase protein by the liver.
Recently published article by Atmaca et al. detected that high levels of acute phase proteins (APP) in T. gondii infected-mice were closely linked to brain lesions and tissue cysts. Briefly, APP is a potentially good marker for T. gondii infection based on correlation values detected its level in serum, tissue cysts’ number and inflammatory score in the brain. This suggests a strong link may exist between acute phase proteins and T. gondii infection [27].
The Parasite and the Host are Under Stress
The infective agents, which are transmitted through vectors as in the case of Plasmodium, Trypanosoma, Filaria come from different environments (i.e. cold-blooded animal vectors) and try to gain access into other environments (i.e. worm-blooded vertebrates), consequently, both the parasite and the host become stressed. The epithelial cells upregulate the heat-shock proteins (highly conserved). Furthermore, the cytolytic activity of the innate cells and γδ T-cells after engagement the heat-shock protein through their NKG2D receptor is augmented [28]. We hypothetically think they can kill the parasites by the same manner: NK or γδ T cells can be activated by IL-12 and TNF-α from macrophage to release IFN-γ or can cause direct lysis of infected cells after enhancement by IFN-α from activated macrophage [29].
Heat shock protein 90 (Hsp90) has been implicated to play a pivotal role in life cycle and growth of Leishmania, Giardia, Toxoplasma and Plasmodium [30-33]. Hsp90 is critical for growth and survival of E. histolytica and its attendance in regulation of phagocytosis and encystation [34-35]. Hsp90 inhibition has been led to the death of Entamoeba trophozoites [35].
Establishment of a beachhead after infection
Intracellular parasites evolved several strategies to evade the host immune attack in a sylvatic host environment before, during, and after entry into the host cell (i.e. they circumvent to complement attack, oxidative, and non-oxidative killing respectively).
Other evasion strategies of Leishmania spp aganist immune defense are δ-amastins. As a consequence of δ-amastins, Leishmania promastigotes set up a secure niche within mammalian host macrophages [36].
Before entry to the safe paradise
Protozoan parasites evade complement pathways employing different strategies. The major obstacle, which faces the obligate intracellular parasites, is the complement attack by lectin and alternative pathways. For examplas, Plasmodium sp, Giardia intestinalis, T. cruzi and Leishmania sp have shown an efficient activation of a lectin pathway [37-41]. The activation of the complement by the parasite surface (non-self surface) leads to the production of a cascade of mediators such as C4b and C3b that bind to CR1 (CD35) receptors on erythrocytes, neutrophils, monocytes, macrophages, eosinophils, follicular dendritic cells, T-cells and B-cells. Additionally, C3bi is the ligand for CR3 (CD11b/18) and CR4 (CD11c/18) on the surface of monocytes, macrophages, natural killers and some T-cells [42]. These complement mediators bind to different ligands on the parasite surfaces and enhancing their internalization. Internalization can be occurred by plasma fibronectin (FN) which has a receptor on Trypanosoma cruzi [43]. Leishmanolysin (gp63) of Leishmania spp. has fibrinonectin-like properties fascilitating parasite internalization by binding fibrinonectin receptor on macrophage [44].
The parasite can evade the complement-mediated killing pathway by different mechanisms:
Inhibiting complement activation: Trypanosoma brucei rhodesiense can avoid complement attack by their thick outer coat as they prevent C5b-9 MAC complex to be inserted on the trypomastigote surface [43]. The trypomastigotes and tachyzoites of American’s trypanosomes, T. cruzi and T. gondii respectively prevent complement cascade activation. C3 convertase (C3bBb or C4b2b) can be inhibited by gp87-93 DAF-like molecule of Trypanosoma cruzi in a way similar to decay-accelerating factor of mammals [45]. In addition to single variant surface glycoprotein, which is expressed by African trypanosome T. brucei; this provides slacking of the complement system [46,47]. The single variant surface glycoprotein that covers on the parasite surface acts as a physical barrier to the host immune system [47-49].
The tachyzoites (the dividing form) of T. gondii causes C3 conversion to C3bi, therefore, limiting the C3 to attach to the parasite surface [50,51]. It is also worth reporting that T. cruzi-induced microvesicle release early in infection from blood cells, leading to the complement C3 convertase stabilization and inhibition [52]. Gp58/68 is an important glycoprotein for trypomastigotes. It enables the parasite to escape complement lysis by the alternative pathway [53]. L. major promastigotes releases the MAC (C5b-9) and during complement activation, MAC (C5b- 9) is deposited to escape host complement system [54].
T. cruzi membrane contains special structures, mucin-like molecules on its surface declining the host protective immunity. It is attached to the terminal β-galactosyl and sialic acid residues, which are transferred from host glycoconjugates [55-57].
Preventing membrane-attack complex (C5b-9 MAC) lysis: Lipophosphoglycan (LPG), a surface glucoconjugate of Leishmania donovani and Leishmania major promastigote, is very long; therefore, it prevents C5b-9 MAC insertion [58].
Circumventing during entry
Intracellular parasites try to acquire access to the intracellular paradise in a way to avoid the receptors associated with the respiratory burst receptors, thus circumventing the oxidative killing mechanisms of the phagocytes. They sort the receptors to select the best for their survival and ignore the old proverb “All roads lead to Rome.”
Mannose-fucose receptor (MFR) on macrophage binds to LPG, and CR1 binds to C3b, LPG, and gp63. Furthermore, β1 integrins on macrophage bind to Leishmanial ligands such as gp63, fibronectin, and laminin and collagen. Gaining access to the intracellular milieu via the complement receptors as in the case of the Leishmanial promastigote which activates the complement on its surface, but it inhibits the final lytic product to attach to its surface facilitating its entry to the macrophage. Additionally, it lives happily inside the phagolysosome as it degrades the killing cascade of lysosomal enzymes by gp63. It was also reported that Trypansoma cruzi gp83 (trans-sialidase-like molecule) could bind to laminin γ-1 receptor on mammalian cells [59]. Apicomplexans such as Toxoplasma, Cryptosporidium, Eimeria, Isospora, Sarcocystis, Babesia, Theileria, Cyclospora and Plasmodium have a crescent-shaped body and exhibit a peculiar type of locomotion to invade the cells actively by gliding motility. The myosin motor complex is attached to the inner membrane complex. The inner membrane complex is in close association with the microtubules. The adhesin molecules secreted by micronemes bind to the Aldolases that in turn link to the actin cytoskeleton. Following actin filament polymerization, it starts to translocate the myosin motor complex posteriorly propelling the parasite forward [60,61]. The membranebound vacuole (parasitophorous vacuole) which wraps the tachyzoites of T. gondii devoids of plasma membrane receptors, therefore both vacuolar acidification and a fusigenic signal (i.e. to fuse with the lysosome) don’t occur [50].
Proline racemase (PRAC) enzymes, potent host B-cell mitogens, released by T. cruzi lead to the generation of a non-specific B-cell proliferation, which results in producing non-specific antibodies; therefore, having an influence on the parasite persistence in the host cells [62-65].
T. cruzi inhibits production of IL-12 owing to the impairment of host dendritic cell functions [66]. T. cruzi mucin-like molecules are important driving factors of the immune system impairment and the invasion of the host. Moreover, infective trypomastigote is protected from host attack mediated by complement factor B and anti-galactosyl antibodies by sialylated mucins, which form a surface coat [67-69].
Recrudescence and intracellular survival of intracellular parasites after the entry of the host cell
Leishmania major Gp63, a surface protease, evades the host cell after entry by inhibiting oxidative respiratory burst. In addition to that, it prevents the chemotatctic events of monocytes and neutrophils [70]. Lysosome fusion is inhibited by LPG, and lysosomal enzymes are hindered by gp63 [71-73].
In the literature, most Leishmania species activate the complement pathway. For examples, L. Enrietti, L. Braziliensis, and L.major enhance the alternative complement pathway; however, L. donovani appears to stimulate the classical pathway [74-76]. Nevertheless, L. Braziliensis, L. Mexicana, L. Major and their promastigotes activate the lectin pathway by binding to mannan-binding lectin [77-80].
A glycosylphosphatidylinositol (GPI) anchor of Leishmanial promastigote contains lipophosphoglycan (LPG) and gp63, which inhibit the action of a non-oxidative killing pathway of the macrophage [50]. The active form, trypomastigote of T. cruzi, invades the host cell within a membrane-bound vacuole, and destroys the vacuole by its membrane-forming protein to escape into the cytoplasm avoiding the non-oxidative killing by hydrolysases [45]. In a similar context, T. cruzi -infected macrophage results in the anti-inflammatory cytokine secretion, for example IL-10 and TGF-ß which favors parasite dissemination. Additionally, T. cruzi interferes with dendritic cell antigen presenting function and suppresses CD4+ T cell responses via host sialic acid-binding Ig-like lectin receptors [81].
Parasitophorous vacuole: Parasitophorous vacuoles (PV), which formed within the host cells are the residence and development niche of apicomplexan parasites. They inhibit the fusion of acidic organelles from the different exo- and endocytic pathways [82-84]. After their active entry into the host cells, T. gondii and N. caninum tachyzoites, Encephalitozoon cuniculi spores, T. cruzi and L. amazonensis amastigotes hide inside the PV and escape killing by humoral immune response [82,85,86].
Wilson et al. showed that LYST/Beige plays an important role at the innate level. They also illustrated that increased expression of LYST/ Beige functions to protect the host cells via restricting Leishmania amastigote growth because of struggling PV expansion [87].
Macrophage (Double-Edged Sword)
Macrophage is a double-edged tool as it is considered one of the safe milieus to many intracellular organisms, such as Leishmania, Trypanosoma cruzi and T. gondii. The best solution for the parasites is to live within macrophages at peace with the host perhaps by being cryptic. However, once “woken,” the macrophage has a number of oxidative and non-oxidative killing mechanisms. Oxidative stress (OS) occurs when there is an imbalance between pro-oxidant-antioxidant levels and is induced by reactive nitrogen species (RNS) and highly reactive oxygen species (ROS) [88,89]. T. gondii expresses antioxidant enzymes, such as catalases and peroxidases to protect itself against ROS activity [90,91]. Increased OS and OS-related neuropathology in T.gondii-infected mice was determinated by Dincel and Atmaca (2015) [92]. In general, therefore, it seems that oxidative killing mechanisms against T. gondii were activated by microglia/macrophages, other glial cells and neurons. Microglia/macrophages might be responsible for both recurrent and systemic T. gondii infections. Tachyzoite- and bradyzoite- hidden mechanisms in the cells act as a “Trojan horse” [93-95]. Therefore, macrophages provide a framework for both in the occurrence and prevention of disease.
Macrophages and neutrophils kill the nematode, Strongyloides stercoralis larvae owing to the activation of IL-4Rα [96]. Overall, intestinal helminth and its larva macrophage killing mechanisms were controlled by type 2 immune cell responses [97-99].
Apoptosis
Programmed cell death also called apoptosis involved in immune response to infections by modulating T. gondii proliferation [100,101]. Dincel and Atmaca (2015) demonstrated that T. gondii and other soluble factors trigger apoptosis employing both instrinsic and extrinsic pathways. T. gondii-mediated apoptosis might associate with the pathogenesis of neurodegeneration and neuropathology [102]. Furthermore, the binding of Plasmodium sporozoites also induced Kupffer cells apoptosis [103].
Non-Specific and Specific Ways that Lead to Macrophage Activation
Parasite products and cytokine-mediators from different cell types such as γδ T cells after parasite insulting can secrete IFN-γ to activate resting macrophages. Moreover, IFN-γ is produced from NK cells after parasite activation or after induction by IL-12 and TNF-α that are secreted from other activated macrophages. On the other side, CD4+ T-cells and CD8+ T-cells produce IFN-γ after antigen processing, presenting and naïve T-cells activation by professional antigen presenting cells [104].
Nitric Oxide Synthase (NOS) Toxic Pathway and Parasitic Evasion: Non-Oxidative Killing Mechanism
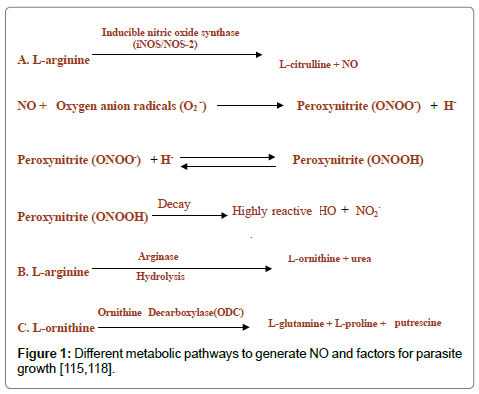
Activated macrophage synthesizes nitric oxide (NO) from L-arginine under the influence of inducible nitric oxide synthase (iNOS/ NOS-2). Nitric oxide and L-ornithine can be produced from L-arginine through hydroxylation and oxidation or hydrolysis in corresponding order. L-ornithine is the precursor for the parasite growth factors such as L-glutamine, L-proline, and putrescine (Figure 1). The nitric oxide toxicity is associated with the production of the reactive hydroxyl group.
NO triggers the development of tissue cysts in T. gondii and Neospora caninum by inhibiting parasite replication. A decrease in NO production might cause tissue cyst reactivation [105-110]. NO dependent mechanisms are responsible for the killing of N. caninum tachyzoites inside macrophages [111]. eNOS, iNOS and nNOS derived NO, produced by microglia/ macrophages, astrocytes and neurons contributes to the development of tissue cysts, and protective immunity [110].
NO derived from nNOS is effective in the elimination of Giardia lamblia infections in mice [112]. NO inhibition causes excystation and encystation of G. lamblia cysts, thereby establishing and continuing the infection process in the small intestine [113]. Giardia has evolved strategies to escape NO-mediated host defenses. For example, epithelial NO production was depleted because of consuming arginine by Giardia [113, 114].
IFN-gamma- and TNF-alpha-mediated activation of NO, which is potent microbicidal agent, importantly derived from inducible nitric oxide synthase in murine macrophages shows a major leishmanicidal effect [115, 116].
T. gondii survives in the activated macrophages, and avoids nitric oxide-killing mechanism. A possible explanation for this might be that a T. gondii patatin-like protein (TgPL1), protects from T. gondii nitric oxide degradation in the activated macrophages [82,117].
Parasites evade the ready-made armory of immune system by avoiding NO harmful effect by depletion of L-arginine through activation of Arginase.
It was reported that NO synthesis (parasite death)/Arginase activation (parasite survival) influenced by the cytokines secreted from the T-helper type 1 (Th1)/T-helper type 2 (Th2) respectively [118]. Cruzipain (major cysteine proteinase) of Trypanosoma cruzi, an intracellular parasitic protozoon, favors Th2 arm of immunity; therefore, evade NOS pathway. Furthermore, cruzipain regulates parasite survival and differentiation through arginase activation [119]. Arginase induction during Leishmania major infection leads to promastigote–amastigote transformation and amastigote replication within the phagolysosome of macrophage [120].
Having considered what is meant by the inducible nitric oxide synthase (NOS2) and the macrophage-secreted NO, we will now move on to briefly shed the light on IFN-γ-inducible IRG proteins, which destroy T. gondii tachyzoites by rupturing of the parasitophorous vacuole (PV) [21,121,122]. Pseudokinase (ROP5) and kinase (ROP18) are secreted by certain T. gondii strains resulting in the formation of ROP5/ROP18 kinase complex, which phosphorylates and inactivates IRG proteins. However, some strains of mice are ROP5/ROP18 kinase complex-resistant [21,123].
Oxidants and Anti-Oxidants
The host innate immune cells such as macrophages, eosinophils, neutrophils, and platelets release reactive oxygen species (ROS) that can kill the adult schistosome. Schistosome, other helminths and protozoa developed countermeasures to avoid oygen-mediated killing. They produce antioxidant enzymes, e.g. superoxide dismutase (SOD) which is responsible for the dismutation of the superoxide anion to hydrogen peroxide (H2O2), Catalase and Glutathione-peroxidase (GPX) which detoxify the hydrogen peroxide, and glutathione-S-transferase (GST) and peroxidoxin which eliminate the hydrogen peroxide. The antioxidants avert the toxic action of the secreted ROS [124,125]. They infectivity differences between non-zoonotic (Trypanosoma brucei brucei) and zoonotic ones (Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense) are due to the absence/presence of serum resistance associated gene (SRA) respectively. Serum lysis, highdensity lipoprotein lytic factor called “TLF1,” can prevent Trypanosoma brucei brucei to infect the human host. TLF1, which uptakes via its ligand {haptoprotein (HPr)} on the parasite surface leads to phagosome formation. Following phagolysosome formation, the acidity enhances the peroxidase activity and eventually cell lysis [126]. We hypothesize that there is a virulence factor which may be the product of RSA gene. It may decrease the acidification of the phagolysosomal vesicle, and deactivate the oxidative killing of the human infective subspecies such asTrypanosoma brucei rhodesiense and Trypanosoma brucei gambiense.
Helminths Deal With the Complement Attack as Well
Schistosomes live within a harsh environment within a blood circulation. They are exposed to immunological falls of killing effectors such as the complement cascade, which can be activated by three pathways (classical “adaptive,” alternative and lectin pathways) [127]. To circumvent the complement attack, they possess a plethora of parasite regulatory proteins as illustrated in the (Table 4).
| Protein | Type of Protein | Affected Pathway | Regulatory Function |
|---|---|---|---|
| Parasite C3 surface receptor | Membrane bound | All pathways | Binds and inactivate C3 |
| DAF-like molecule | Membrane bound | All pathways | Accelerates the C3 convertase (C3bBb and C4b2a) dissociation |
| Parasite C2 receptor | Membrane bound | Lectin and Classical pathway | Interferes with C3 convertase |
| C1q-binding protein | Membrane bound | Classical pathways | Prevents complement activation through binding to C1q |
| Surface antibody bound through Fc | Membrane bound | Classical pathways | Docking site becomes unavailable to bind to C1q |
| C8-C9-binding protein | Membrane bound | All pathways | Acts like CD59 |
Table 4: Schistosomal components that regulate complement system [42,127].
The production of IL-10, IL-6, and TNF-α from monocyte is enhanced by glycolipids of female schistosome egg [128]. The oncosphere larva of Echinococcus granulosus (hydatid disease) penetrates the intestinal wall, and migrates to liver and lung where transformation occurs to hydatid cyst. The latter consists of outer layer and inner laminated layer lined with germinal epithelium. The germinal layers give rise to the daughter cyst “brood capsules,” which contains protoscolices “hydatid sands.” The hydatid cyst wall acts as a barrier against the immunologically hostile milieu. The acquisition of host myo-Inositol hexakisphosphate (IP6) at the surface of the hydatid cyst wall inhibits C3 convertase factor I, which cleaves C3ba and C4b [129]. The hydatid disease is divided into two stages:
Precystic stage (establishment phase): This can occur after egg infection, and oncoshpere migration in the intermediate host (i.e. primary infection) or protoscolex migration after cyst infection in the definitive host (i.e. secondary infection). In these two cases oncospheres and protoscoleces undergo transformation into the cyst stage. Protoscoleces (PSCs) can trigger alternative complement pathway and lead to inflammatory responses.
Cystic stage (inert phase): the laminated layer of the hydatid cyst is acellular, mechanically resistant, permeable for a macromolecule, and carbohydrate rich (galactose and N-acetyl galactosamine). It is lined with parasite living syncytial germinal layer. Although the hydatid cyst wall (HCW) is rich in carbohydrate, the lectin pathway can’t be activated. The reason could be low affinity of binding between the mannose binding lectin and hydatid cyst wall sugar or poor recruitment of inflammatory cells. HCW can capture the host H factor that binds to C3b leading to blocking C3 convertase (C3bBb). The same mechanism has been reported for Taenia taeniformis (Rat tape worm), where the membrane associated inhibitor molecules can block the final lytic pathway of the complement [130].
Parasitic helminths “Masters of the immunoregulation”
Helminthic parasites have evolved to be protected from host immune systems. Parasitic helminths have an inherent ability to elicit Th2-dominated immune response characterized by Th2 cytokines, robust immunoglobulin E (IgE), eosinophil, and mast cell responses [131]. Many species of parasitic helminths and helminth-derived molecules are instrumental in the shaping of the immune response of the host by creating regulatory environment [132]. Th2 cytokines and chemokines are most important and increased after T. spiralis infection in mesenteric lymph node [133]. Moreover, TLR4 and TLR9 are overexpressed during T. spiralis-infected mice in small intestine and muscle tissue [134]. Elevated levels of regulatory T cells (Tregs), regulatory B cells (Bregs), alternatively activated macrophages (AAMs), IL-10 and transforming growth factor (TGF) -ß have been observed after infection with parasitic helminths [135-138]. In a similar context, the adoptive transfer of intestinal DCs from Heligmosomoides polygyrus bakeri-infected mouse to naïve one provides protection from colitis [139]. Further, Fasciola hepatica total extract (TE) endorses CpG-ODN (CpG)-activated DCs to diminish exacerbated immune response in collagen-induced arthritis (CIA) [140], and human DCs primed with soluble products (SPs) of Trichuris suis induce a T helper 2 (Th2) response and suppress TLR-induced Th1 and Th17 responses [141]. Furthermore, it has been demonstrated that dinitrobenzene sulfonic acid (DNBS)-induced colitis was reduced in mice by using in vitro IL-4 -derived alternatively activated macrophages (AAMs) from Hymenolepis diminuta-infected mice [142]. A significant analysis and discussion on the rule of helminths and their products that could dampen the inflammatory and autoimmune disorders were presented by [139,143-149].
Trichinella spiralis paramyosin (Ts-Pmy) is exhibited on the outer membrane of both larvae and adults [150]. Ts-Pmy is linked to complement components C8 and C9, which have a considerable impact on of the membrane attack complex (MAC) and the complement activation cascade. Ts-Pmy inhibits the formation of MAC via interfering with the complement component C9, thus evading the attack of the host complement system [151].
Further humoral molecules of innate immune system
Lectins are carbohydrate binding proteins; host galectins and C-type lectins are associated with a variety of immune processes, such as cell adhesion and T cell polarisation [152]. Collectins are C-type lectins (C-TLs) or Ca2+ dependent lectins include mannanbinding lectin (MBL), surfactant protein A and D (SP-A and SP-D). They consist of an N-terminal collagenous domain and C-terminal carbohydrate-recognition domain (CRD). They differ from Ficolins (L-ficolin, M-ficolin, and H-ficolin), which contain a fibrinogen-like domain instead of CRD. Collectins are found in mucosal surfaces and on the blood. MBL and Ficolin are PRRs and recognize PAMPs. They can launch the lectin pathway. SP-A and SP-D can opsonize, agglutinate, and neutralize the bacteria and viruses [153]. The majority of collectins and ficolins are homomultimeric to increase the avidity of binding with their ligands. Other examples of C-type lectins (C-TLs) include:
Mucosal addressin cell adhesion molecule (MAdCAM) is a ligand for L-selectin.
Macrophage mannose receptor, DEC-205 and galactose-GalNAc receptor bind to glycan ligand and facilitate endocytosis.
NK cell receptors are integeral membrane proteins that include Ly-49, NKRP-1, and CD94. They prevent lysis of the target after engagement of MHC class I molecule.
CD23 receptor that engages IgE on haematopoeitc cells.
T. cruzi is compelled evade the cytolytic effects of the complement system. At this point, L-Ficolin binding to Trypanosoma cruzi calreticulin (TcCRT) undertakes the influential task. TcCRT can bind C1 and mannan-binding lectin/ficolins inhibiting the prominrnt lectin complement and classical pathway activation. This clearly shows that the role of TcCRT/ T. cruzi evasion strategy to inhibit an important innate immune response pathway [154,155].
Parasites evade the CTLs
Helminths also secrete C-type lectins as a way to evade the host C-type lectins. CTLs of nematodes compete for L-selectin ligand (MAdCAM), which is upregulated during the inflammatory response inhibiting the extravasation of the leukocytes. Schistomal CTLs play a crucial role in molecular mimicry; the parasite covers its surface by C-type lectins [152].
Eosinophil-mediated parasite killing
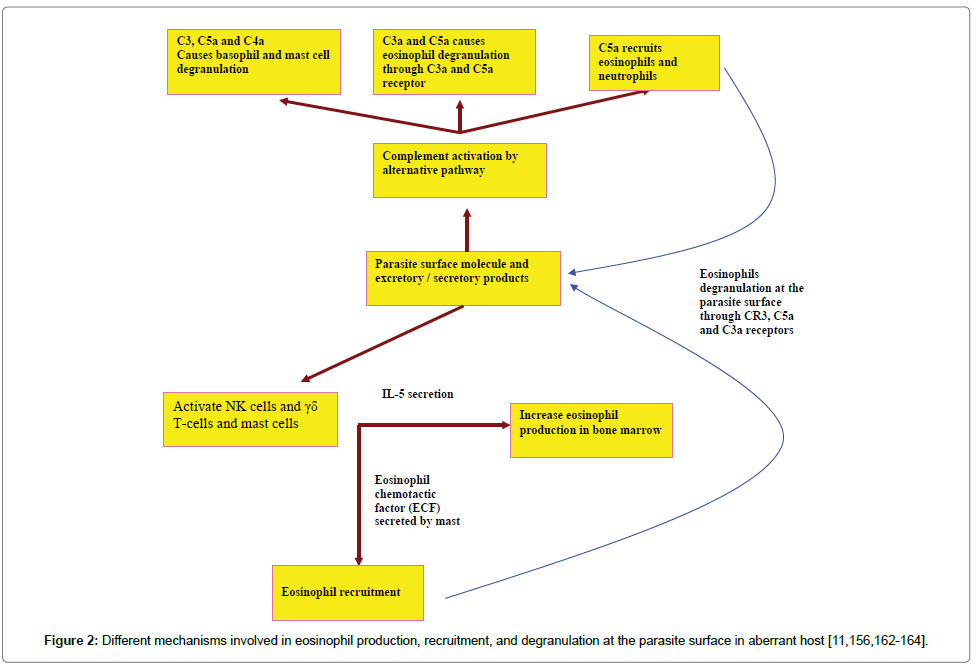
Eosinophils are non-specific killer polymorphic granulocytes. It was reported that the innate immune response was efficient to eliminate the parasite [156]. Eosinophil-mediated parasite killing can result in parasite clearance. Eosinophil production, recruitment, and activation can occur by different ways in incompatible hosts as can be seen from (Figure 2).
IL-5, constitutive production by eosinophils, plays an important role in the immune system, such as eosinophil differentiation from the bone marrow and critical for healing and recruiting of the eosinophils following nematode infection [157]. Increased expression of IL-5 leads to the reduction of N. brasiliensis worm recovery from the lungs and strongly advocates that eosinophils play a key role in the augmentation of immunity against N. brasiliensis infection and protection against its migrating larvae [158].
Eosinophils are important immune cells for T. spiralis larvae survival. IL-10-secreting DCs, CD4+ T cells and IL-10 control the activation of macrophages and neutrophils in the context of T. spiralis larvae tissue invasion [159-161].
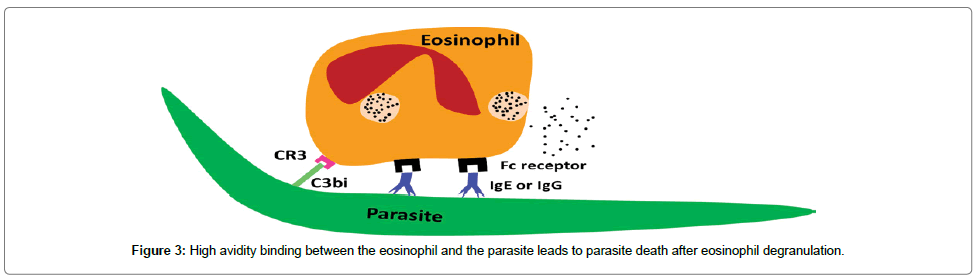
Owing to host measure and the parasite counter-measure to reach a state of equilibrium (adaptation/long persistence/chronicity), the innate immune response is inefficient to get rid of infected parasite larvae in susceptible hosts. Specific molecules are required to increase the avidity (i.e. the overall binding strength) of binding between the eosinophils and the parasite surface either directly (eosinophil Fc-specific ab-Ag complexes) or indirectly (classical pathway complement activation). Augmentation of the eosinophil’s production can occur by specific IL-5 (i.e. CD4+ Th2) [162]. All of host mediators can act synergistically to clear the parasite larvae before their ability to evade the immune responses [156]. Eosinophils bind to either IgE or IgG through Fc receptor and to the epitope on the parasite surface via Fab. CR3 receptor binds to C3bi, which coats the parasite surface. This high avidity of binding leads to eosinophil degranulation on the parasite surface (Figure 3).
Linking innate and adaptive immune response
Apart from antigen processing and presentation by antigen presenting cells such as macrophages, dentritic cells and B cells, mediators are released from mast cells either as a response to anaphylatoxins (C3a, C4a and C5a) or through IgE-dependent degranulation [163,164]. C5a, eosinophil chemotact factor (released from mast), and IL-5 enhance eosinophil production and recruitment to the parasite surface. IgE, IgG, and complement can mediate eosinophil killing by releasing toxic granules. IgE can lead to functional changes in macrophage and mediate macrophage-killing mechanism. Moreover, neutrophils can mediate killing through respiratory burst or Ab (IgG) mediated degranulation and liberation of toxic granules [165].
Neutrophil extracellular traps (NETs) and their microbicidal molecules are strategies exploited by neutrophils to kill T. gondii tachyzoites and L. donovani and Leishmania amazonensis promastigotes [166-168].
Myeloperoxidase is a heme protein synthesized during myeloid differentiation and most abundantly expressed in neutrophils and to a lesser extent activated macrophages during the primary immune response. It has a considerable impact on larval killing, such as S. stercoralis. Neutrophils, macrophages and complement also killed the adult worms [96,169-172]. Importantly, NETs as well play a role in the control of the extracellular parasite, for example, S. stercoralis [172].
Cercarial dermatitis (swimmer’s itch/hunter’s itch) as an example of linking innate and acquired immunity
Three genera of non-human schistosomes (cattle, rodent and avian schistosomes) can cause cercaial dermatitis namely, Ornithobilharzia, Gigatobilherzia and Trichobilharzi [173]. Trichobilharzia can be categorized into visceral and nasal species according to the migratory route [174]. Trichobilharzia regenti is a neurotropic metazoan parasite (nasal species) that infects the avian host. Following skin penetration, the cercariae undergo transformation to schistosomula. The secretion of cathepsin B-like cysteine peptidases by schistosomula results in myelin basic protein degradation. The immature stage migrates through the nervous tissue of the brain. Adult stage resides in the nasal tissue of the duck in- and out-sides of the blood vessels. Cercarial dermatitis represents an allergic reaction (type I hypersensitivity reaction/ an immediate hypersensitivity) during cercarial penetration in the primed or unprimed incompatible animal host. Moreover, the late phase inflammatory reaction is due to the persistence of dead cercariae in the skin. In immunologically naïve animal, histamine is released from mast cells in IgE independent manner, and cause vascular permeability, and oedema after cercarial penetration. However, in immunologically primed animal, histamine and IL-4 secretions are elevated in IgE-dependent way from mast cell (i.e. through tertiary event). Additionally, the late phase response is due to leukocytes and CD4+ T cell recruitment to the site of penetration. Dendritic cells and monocytes have H2 receptor (H2R) for histamine binding leading to IL-12 release inhibition and IL-10 enhancement. This can be bias the immune response toward Th2, IgG1 and IgE class switching [175].
Concluding remarks and perspectives
This review provides an overview of the host-oriented inherent measures and eukaryotic parasite countermeasures, and the foundation of the molecular pathways of these interactions. A reasonable approach to exploit this information could be to discover new therapeutic and prophylactic intervention points for broad-spectrum host –oriented measures and parasite counter- measures, and to determine the infection outcome.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was supported by the National Key Basic Research Program (973) of China (2015CB150300), the Special Fund for Agro-scientific Research in the Public Interest (201303042), and the Open Project of the State Key Laboratory of Veterinary Etiological Biology (SKLVEB2011KFKT001).
References
- Mohan K1, Moulin P, Stevenson MM (1997) Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol 159: 4990-4998.
- Roland J, Soulard V, Sellier C, Drapier AM, Di Santo JP, et al. (2006) NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J Immunol 177: 1229-1239.
- Stevenson MM, Riley EM (2004) Innate immunity to malaria. Nat Rev Immunol 4: 169-180.
- Filtjens J, Foquet L, Taveirne S, Van Ammel E, Vanhees M, et al. (2014) Contribution of the Ly49E natural killer receptor in the immune response to Plasmodium berghei infection and control of hepatic parasite development. PLoS One 9: 87463.
- McKerrow JH1, Caffrey C, Kelly B, Loke P, Sajid M (2006) Proteases in parasitic diseases. Annu Rev Pathol 1: 497-536.
- Kappe SH1, Buscaglia CA, Nussenzweig V (2004) Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev Biol 20: 29-59.
- Sidjanski SP, Vanderberg JP, Sinnis P (1997) Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol 90: 33-41.
- Elphick DA1, Mahida YR (2005) Paneth cells: their role in innate immunity and inflammatory disease. Gut 54: 1802-1809.
- Roxström-Lindquist K1, Palm D, Reiner D, Ringqvist E, Svärd SG (2006) Giardia immunity--an update. Trends Parasitol 22: 26-31.
- Akira S1, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4: 499-511.
- Beutler B1 (2004) Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430: 257-263.
- Adachi K, Tsutsui H, Kashiwamura S, Seki E, Nakano H, et al. (2001) Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J Immunol 167: 5928-5934.
- Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, et al. (2002) Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 168: 5997-6001.
- Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, et al. (2005) Immunomodulation via novel use of TLR4 by the filarial nematode phosphoryl-choline-containing secreted product, ES-62. J Immunol 174: 284-293.
- Aranzamendi C, Fransen F, Langelaar M, Franssen F, Ley PVD, et al. (2012) Trichinella spiralis-secreted products modulate DC functionality and expand regulatory T cells in vitro. Parasite Immunol 34: 210-223.
- Cho MK1, Park MK, Kang SA, Park SK, Lyu JH, et al. (2015) TLR2-dependent amelioration of allergic airway inflammation by parasitic nematode type II MIF in mice. Parasite Immunol 37: 180-191.
- Venugopal PG1, Nutman TB, Semnani RT (2009) Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol Res 43: 252-263.
- Gazzinelli RT1, Denkers EY (2006) Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. See comment in PubMed Commons below Nat Rev Immunol 6: 895-906.
- Andrade WA, Souza Mdo C, Ramos-Martinez E, Nagpal K, Dutra MS, et al. (2013) Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13: 42-53.
- Koblansky AA1, Jankovic D, Oh H, Hieny S, Sungnak W, et al. (2013) Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38: 119-130.
- Gazzinelli RT1, Mendonça-Neto R2, Lilue J3, Howard J4, Sher A5 (2014) Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe 15: 132-138.
- Atmaca HT, Kul O, Karakus E, Terzi OS, Canpolat S, et al. (2014) Astrocytes, microglia/macrophages, and neurons expressing Toll-like receptor 11 contribute to innate immunity against encephalitic Toxoplasma gondii infection. Neuroscience 269: 184-91.
- Tuon FF1, Amato VS, Bacha HA, Almusawi T, Duarte MI, et al. (2008) Toll-like receptors and leishmaniasis. Infect Immun 76: 866-872.
- de Veer MJ1, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, et al. (2003) MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol 33: 2822-2831.
- Flandin JF, Chano F, Descoteaux A (2006) RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol 36: 411-420.
- Hosein S, Rodríguez-Cortés A, Blake DP, Allenspach K, Alberola J, et al. (2015) Transcription of Toll-Like Receptors 2, 3, 4 and 9, FoxP3 and Th17 Cytokines in a Susceptible Experimental Model of Canine Leishmania infantum Infection. PLoS One 10: e0140325.
- Atmaca HT, Gazyagci AN, Dincel GC, Canpolat S, Terzi OS, et al. (2015) Relation of tissue cyst number, histopathology score and systemic acute phase proteins in mice infected experimentally with Toxoplasma gondii (ME49 Strain). J Comp Pathol 152: 82.
- Pardoll DM1 (2001) Immunology. Stress, NK receptors, and immune surveillance. Science 294: 534-536.
- Scharton-Kersten TM1, Sher A (1997) Role of natural killer cells in innate resistance to protozoan infections. Curr Opin Immunol 9: 44-51.
- Wiesgigl M1, Clos J (2001) Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani. Mol Biol Cell 12: 3307-3316.
- Banumathy G1, Singh V, Pavithra SR, Tatu U (2003) Heat shock protein 90 function is essential for Plasmodium falciparum growth in human erythrocytes. J Biol Chem 278: 18336-18345.
- Echeverria PC, Matrajt M, Harb OS, Zappia MP, Costas MA, et al. (2005) Toxoplasma gondii Hsp90 is a potential drug target whose expression and subcellular localization are developmentally regulated. J Mol Biol 350: 723-734.
- Nageshan RK, Roy N, Ranade S, Tatu U (2014) Trans-spliced heat shock protein 90 modulates encystation in Giardia lamblia. PLoS Negl Trop Dis 8: e2829.
- Debnath A, Shahinas D, Bryant C, Hirata K, Miyamoto Y, et al. (2014) Hsp90 inhibitors as new leads to target parasitic diarrhea diseases. Antimicrob Agents Chemother 58: 4138-4144.
- Singh M, Shah V, Tatu U (2014) A novel C-terminal homologue of Aha co-chaperone binds to heat shock protein 90 and stimulates its ATPase activity in Entamoeba histolytica. J Mol Biol 426: 1786-1798.
- Jackson AP (2010) The evolution of amastin surface glycoproteins in trypanosomatid parasites. Mol Biol Evol 27: 33-45.
- Ambrosio AR, De Messias-Reason IJ (2005) Leishmania (Viannia) braziliensis: interaction of mannose-binding lectin with surface glycoconjugates and complement activation. An antibody-independent defence mechanism. Parasit Immunol 27: 333-340.
- Holmberg V, Schuster F, Dietz E, Visconti JCS, Anemana SD, et al. (2008) Mannose-binding lectin variant associated with severe malaria in young African children. Microbes Infect 10: 342-348.
- Lambris JD, Ricklin D, Geisbrecht BV (2008) Complement evasion by human pathogens. Nat Rev Microbiol 6: 132-142.
- Cestari Idos S1, Krarup A, Sim RB, Inal JM, Ramirez MI (2009) Role of early lectin pathway activation in the complement-mediated killing of Trypanosoma cruzi. Mol Immunol 47: 426-437.
- Evans-Osses I, Ansa-Addo EA, Inal JM, Ramirez MI (2010) Involvement of lectin pathway activation in the complement killing of Giardia intestinalis. Biochem Biophys Res Commun 395: 382-386.
- Kindt, TJ, Goldsby RA, Osborne BA, Kuby J (2007) Kuby Immunology. New York: W.H. Freeman; 6: p574.
- Wirth JJ, Kierszenbaum F (1984) Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol 133: 460-464.
- Brittingham A, Chen G, McGwire BS, Chang KP, Mosser DM (1999) Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect Immun 67: 4477-4484.
- Hall BF, Joiner KA (1993) Developmentally-regulated virulence factors of Trypanosoma cruzi and their relationship to evasion of host defenses. J Eukaryot Microbiol 40: 207-213.
- Russo DCW, Williams DJL, Grab DJ (1994) Mechanisms for the elimination of potentially lytic complement-fixing variable surface glycoprotein antibody-complexes in Trypanosoma brucei. Parasitol Res 80: 487-492.
- Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, et al. (2007) Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell 131: 505-515.
- Pays E, Vanhollebeke B (2009) Human innate immunity against African trypanosomes. Curr Opin Immunol 21: 493-498.
- Schwede A, Carrington M (2010) Bloodstream form trypanosome plasma membrane proteins: antigenic variation and invariant antigens. Parasitology 137: 2029-2039.
- Hall BF, Joiner KA (1991) Strategies of obligate intracellular parasites for evading host defences. Immunol Today 12: A22-27.
- Fuhrman SA, Joiner KA (1989) Toxoplasma gondii: mechanism of resistance to complement-mediated killing. J Immunol 142: 940-947.
- Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI (2012) Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol 188: 1942-1952.
- Fischer E, Ouaissi MA, Velge P, Cornette J, Kazatchkine MD (1988) Gp 58/68, a parasite component that contributes to the escape of the trypomastigote form of T. cruzi from damage by the human alternative complement pathway. Immunology 65: 299-303.
- Puentes SM, Da Silva RP, Sacks DL, Hammer CH, Joiner KA (1990) Serum resistance of metacyclic stage Leishmania major promastigotes is due to release of C5b-9. J Immunol 145: 4311-4316.
- Schenkman S, Jiang MS, Hart GW, Nussenzweig V (1991) A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell 65: 1117-1125.
- Schenkman S, Eichinger D, Pereira ME, Nussenzweig V (1994) Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol 48: 499-523.
- Previato JO, Jones C, Xavier MT, Wait R, Travassos LR, et al (1995) Structural characterization of the major glycosylphosphatidylinositol membrane-anchored glycoprotein from epimastigote forms of Trypanosoma cruzi Y-strain. J Biol Chem 270: 7241-7250.
- Sacks DL, Pimenta PF, McConville MJ, Schneider P, Turco SJ (1995) Stage-specific binding of Leishmania donovani to the sand fly vector midgut is regulated by conformational changes in the abundant surface lipophosphoglycan. J Exp Med 181: 685-697.
- Nde PN, Simmons KJ, Kleshchenko YY, Pratap S, Lima MF, et al. (2006) Silencing of the laminin gamma-1 gene blocks Trypanosoma cruzi infection. Infect Immun 74: 1643-1648.
- Dobrowolski JM, Sibley LD (1996) Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84: 933-939.
- Sibley LD (2004) Intracellular parasite invasion strategies. Science 304: 248-253.
- Reina-San-Martín B, Degrave W, Rougeot C, Cosson A, Chamond N, et al. (2000) A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat Med 6: 890-897.
- Buschiazzo A, Goytia M, Schaeffer F, Degrave W, Shepard W, et al. (2006) Crystal structure, catalytic mechanism, and mitogenic properties of Trypanosoma cruzi proline racemase. Proc Natl Acad Sci USA 103: 1705-1110.
- Chamond N, Cosson A, Coatnoan N, Minoprio P (2009) Proline racemases are conserved mitogens: characterization of a Trypanosoma vivax proline racemase. Mol Biochem Parasitol 165: 170-179.
- Caballero ZC, Costa-Martins AG, Ferreira RC, Alves JMP, Serrano MG, et al. (2009) Phylogenetic and syntenic data support a single horizontal transference to a Trypanosoma ancestor of a prokaryotic proline racemase implicated in parasite evasion from host defences. Parasit Vectors 8: 222.
- Erdmann CH, Steeg F, Koch-Nolte, Fleischer B, Jacobs T (2009) Sialylated ligands on pathogenic Trypanosoma cruzi interact with Siglec-E (sialic acid-binding Ig-like lectin-E). Cell Microbiol 11: 1600-1611.
- Joiner K, Sher A, Gaither T, Hammer C (1986) Evasion of alternative complement pathway by Trypanosoma cruzi results from inefficient binding of factor B. Proc Natl Acad Sci USA 83: 6593-6597.
- Gazzinelli RT, Pereira MES, Romanha A, Gazzinelli G, Brener Z (1991) Direct lysis of Trypanosoma cruzi: a novel effector mechanism of protection mediated by human anti-gal antibodies. Parasite Immunol 13: 345-356.
- Pereira-Chioccola VL, Acosta-Serrano A, Correia de Almeida I, Ferguson MA, Souto-Padron T, et al. (2000) Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-???-galactosyl antibodies. J Cell Sci 113: 1299-1307.
- Sørensen AL, Hey AS, Kharazmi A (1994) Leishmania major surface protease Gp63 interferes with the function of human monocytes and neutrophils in vitro. APMIS 102: 265-271.
- Chaudhuri G, Chaudhuri M, Pan A, Chang KP (1989) Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J Biol Chem 264: 7483-7489.
- Seay MB, Heard PL, Chaudhuri G (1996) Surface Zn-proteinase as a molecule for defense of Leishmania mexicana amazonensis promastigotes against cytolysis inside macrophage phagolysosomes. Infect Immun 64: 5129-5137.
- Desjardins M, Descoteaux A (1997) Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J Exp Med 185: 2061-2068.
- Pearson RD, Steigbigel RT (1980) Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol 125: 2195-2201.
- Mosser DM, Edelson PJ (1984) Activation of the alternative complement pathway by Leishmania promastigotes: parasite lysis and attachment to macrophages. J. Immunol 132: 1501-1505.
- Mosser DM, Burke SK, Coutavas EE, Wedgwood JF, Edelson PJ (1986) Leishmania species: mechanisms of complement activation by five strains of promastigotes. Exp Parasitol 62: 394-404.
- Claus DR, Siegel J, Petras K, Osmand AP, Gewurz H (1977) Interactions of C-reactive protein with the first component of human complement. J Immunol 119: 187-192.
- Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I (1987) Serum lectin with known structure activates complement through the classical pathway. J Biol Chem 262: 7451-7454.
- Green PJ, Feizi T, Stoll MS, Thiel S, Prescott A, et al. (1994) Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Mol Biochem Parasitol 66: 319-328.
- Ambrosio AR, De Messias-Reason IJ (2005) Leishmania (Viannia) braziliensis: interaction of mannose-binding lectin with surface glycoconjugates and complement activation. An antibody-independent defence mechanism. Parasite Immunol 27: 333-340.
- Flávia Nardy A, Freire-de-Lima CG, Morrot A (2015) Immune Evasion Strategies of Trypanosoma cruzi. J Immunol Res 2015: 178947.
- Mordue DG, Sibley LD (1997) Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol 159: 4452-4459.
- Akerman SE, Müller S (2005) Peroxiredoxin-linked detoxification of hydroperoxides in Toxoplasma gondii. J Biol Chem 280: 564-570.
- Carruthers V, Boothroyd JC (2007) Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol 10: 83-89.
- Sacks D1, Sher A (2002) Evasion of innate immunity by parasitic protozoa. Nat Immunol 3: 1041-1047.
- Moreira-Souza AC, Marinho Y, Correa G, Santoro GF, Coutinho CM, et al. (2015) Pyrimidinergic Receptor Activation Controls Toxoplasma gondii Infection in Macrophages. PLoS One 10: e0133502.
- Wilson J, Huynh C, Kennedy KA, Ward DM, Kaplan J, et al. (2008) Control of parasitophorous vacuole expansion by LYST/Beige restricts the intracellular growth of Leishmania amazonensis. PLoS Pathog 4: e1000179.
- Halliwell B (1999) Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res 443: 37-52.
- Mariani E, Polidori MC, Cherubini A, Mecocci P (2005) Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci 827: 65-75.
- Denkers EY (2003) From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol Med Microbiol 39: 193-203.
- Ding M, Kwok LY, Schlüter D, Clayton C, Soldati D (2004) The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol Microbiol 51: 47-61.
- Dincel GC, Atmaca HT ROLE OF OXIDATIVE STRESS IN THE PATHOPHYSIOLOGY OF TOXOPLASMA GONDII INFECTION 33rd Annual Meeting of The European Society of Veterinary Pathology, the 26th Annual Meeting of The European College of Veterinary Pathologists, and the 39th Annual Meeting of Nordic Society for Veterinary Pathology Annual Meeting. 2-5 September 2015 Helsinki, Finland.
- Da Gama LM, Ribeiro-Gomes FL, Guimar~aes JrU, Arnholdt AC (2004) Reduction in adhesiveness to extracellular matrix components, modulation of adhesion molecules and in vivo migration of murine macrophages infected with Toxoplasma gondii. Microbes Infect 6: 1287-1296.
- Barragan A, Hitziger N (2008) Transepithelial migration by Toxoplasma. Subcell Biochem 47: 198-207.
- Dellacasa-Lindberg I, Fuks JM, Arrighi RB, Lambert H, Wallin RP, et al. (2011) Migratory activation of primary cortical microglia upon infection with Toxoplasma gondii. Infect Immun 79: 3046-3052.
- Bonne-Année S, Kerepesi LA, Hess JA, O’Connell AE, Lok JB, et al. (2013) Human and mouse macrophages collaborate with neutrophils to kill larval Strongyloides stercoralis. Infect Immun 81: 3346–3355.
- Kreider T, Anthony RM, Urban JF Jr, Gause WC (2007) Alternatively activated macrophages in helminth infections. Curr Opin Immunol 19: 448-453.
- Bieren JEV, Mosconi I, Guiet R, Piersgilli A, Volpe B, et al. (2013) Antibodies trap tissue migrating helminth larvae and prevent tissue damage by driving IL-4Ra-independent alternative differentiation of macrophages. PLoS Pathog 9: e1003771.
- Filbey KJ, Grainger JR, Smith KA, Boon L, Van Rooijen N, et al. (2014) Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunol Cell Biol 92: 436–448.
- Denkers EY, Kim L, Butcher BA (2003) In the belly of the beast: subversion of macrophage proinflammatory signalling cascades during Toxoplasma gondii infection. Cell Microbiol 5: 75-83.
- Bertaux L, Mevelec MN, Dion S, Suraud V, Gregoire M, et al. (2008) Apoptotic pulsed dendritic cells induce a protective immune response against Toxoplasma gondii. Parasite Immunol 30: 620-629.
- Dincel GC, Atmaca HT (2015) Increased expressions of ADAMTS-13 and apoptosis contribute to neuropathology during Toxoplasma gondii encephalitis in mice. Neuropathology 36: 211–226.
- Klotz C, Frevert U (2008) Plasmodium yoelii sporozoites modulate cytokine profile and induce apoptosis in murine Kupffer cells. Int J Parasitol 38: 1639-1650.
- Preston P, Hall R, Glass E, Campbell J, Darghouth M, et al. Innate and adaptive immune responses co-operate to protect cattle against Theileria annulata. Parasitol Today 15: 268-274.
- Gazzinelli RT, Eltoum I, Wynn TA, Sher A (1993) Acute cerebral toxoplasmosis is induced by in vivo neutralization and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol 151: 3672-3681.
- Khan IA, Schwartzman JD, Matsuura T, Kasper LH (1997) A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci U S A 94: 13955-13960.
- Schlüter D, Deckert-Schlüter M, Lorenz E, Meyer T, Rollinghoff M, et al. (1999) Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J Immunol 162: 3512-3518.
- Bohne W, Heesemann J, Gross U (1994) Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun 62: 1761-1767.
- Ibrahim HM, Bannai H, Xuan X, Nishikawa Y (2009) Toxoplasma gondii cyclophilin 18-mediated production of nitric oxide induces bradyzoite conversion in a CCR5-dependent manner. Infect Immun 77: 3686-3695.
- Dincel GC, Atmaca HT (2015) Nitric oxide production increases during Toxoplasma gondii encephalitis in mice. Exp Parasitol 156: 104-112.
- Tanaka T, Nagasawa H, Fujisaki K, Suzuki N, Mikami T (2000) Growth-inhibitory effects of interferon-gamma on Neospora caninum in murine macrophages by a nitric oxide mechanism. Parasitol Res 86: 768-771.
- Li E, Zhou P, Singer SM (2006) Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J Immunol 176: 516-521.
- Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, et al. (2000) Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J Immunol 164: 1478–1487.
- Pavanelli WR, Gutierrez FR, Silva JJ, Costa IC, Menezes MC, et al. (2010) The effects of nitric oxide on the immune response during giardiasis. Braz J Infect Dis 14: 606-612.
- Liew FY, O'Donnell CA (1993) Immunology of leishmaniasis. Adv Parasitol 32: 161-259.
- Bogdan C, Rollinghoff M, Diefenbach A (2000) The role of nitric oxide in innate immunity. Immunol Rev 173: 17-26.
- Tobin C, Pollard A, Knoll L (2010) Toxoplasma gondii cyst wall formation in activated bone marrow-derived macrophages and bradyzoite conditions. J Vis Exp 42: 2091.
- Vincendeau P, Gobert AP, Daulouède S, Moynet D, Mossalayi MD (2003) Arginases in parasitic diseases. Trends Parasitol 19: 9-12.
- Aoki MP, Guiñazú NL, Pellegrini AV, Gotoh T, Masih DT, et al. (2004) Cruzipain, a major Trypanosoma cruzi antigen, promotes arginase-2 expression and survival of neonatal mouse cardiomyocytes. Am J Physiol Cell Physiol 286: 206-212.
- Iniesta V, Carcelén J, Molano I, Peixoto PMV, Redondo E, et al. (2005) Arginase I induction during Leishmania major infection mediates the development of disease. Infect Immun 73: 6085-6090.
- Howard JC, Hunn JP, Steinfeldt T (2011) The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol 14: 414-421.
- Hunter CA, Sibley LD (2012) Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol 10: 766-778.
- Lilue J, Muller UB, Steinfeldt T, Howard JC (2013) Reciprocal virulence and resistance polymorphism in the relationship between Toxoplasma gondii and the house mouse. Elife 2: e01298.
- Mohamed AM, Metwally NM, Mahmoud SS (2005) Sativa seeds against Schistosoma mansoni different stages. Memorias do Instituto Oswaldo Cruz 100: 205-11.
- McGonigle S, Dalton JP, James ER (1998) Peroxidoxins: a new antioxidant family. Parasitol Today 14: 139-145.
- Tomlinson S, Raper J (1998) Natural human immunity to trypanosomes. Parasitol Today 14: 354-359.
- Skelly PJ1 (2004) Intravascular schistosomes and complement. See comment in PubMed Commons below Trends Parasitol 20: 370-374.
- Van der Kleij D, Van Remoortere A, Schuitemaker JHN, Kapsenberg ML, Deelder AM, et al. (2002) Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAc beta 1-4(Fuc alpha 1-2Fuc alpha 1-3) GlcNAc. J infect dis 185: 531-9.
- Ferreira AM, Diaz A, Fernandez C, Sim RB (2001) Assessment of in vivo complement activation on the Echinococcus granulosus hydatid cyst wall. Parasite Immunol 23: 655-658.
- Ferreira AM, Irigoín F, Breijo M, Sim RB, Diáz A (2000) How Echinococcus granulosus deals with complement. Parasitol Today 16: 168-172.
- McSorley HJ, Maizels RM (2012) Helminth infections and host immune regulation. Clin Microbiol Rev 25: 585-608.
- Heylen M, Ruyssers NE, Gielis EM, Vanhomwegen E, Pelckmans PA, et al. (2014) Of worms, mice and man: an overview of experimental and clinical helminth-based therapy for inflammatory bowel disease. Pharmacol Ther 143: 153-167.
- Kang SA, Cho MK, Park MK, Kim DH, Hong YC, et al. (2012) Alteration of helper T-cell related cytokine production in splenocytes during Trichinella spiralis infection. Vet Parasitol 186: 319-327.
- Kim S, Park MK, Yu HS, et al. (2015) Toll-Like Receptor Gene Expression during Trichinella spiralis Infection. Korean J Parasitol 53: 431-438.
- Maizels RM1, Balic A, Gomez-Escobar N, Nair M, Taylor MD, et al. (2004) Helminth parasites--masters of regulation. See comment in PubMed Commons below Immunol Rev 201: 89-116.
- McSorley HJ1, Hewitson JP, Maizels RM (2013) Immunomodulation by helminth parasites: defining mechanisms and mediators. See comment in PubMed Commons below Int J Parasitol 43: 301-310.
- Van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, Kremsner PG, Yazdanbakhsh M, et al. (2014) Interleukin 10 (IL-10) producing CD1dhi regulatory B cells from Schistosoma haematobium-infected individuals induce IL-10 positive and suppress effector T-cell cytokines. J Infect Dis 210: 1207-1216.
- Mckay DM (2015) Not all parasites are protective. Parasite Immunol 37: 324-332.
- Blum AM, Hang L, Setiawan T, Urban JP Jr, Stoyanoff KM, et al. (2012) Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J Immunol 189: 2512-2520.
- Carranza F, Falcón CR, Nuñez N, Knubel C, Correa SG, et al. (2012) Helminth antigens enable CpG-activated dendritic cells to inhibit the symptoms of collagen-induced arthritis through Foxp3+regulatory T cells. PLoS One 7: e40356.
- Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, et al. (2013) Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. See comment in PubMed Commons below Int J Parasitol 43: 191-200.
- Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, et al. (2010) In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterol 138: 1395-405.
- Donskow-Lysoniewska K, Majewski P, Brodaczewska K, Jozwicka K, Doligalska M (2012) Heligmosmoides polygyrus fourth stages induce protection against DSS-induced colitis and change opioid expression in the intestine. Parasite Immunol 34: 536-46.
- Leung J, Hang L, Blum A, Setiawan T, Stoyanoff K, et al. (2012) Heligmosomoides polygyrus abrogates antigen-specific gut injury in a murine model of inflammatory bowel disease. Inflamm Bowel Dis 18: 1447-55.
- Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, et al. (2012) Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PloS Pathog 8: e1003000.
- Adisakwattana P, Nuamtanong S, Kusolsuk T, Chairoj M, Yenchitsomanas PT, et al. (2013) Non-encapsulated Trichinella spp., T. papuae, diminishes severity of DSS-induced colitis in mice. Asian Pac J Allergy Immunol 31: 106-114.
- Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry-Wheeler A, et al. (2013) Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Aliment Pharmacol Ther 38: 255-263.
- Ferreira I, Smyth D, Gaze S, Aziz A, Giacomin P, et al. (2013) Hookworm excretory/secretory products induce interleukin-4 (IL-4)+ IL-10+ CD4+ T cell responses and suppress pathology in a mouse model of colitis. Infect Immun 81: 2104-2111.
- Heylen M, Ruyssers N, De Man JG, Pelckmans P, De Winter B, et al. (2013) The therapeutic potential of helminth proteins in a chronic colitis transfer model. Acta Gastroenterol Belg 74: I02.
- Zhang Z, Yang J, Wei J, Yang Y, Chen X, et al. (2011) Trichinella spiralis paramyosin binds to C8 and C9 and protects the tissue-dwelling nematode from being attacked by host complement. PLoS Negl Trop Dis 5: 1225.
- Zhao X, Hao Y, Yang J, Gu Y, Zhu X (2014) Mapping of the complement C9 binding domain on Trichinella spiralis paramyosin. Parasit Vectors 7: 80.
- Loukas A, Maizels RM (2000) Helminth C-type lectins and host-parasite interactions. Parasitol Today 16: 333-339.
- Holmskov U1, Thiel S, Jensenius JC (2003) Collections and ficolins: humoral lectins of the innate immune defense. See comment in PubMed Commons below Annu Rev Immunol 21: 547-578.
- Sánchez Valdéz FJ, Pérez Brandán C, Zago MP, Labriola C, Ferreira A, et al. (2013) Trypanosoma cruzi carrying a monoallelic deletion of the calreticulin (TcCRT) gene are susceptible to complement mediated killing and defective in their metacyclogenesis. Mol Immunol 53: 198-205.
- Sosoniuk E, Vallejos G, Kenawy H, Gaboriaud C, Thielens N, et al. (2014) Trypanosoma cruzi calreticulin inhibits the complement lectin pathway activation by direct interaction with L-Ficolin. Mol Immunol 60: 80-85.
- Meeusen EN, Balic A (2000) Do eosinophils have a role in the killing of helminth parasites? Parasitol Today 16: 95-101.
- Rothenberg ME, Hogan SP (2006) The eosinophil. See comment in PubMed Commons below Annu Rev Immunol 24: 147-174.
- Shin EH, Osada Y, Chai JY, Matsumoto N, Takatsu K, et al. (1997) Protective roles of eosinophils in Nippostrongylus brasiliensis infection. Int Arch Allergy Immunol 1: 45-50.
- Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, et al. (2007) Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol 178: 1039-1047.
- Beiting DP, Bliss SK, Schlafer DH, Roberts VL, Appleton JA (2004) Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect Immun 72: 3129-3137.
- Huang L, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, et al. (2014) Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol 193: 4178-4187.
- Seder RA, Paul WE, Ben-Sasson SZ, LeGros GS, Kagey-Sobotka A, et al. (1991) Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol 94: 137-140.
- DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P (1999) A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol 162: 1127-1136.
- Egesten A, Blom M, Calafat J, Janssen H, Knol EF (1998) Eosinophil granulocyte interaction with serum-opsonized particles: Binding and degranulation are enhanced by tumor necrosis factor alpha. Int Arch Allergy Immunol 115: 121-128.
- Butterworth AE (1984) Cell-mediated damage to helminths. Adv Parasitol 23: 143-235.
- Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, et al. (2009) Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci USA 106: 6748–6753.
- Gabriel C, McMaster WR, Girard D, Descoteaux A (2010) Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J Immunol 185: 4319-4327.
- Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, et al. (2012) Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect Immun 80: 768-777.
- Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, et al. (2006) Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect Immun 74: 5730-5738.
- Padigel UM, Stein L, Redding K, Lee JJ, Nolan TJ, et al. (2007) Signaling through Gαi2 protein is required for recruitment of neutrophils for antibody-mediated elimination of larval Strongyloides stercoralis in mice. J Leukoc Biol 81: 1120-1126.
- O’Connell AE, Redding KM, Hess JA, Lok JB, Nolan TJ, et al. (2011) Soluble extract from the nematode Strongyloides stercoralis induces CXCR2 dependent/IL-17 independent neutrophil recruitment. Microbes Infect 13: 536-544.
- Bonne-Année S, Kerepesi LA, Hess JA, Wesolowski J, Paumet F, et al. (2014) Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect 16: 502-511.
- Hafeez MD (2003) Helminth Parasites of Public Health Importance -Trematodes. J Parasit Dis 27: 69-75.
- Horák P, Kolárová L, Adema CM (2002) Biology of the schistosome genus Trichobilharzia. Adv Parasitol 52: 155-233.
- Kourilová P, Hogg KG, Kolárová L, Mountford AP (2004) Cercarial dermatitis caused by bird schistosomes comprises both immediate and late phase cutaneous hypersensitivity reactions. J Immunol 172: 3766-3774.
Relevant Topics
- Bacteriostatic antibiotics
- Cell signaling and activation
- Chemokines
- Class I MHC molecules
- Class II MHC molecule
- Colitis Antibiotics
- Immune response
- Immunochemistry
- Immunogenicity of biopharmaceuticals
- Immunogenomics
- Immunoglobulins
- Immunoglycomics
- Immunomodulatory xenobiotics
- Immunopharmacology
- Immunoproteomics
- Immunosenescence
- Immunotolerance
- Molecular Immunology
- Non classical MHC class I molecules
Recommended Journals
Article Tools
Article Usage
- Total views: 5616
- [From(publication date):
June-2017 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 4694
- PDF downloads : 922