Review Article Open Access
HMGB1-TLR Signaling in Rasmussens Encephalitis
Tianfu Li1,3,4,*, Qing Gao3 and Guoming Luan2,3,41Department of Neurology, Beijing Sanbo Brain Hospital, Capital Medical University, Beijing, China
2Department of Neurosurgery, Beijing Sanbo Brain Hospital, Capital Medical University, Beijing, China
3Beijing Key labartory of Epilepsy, Beijing, China
4Center of Epilepsy, Beijing Institute for Brain Disorders, Beijing, China
- *Corresponding Author:
- Tianfu Li
Professor and Chief Physician, Department of Neurology
Beijing Sanbo Brain Hospital, Capital Medical University
Xiangshan Yikesong 50, Haidian district
Beijing, 100093, P.R. China
Tel: +86 1062856761
Fax: +86-10-62856902
E-mail: tianfuli66@126.com
Received date: July 25, 2016; Accepted date: August 09, 2016; Published date: August 12, 2016
Citation: Li T, Gao Q, Luan G (2016) HMGB1-TLR Signaling in Rasmussens’ Encephalitis. J Neuroinfect Dis 7: 223. doi: 10.4172/2314-7326.1000223
Copyright: © 2016 Li T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Rasmussen’s encephalitis is neurological disorder of childhood characterized by uni-hemispheric inflammation, intractable focal epilepsy and progressive cognitive and neurological deficits. Currently, the pathogenesis of Rasmussen’s encephalitis is still enigmatic and hemispherectomy is the only effective method to control the seizures associated with Rasmussen’s encephalitis. Recently data indicated that intrinsic activation of endogenous proinflammation high-mobility group box-1 (HMGB1) and Toll-like receptor (TLR) is involved in the development of Rasmussen’s encephalitis. Activation of HMGB1-TLR signaling plays a critical role in brain inflammation, development of epilepsy and cognitive dysfunction. Targeted therapy on HMGB1-TLR signaling might be a novel strategy with anti-inflammation, anti-epilepsy as well as improving cognitive dysfunction associated with epilepsy in Rasmussen’s encephalitis.
Keywords
Rasmussen’s encephalitis; Epilepsy; Inflammation; Cognition; HMGB1
Introduction
Rasmussen’s encephalitis is a very rare chronic progressive inflammatory neurological disorder of uncertain etiology affecting mostly children and associated with hemispheric atrophy, focal epilepsy (epilepsia partialis continua), cognitive deterioration and progressive neurological deficits, resulting from progressive loss of function subserved by the involved cerebral hemisphere [1-3]. The etiology and pathogenesis of Rasmussen’s encephalitis, in particular, the factors responsible for the characteristic of asymmetry are still elusive. Currently, hemispherectomy is the only effective method to control the seizures associated with Rasmussen’s encephalitis. Increasing experimental and clinical evidence supports a link among inflammation, epilepsy (both in terms of epileptogenesis and the longterm consequences of seizures) and cognitive dysfunction associated with epilepsy, which indicates that activation of inflammatory processes in the brain is a common feature of various epileptic disorders [4]. Recently study demonstrates that intrinsic activation of endogenous pro-inflammation high-mobility group box-1 (HMGB1) and Toll-like receptor (TLR) are involved in the development of Rasmussen’s encephalitis [5]. Activation of HMGB1-TLR plays a critical role in brain inflammation, development of epilepsy and cognitive dysfunction, and inhibition of HMGB1-TLR signaling demonstrates anti-inflammation, anti-epilepsy as well as improving cognitive dysfunction associated with epilepsy [4].
HMGB1-TLR Signaling and Brain Inflammation
HMGB1-TLR may represent a novel pro-inflammatory axis following sterile brain injury [6]. Activation of proinflammatory cytokinesespecially HMGB1-TLR signaling involves in immune response to inflammation associated with many neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis and epilepsy [4,7], and subserve neuromodulatory functions implicated in brain physiology and may contribute to acute and chronic neurodegeneration. HMGB-1, characterized as a key cytokine [8], is a 216 amino acids (29 kDa) DNA-binding protein with a highly conserved structure in several species [9]. HMGB-1 participates in nucleosome formation and regulation of gene transcription [10,11], including proinflammatory genes [12]. HMGB1 acts as a “danger signal” and alerts the immune system to damaged or dying cells. In response to inflammatory stimuli, HMGB-1 is secreted by activated macrophages [13], natural killer cells [14], myeloid dendritic cells [8] and astrocytes [15] binding to RAGE and TLR, including TLR2 and TLR4. The hyperacetylated form of HMGB1 regulates transcription of various pro-inflammatory cytokines, including IL-1β, through binding to TLR2, TLR4 and also to RAGE [12,16]. In human brain cortex, HMGB1 immunoreactivity expressed in nuclei of neurons and glia cells as reported by Zurolo et al. in 2011 and Luan et al. in 2016. When under immune/inflammatory challenges or injurious conditions, HMGB-1, normally residing in nuclei, translocates to the cytoplasm and/or extracellular space [12,17]. Extracellular HMGB1 acts as a ‘danger signal’ to orchestrate a homeostatic defensive response in challenged tissues; however, under circumstances that remain poorly understood, the resolution of inflammation is compromised, its pro-inflammatory properties, acquired upon its cellular release and consequent TLR/RAGE stimulation, appear to contribute to the pathogenesis of various inflammatory and CNS diseases [18].
TLR4 and TLR2 are preferentially expressed on cells that play central roles in innate immune responses, including macrophages and microglia, but it is present at very low or undetectable levels on neurons and astrocytes, [5,19,20]. As the major initial sensors of danger or stranger signals recognized by TLR4, Microglia secretes inflammatory cytokines to induce secondary inflammatory responses by acting on astrocytes [21]. Therefore, Microglia plays critical roles in establishing and maintaining inflammatory responses in the context of neurodegenerative diseases [7]. TLRs play a key role in pathogen recognition, which recognize a diverse set of pathogen-associated molecules that are not present in the host, and culminate in activation of the transcription factor nuclear factor-kappa B, which controls the expression of an array of inflammatory cytokine genes [22]. In the absence of pathogens, TLR signalling can be activated by molecules released by injured tissue, namely damage-associated molecular patterns, which include HMGB1 [12]. Activation of TLR2 and TLR4 pathway plays an important role in the pathogenesis of several chronic inflammatory diseases in animal models, contributing to inflammatory programs associated with neurodegenerative disease including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis and epilepsy [4,7].
HMGB1/TLR Signaling and Epilepsy
Extensive experimental and clinical evidence supports a link between inflammation and epilepsy, both in terms of epileptogenesis and the long-term consequences of seizures, which indicates that activation of inflammatory processes in the brain is a common feature of various epileptic disorders [4,16,23,24]. Therefore, brain inflammation is regarded as a diagnostic, prognostic or therapeutic biomarker for epilepsy [25]. Recent studies demonstrate that activation of HMGB1/TLR signaling pathways plays a crucial role in the development of epilepsy. Activation of HMGB1–TLR signaling has been recently demonstrated in epileptogenic brain tissue from drugresistant epilepsy patients with Rasmussen's encephalitis [5], the prototype of inflammatory and immune-mediated epileptic encephalopathy, but also with temporal lobe epilepsy [15,16] and focal malformations of cortical development [19] without infectious or immune-mediated etiology. In particular, antagonists of HMGB1 and TLR4 retard seizure precipitation and decrease acute and chronic seizure recurrence, which highly suggest that activation of HMGB1– TLR-RAGE pathway possibly represents a common mechanism of epileptogenesis [26].
Rasmussen's encephalitis is a rare chronic progressive inflammatory neurological disorder with pharmacoresistant focal epilepsy (epilepsia partialis continua) [1,2,27]. There might be a linking between activation of the innate immune system and consequent inflammation to epilepsy of Rasmussen's encephalitis. The chronic inflammation is— at least partly—the consequence of a cycle in which inflammation induced cell injury leads to the release of endogenous dangerassociated molecular pattern that drives the inflammatory response, causing further damage and inherent epileptogenicity of brain lesions, pathogenic or otherwise [6]. The intralesional overexpression and cellular distribution of HMGB1 and its cognate receptors TLR2, TLR4 and RAGE in specimens from Rasmussen's encephalitis patients with medically intractable epilepsy has been demonstrated (5): (i) Cytoplasmic translocation of HMGB1 was detected in the neurons, reactive astrocytes and reactive microglial cells within the lesions; (ii) Increases in the immunoreactivity of HMGB1 in reactive astrocytes (predominant in cytoplasm) were observed; (iii) Intralesions expression of RAGE, TLR4 and TLR2 was also elevated in both neurons, reactive astrocytes and reactive microglial cells, particularly in reactive astrocytes. Although HMGB1 release and signaling may be a general feature of all epilepsies, expression of HMGB1, TLR2, TLR4 and RAGE was more markedly increased in perivascular areas, and endothelial cells in walls of blood vessels within the lesions cortex displayed immunoreactivity in Rasmussen's encephalitis patients. These findings are concomitant with the reactive astrogliosis, neuron loss and inflammation (i.e. CD8-positive, CD3-positive T lymphocyte). The evidence further supported the role of HMGB1-TLR pathway in activation of immune and endothelial cells in the pathogenesis of Rasmussen's encephalitis [5].
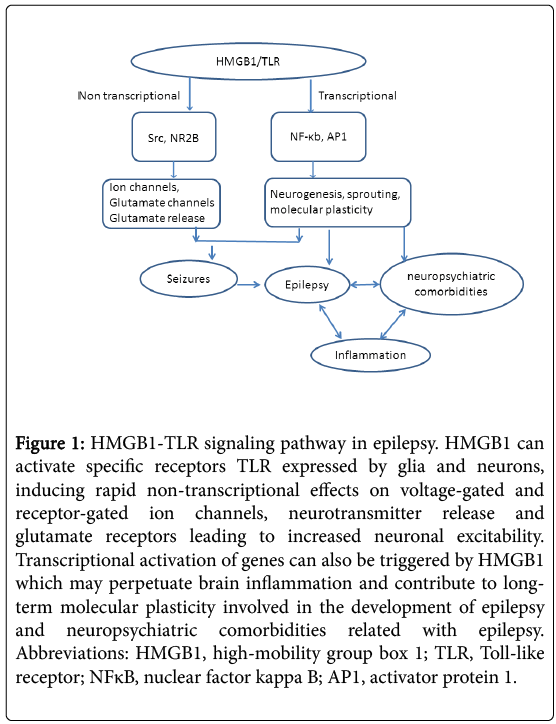
Transcriptional activation of genes can be triggered by HMGB1 via nuclear factor-κB and/or activator protein-1–dependent transcription of inflammatory genes, which may perpetuate brain inflammation and contribute to long-term molecular plasticity involved in epileptogenesis and neuropsychiatric comorbidities (Figure 1). It has been shown that repressor element 1-silencing transcription factor (REST) may control the fundamental transcription patterns that drive circuit excitability, seizures and epilepsy via recruitment of histone methyltransferase G9a [28]. As G9a is essential for the establishment and maintenance of H3K9me2 [29], aberrant distribution of heterochromatin marker and alteration of chromatin structure may directly leads to the initiation and deterioration of seizures and epilepsy. Interestingly, as histone modifier G9a also contributes to the maintenance of genomic imprinting [30], the REST-G9a-imprinted genes axis may explain the genetic tendency in the family suffered seizures and epilepsy. Therefore, understanding the epigenetic mechanisms involved in seizures and epilepsy may shed light for a better treatment of these disorders.
Figure 1: HMGB1-TLR signaling pathway in epilepsy. HMGB1 can activate specific receptors TLR expressed by glia and neurons, inducing rapid non-transcriptional effects on voltage-gated and receptor-gated ion channels, neurotransmitter release and glutamate receptors leading to increased neuronal excitability. Transcriptional activation of genes can also be triggered by HMGB1 which may perpetuate brain inflammation and contribute to longterm molecular plasticity involved in the development of epilepsy and neuropsychiatric comorbidities related with epilepsy. Abbreviations: HMGB1, high-mobility group box 1; TLR, Toll-like receptor; NFκB, nuclear factor kappa B; AP1, activator protein 1.
HMGB1/TLR Signaling and Cognition
Brain inflammation has been implicated in the pathophysiology of several neuropsychiatric conditions. Therefore, inflammatory processes which are triggered in the brain by an epileptogenic insult may, concurrently with seizures, lead to the development of neuropsychiatric abnormalities [4]. As a crucial regulator mediating epilepsy development and brain inflammation, HMGB1/TLR signaling also has been implicated in the pathophysiology of cognitive and psychiatric phenotypes. RAGE mediated inflammatory pathway plays an important negative regulator of learning and memory. In particular, the increased RAGE signaling has been implicated in mechanisms of memory impairments in Alzheimer's disease, and RAGE is regarded as a potential therapeutic target to ameliorate cellular dysfunction and cognitive dysfunction [31]. Recent evidence indicated that HMGB1 and its corresponding receptors are upregulated in the inflammation pathway in epileptic tissue and may play a crucial role in the development and perpetuation of seizures and cognitive dysfunction associated with epilepsy [4,15]. To elevate the brain levels of HMGB1 via intracerebroventricular administration has been proven to precipitate seizures and disrupted object memory mediated by either TLR4, or RAGE [32]. Refractory focal seizures and cognitive deterioration are the clinical features of Rasmussen's encephalitis, a rare progressive inflammatory disorder of uncertain etiology [1,2]. Upregulation of HMGB1, TLR4 and RAGE in reactive microglia, reactive astrocytes, neurons and vascular endothelial cells has been demonstrated in the brain lesion area of Rasmussen's encephalitis. Therefore, HMGB1-induced overstimulation of RAGE or TLR4 might contribute to memory deficits in Rasmussen's encephalitis.
Currently, we still have no clear answer for the etiology and pathogenesis of this destructive inflammatory response in Rasmussen's encephalitis; in particular, the factors responsible for the characteristic of asymmetry are still elusive. We are uncertain of the possible initial antigens to trigger the inflammatory response and why the process is unilateral. So far, hemispherectomy is the only effective method to control the seizures associated with Rasmussen’s encephalitis. To date anti-inflammatory therapy has not produced lasting responses in Rasmussen's encephalitis, such as corticosteroids pulses and intravenous immunoglobulins. Therefore, imperfectly effective immunological interventions might unacceptably postpone hemispherectomy. Evidence demonstrated HMGB1-TLR pathway in activation of immune and endothelial cells in the pathogenesis of Rasmussen's encephalitis. Therefore, perhaps more specifically targeted therapy such as targeting on HMGB1-TLR might produce lasting and promising clinical responses.
Acknowledgment
This Project was supported by the Grant from the BIBDPXM2013_ 014226_07_000084, National Natural Science Foundation of China (81571275), Scientific Research Common Program of Beijing Commission of Education (KM201410025027). We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, et al. (2005) Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 128:454-471.
- Rasmussen T, Olszewski J, Lloydsmith D (1958) Focal seizures due to chronic localized encephalitis. Neurology 8: 435-445.
- Bien CG, Widman G, Urbach H, Sassen R, Kuczaty S, et al. (2002) The natural history of Rasmussen's encephalitis. Brain 125: 1751-1759.
- Vezzani A, Aronica E, Mazarati A, Pittman QJ (2013) Epilepsy and brain inflammation. Exp Neurol 244: 11-21.
- Luan G, Gao Q, Zhai F, Chen Y, Li T (2016) Upregulation of HMGB1, toll-like receptor and RAGE in human Rasmussen's encephalitis. Epilepsy Res 123: 36-49.
- Walker L, Sills GJ (2012) Inflammation and epilepsy: the foundations for a new therapeutic approach in epilepsy? Epilepsy Curr 12: 8-12.
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140: 918-934.
- Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331-342.
- Thomas JO (2001) HMG1 and HMGB2: architectural DNA-binding proteins. Biochem Soc Trans 29: 395-401
- Park JS, Arcaroli J, Yum HK, Yang H, Wang H, et al. (2003) Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 284: C870-879.
- Stros M, Ozaki T, Bacikova A, Kageyama H, Nakagawara A (2002) HMGB1 and HMGB2 cell-specifically down-regulate the p53- and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J Biol Chem 277: 7157-7164.
- Bianchi ME, Manfredi AA (2009) Immunology. Dangers in and out. Science 323: 1683-1684.
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, et al. (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22: 5551-5560.
- Semino C, Angelini G, Poggi A, Rubartelli A (2005) NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 106: 609-616.
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, et al. (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16: 413-419.
- Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, et al. (2011) Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J Intern Med 270: 319-326.
- Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191-195.
- Bianchi ME, Manfredi AA (2007) High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 220: 35-46.
- Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, et al. (2011) Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain 134: 1015-1032.
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, et al. (2000) Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 20: 6309-6316.
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, et al. (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137: 47-59.
- Kawai T, Akira S (2007) Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13: 460-469.
- Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7: 31-40.
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T (2011) IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 25: 1281-1289.
- Vezzani A, Friedman A (2011) Brain inflammation as a biomarker in epilepsy. Biomark Med 5: 607-614.
- Ravizza T, Balosso S, Vezzani A (2011) Inflammation and prevention of epileptogenesis. Neurosci Lett 497: 223-230.
- Takei H, Wilfong A, Malphrus A, Yoshor D, Hunter JV, et al. (2010) Dual pathology in Rasmussen's encephalitis: a study of seven cases and review of the literature. Neuropathology 30: 381-391.
- Roopra A, Dingledine R, Hsieh J (2012) Epigenetics and epilepsy. Epilepsia 9: 2-10.
- Tachibana M, Sugimoto K, Fukushima T, Shinkai Y (2001) Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem 276: 25309-25317.
- Zhang T, Termanis A, Ozkan B, Bao XX, Culley J, et al. (2016) G9a/GLP complex maintains imprinted DNA methylation in embryonic stem cells. Cell Rep 15: 77-85.
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, et al. (2004) RAGE potentiates a beta-induced perturbation of neuronal function in transgenic mice. EMBO J 23: 4096-4105.
- Mazarati A, Maroso M, Iori V, Vezzani A, Carli M (2011) High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and Receptor for Advanced Glycation End Products. Exp Neurol 232: 143-148.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11901
- [From(publication date):
September-2016 - Apr 10, 2025] - Breakdown by view type
- HTML page views : 11007
- PDF downloads : 894