Short Communication Open Access
HIV and Pneumocystis Pneumonia (PCP): An Upto date
| Yanagisawa K* and Nojima Y | |
| Department of Medicine and Clinical Science, Gunma University Graduate School of Medicine, Gunma, Japan | |
| Corresponding Author : | Yanagisawa K Department of Medicine and Clinical Science Gunma University Graduate School of Medicine Maebashi, Gunma, Japan Tel: +81272208166 E-mail: kyanagi@gunma-u.ac.jp |
| Received: November 06, 2015; Accepted: December 28, 2015; Published: December 31, 2015 | |
| Citation: Yanagisawa K and Nojima Y (2015) HIV and Pneumocystis Pneumonia (PCP): An Upto date. J Infect Dis Ther 3:257. doi:10.4172/2332-0877.1000257 | |
| Copyright: © 2015 Yanagisawa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Pneumocystis pneumonia (PCP) is one of an important opportunistic infections in patients with human immunodeficiency virus (HIV) infection or taking immunosuppressive agents. The diagnosis, therapy and prophylaxis have been well-established. The counts of CD4 positive T cells (CD4) is a risk factor of PCP in HIV-infected patients, and the prevalence of PCP in the patients having CD4<200/µl is almost a half of them in Japan. However, among HIV-infected patients, risk factor other than CD4 have not been established, although in patients with rheumatic diseases some factors have been reported. Recently, our team reported the association between the genotypes in gene coding mannose-binding lectin (MBL) and the prevalence of PCP in HIV-infected patients. It may confer the strategy of PCP prophylaxis in the immunocompromised patients.
| Keywords |
| HIV; AIDS; Pneumocystis pneumonia; Mannose-binding lectin (MBL) |
| Introduction |
| The Pneumocystis pneumonia (PCP) is an opportunistic infections caused by Pneumocystis jirovecii well-known as a major manifestations of acquired immunodeficiency syndrome (AIDS) defining diseases. The number of CD4 positive T lymphocytes (CD4) <200/μl has been established as a risk factor of PCP. However, among the patients developed AIDS due to advanced human immunodeficiency virus (HIV) infection, the prevalence of PCP is varied in between countries; around 10% to 50% [1,2]. The frequency of PCP in African countries tend to be low than that of Western countries [3], and it may be affected by the limited medical resources for diagnosis in the developing or low-income countries. However, a study from Netherland reported the low prevalence of PCP in HIV-infected African race compared to that of European race, living in same country. The authors suggested that some genetic host factors rather than the environmental factor may confer the development of PCP [4]. Furthermore, one report from Japan reveals relatively high prevalence of PCP compared to those of Western countries among patients used TNF inhibitor for their rheumatoid arthritis [5]. Taken together those evidences, we can hypothesize that some variety of immunity based on the patients’ genetic background may confer the frequency of PCP. Furthermore, the development of immunosuppressive therapy and increase of the immunocompromised patients require that appropriate evaluation of the risk factor of PCP to establish the prophylaxis and therapy. |
| For the diagnosis, detection of Pneumocystis organisms in the specimens from respiratory tract is crucial; for example, Giemsa staining or PCR detection from broncho-alveolar lavage fluid. The elevation of serum (1-->3) beta-D-glucan is a useful diagnostic biomarker with a high sensitivity [6]. In the therapeutic and prophylaxis options, trimethoprim−sulfamethoxazole is established for the efficacy against PCP, and intravenous pentamidine is selected as an alternative therapeutic options (inhalation is recommended only for prophylaxis). Atovaquone has been developed as a choice for PCP therapy and prophylaxis in patient who cannot tolerate trimethoprim −sulfamethoxazole [7]. |
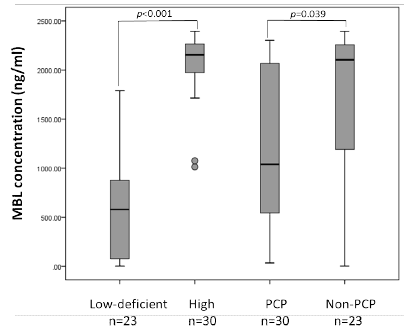
| Risk factors of PCP have been also discussed in the field of rheumatic diseases; older age, baseline pulmonary diseases, higher prednisolone dose, lower counts of lymphocytes [8,9]. However, among HIV-infected patients, the host’s risk factors other than CD4 have not been well- established. Recently, our team reported the association of Mannose-bindin lectin (MBL) genotypes and the prevalence of PCP in HIV-infected patients in Japan [10]. MBL is one of well-studied pattern recognition receptors (PRR) [11,12], plays a pivotal role in innate immunity by directly opsonizing pathogens or by activating complement system via the mannose-associated serine protease (MASP), called lectin pathway [13,14]. Most mammals have two genes (MBL1 and MBL2) that encode functional MBL; however, MBL1 is a pseudogene in humans. Genetic mutations in promoter and exon1 of MBL2 influence functional MBL levels in human serum [13,14]. Polymorphisms in exon1 of MBL2 one each in codons 52, 54, and 57; these single nucleotide polymorphisms (SNPs) rs5030737, rs1800450, and rs1800451 are referred to as variants D, B and C respectively; the wild type is designated A. Additionally, three SNP sites in the promoter to the 5’-untranslated region (5’-UTR) at nt-550, nt-221, and nt+4 (rs11003125, rs7096206, and rs7095891, referred to as H/L, Y/X, and P/Q, respectively) also affect MBL production. From these six alleles, seven typical haplotypes have been determined as follows: HYPA and LYQA result in high serum MBL levels; LYPA results in intermediate levels; LXPA results in low levels; LYPB, HYPD, and LYQC result in MBL deficiency [15]. With these seven haplotypes, we can categorize the patients’ MBL productivity into two classes: 1) high production and 2) low-deficient [16]. We confirmed that genetic variety of MBL coding gene confer the serum levels of MBL, and lowdeficient genotypes were associated with the higher prevalence of PCP in baseline CD4 counts <200/μl (Figure 1) [10]. |
| Low to deficient and high-producing genotype groups were compared with regard to median MBL levels; the PCP and non-PCP groups were also compared with regard to median MBL levels. All serum samples were collected at least 3 months after the onset of PCP to avoid acute phase reactions, and measured by MBL-oligomer ELISA kits (BioPorto, Gentofte, Denmark). The Mann-Whitney U test was used to assess between-group differences, and p values <0.05 was determined and considered significant. |
| Of course our results are insufficient to explain the all risks or molecular mechanisms of the PCP, some host factors, microorganism factors, and environmental factors other than MBL genotypes may confer the susceptibility against PCP. Ambient air pollution have been suggested to be as a risk factor of PCP [17]. However, we elucidated at least one component of the anti-PCP mechanisms of MBL in advanced HIV-infection. MBL genotypes and serum levels can be a useful and novel predictive factor for PCP, in addition with CD4 cell levels. Further study is needed in large sample size and among other ethnic groups to confirm this clinical issue. |
References
- Morrow BM, Samuel CM, Zampoli M, Whitelaw A, Zar HJ (2014) Pneumocystis pneumonia in South African children diagnosed by molecular methods. BMC Res Notes 7: 26.
- Huang L, Cattamanchi A, Davis JL, den Boon S, Kovacs J, et al. (2011) HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc 8: 294-300.
- Nowaseb V, Gaeb E, Fraczek MG, Richardson MD, Denning DW (2014) Frequency of Pneumocystis jirovecii in sputum from HIV and TB patients in Namibia. J Infect Dev Ctries 8: 349-357.
- Schoffelen AF, van Lelyveld SF, Barth RE, Gras L, de Wolf F, et al. (2013) Lower incidence of Pneumocystis jirovecii pneumonia among Africans in the Netherlands host or environmental factors? AIDS 27: 1179-1184.
- Takeuchi T, Kameda H (2010) The Japanese experience with biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 6: 644-652.
- Watanabe T, Yasuoka A, Tanuma J, Yazaki H, Honda H, et al. (2009) Serum (1-->3) beta-D-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin Infect Dis 49: 1128-1131.
- Aberg J, Powderly W (2010) HIV: primary and secondary prophylaxis for opportunistic infections. BMJ Clin Evid 2010.
- Mori S, Sugimoto M2 (2015) Pneumocystis jirovecii Pneumonia in Rheumatoid Arthritis Patients: Risks and Prophylaxis Recommendations. Clin Med Insights Circ Respir Pulm Med 9: 29-40.
- Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y (2014) Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 16:R43.
- Yanagisawa K, Ogawa Y, Uchiumi H, Gohda F, Mawatari M, et al. (2015) Gene polymorphisms of mannose-binding lectin confer susceptibility to Pneumocystis pneumonia in HIV-infected patients. J Infect Chemother 21: 769-775.
- Willment JA, Brown GD (2008) C-type lectin receptors in antifungal immunity. Trends Microbiol 16: 27-32.
- Kerrigan AM, Brown GD (2009) C-type lectins and phagocytosis. Immunobiology 214: 562-575.
- Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO (2006) Mannose-binding lectin and its genetic variants. Genes Immun 7: 85-94.
- Eisen DP, Minchinton RM (2003) Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis 37: 1496-1505.
- Verdu P, Barreiro LB, Patin E, Gessain A, Cassar O, et al. (2006) Evolutionary insights into the high worldwide prevalence of MBL2 deficiency alleles. Hum Mol Genet 15: 2650-2658.
- Ou XT, Wu JQ, Zhu LP, Guan M, Xu B, et al. (2011) Genotypes coding for mannose-binding lectin deficiency correlated with cryptococcal meningitis in HIV-uninfected Chinese patients. J Infect Dis 203: 1686-1691.
- Alvaro-Meca A, Palomares-Sancho I2, Diaz A3,4, Resino R5, et al. (2015) Pneumocystis pneumonia in HIV-positive patients in Spain: epidemiology and environmental risk factors. J Int AIDS Soc 18: 19906.
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11587
- [From(publication date):
December-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10606
- PDF downloads : 981
