Research Article Open Access
Histopathological and Biochemical Assessment of Chronic Oral Administration of Aqueous Leaf Extract of Thymus Serrulatus in Mice
Nigatu Debelo1, Mekbib Afework2, Asfaw Debella3, Eyasu Makonnen4, Wondwossen Ergete5, and Bekesho Geleta3*
1Department of Anatomy, College of Medicine and Health Sciences, Ambo University, Ethiopia
2Department of Anatomy, School of Medicine, College of Health Sciences, Addis Ababa University, Ethiopia
3Directorate of Traditional and Modern Medicine Research, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
4Department of Pharmacology, School of Medicine, College of Health Sciences, Addis Ababa University, Ethiopia
5Department of Pathology, School of Medicine, College of Health Sciences, Addis Ababa University, Ethiopia
- *Corresponding Author:
- Bekesho Geleta
Ethiopian Public Health Institute
P.O Box 1242, Addis Ababa, Ethiopia
Tel: 251911091969
E-mail: bekeshog@gmail.com
Received Date: October 19, 2015 Accepted Date: November 17, 2015 Published Date: November 17, 2015;
Citation: Debelo N, Afework M, Debella A, Makonnen E, Ergete W, et al. (2015) Histopathological and Biochemical Assessment of Chronic Oral Administration of Aqueous Leaf Extract of Thymus Serrulatus in Mice. J Clin Exp Pathol 5:258. doi: 10.4172/2161-0681.1000258
Copyright: © 2015, Debelo N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Background: Medicinal plants are the source of treatment for many diseases throughout the developing world. The provision of safe herbal therapies could, thus, become a critical tool to increase access to health care in minimizing the undesirable effects. Thymus serrulatus is among commonly used herbal remedy in Ethiopia in addition to its use for spicing a food and as infusion in tea. This study was designed to investigate toxic effects of the aqueous leaf extract of Thymus serrulatus in mice.
Methods: In this study, the mice were randomly divided into control and treatment groups that received distilled water and oral administration of single dose of 300, 2000, 5000 and 10,000 mg/kg (n=5) extract for acute toxicity, respectively and followed for 14 days. The chronic toxicity was evaluated through daily oral administration of extracts at doses of 200 and 600 mg/kg for 90 days in mice (n=6). On the 91st day, blood samples were collected for hematological and biochemical analysis. Liver and kidney were harvested for histopathological examination by tissue section preparation and microscopic examination.
Results: The acute toxicity study found no signs of toxicity; hence LD50 was greater than 10,000 mg/kg. After 90 days of extract administration, there was no significant change in body weight, and most of the evaluated hematological and biochemical parameters. There was a significant decrease in count of basophils in male mice treated at dose 600 mg/kg. In addition, creatinine level of female mice at both doses was also significantly reduced. No death was recorded, and body weight change remained normal compared to the control. In gross observations, the kidneys and liver appear normal compared to the control. Histopathological examination of liver and kidney were essentially normal.
Conclusions: Thymus serrulatus is relatively safe. However, mild mononuclear leukocytic infiltration was observed in mice treated at higher doses.
Keywords
Thymus serrulatus ; Histopathology; Acute toxicity; Chronic toxicity
Background
Medicinal plants are the source of treatment for many diseases and ailments throughout the developing world [1] because they contain various bioactive principles which have the potential to cause beneficial and/or detrimental effects [2]. They are often used without satisfactory demonstration of their pharmacological activities and toxicological evaluation. Traditionally, many people believe that medicinal herbs being natural are safe and free from undesirable effects, failing to recognize that herbs are composed of bioactive chemicals some of which may be toxic. In fact, the adverse effects of phytomedicines, as well as its adulteration, toxicity, and drug interaction are common problems related to public health [3].
Although there is increased acceptance and consumption of herbal remedies worldwide, care must be taken not to consume harmful plants or high doses of plant extracts that could have deleterious effects on vital body organs either in short term or long term. Concerns by medical personnel indicate that herbal medicines may be harmful to vital organs such as liver and kidneys [4]. Toxic effects due to herbal medicine may manifest in a number of organs such as kidney, liver, stomach, nervous system and blood. The liver is a vital organ for maintaining of metabolic functions and detoxification from exogenous and endogenous substances like xenobiotics, drugs and viral infections [5-6]. When the liver is exposed to such substances, its protective mechanisms are overpowered due to cellular necrosis and increase in serum levels of biochemical parameters like Alanine aminotransferase (ALP) and Aspartate aminotransferase (AST) [7-8]. Determination of efficacy and safety of herbal remedies is necessary as many people use them for self-medication [9-10]. For majority of herbal products in use, very little is known about their active and/or toxic constituents. Evaluating the prolonged effects of medicinal plant extracts used in humans is useful in assessing the potential toxic effects. This increases the confidence in their safety to humans, particularly for use in the development of pharmaceuticals. The provision of safe and effective herbal therapies could, thus, become a critical tool to increase access to health care [11].
Among numerous plants used traditionally for medicinal purpose Thymus serrulatus is the most common one in Ethiopia. It is also T. serrulatus is a plant with genius Thymus and indigenous to Ethiopia, locally known as “Tosign” and growing on edges of roads, in open grassland, and on bear rocks [12]. Its leaf is used for treatment of renal diseases, hypertension, and Tinea capitis [13-14]. In addition the essential oil is found to be useful for overcoming fatigue, joint pain, backache, sciatica and physical weakness in a hot bath and also used as antimicrobial, antibacterial, antifungal, antiviral and germicidal. Inhaled, thyme oil uplifts the spirit, relieves depression and is an excellent decongestant and cold treatment [15-16]. These activities may be due to the presence of active ingredients such as p-cymene, γ- terpine, carvacrol, rosmarinic acid, eugenol and thymol [17-18]. The leaves of T. serrulatus are used in Ethiopia as spices to flavor a wide range of food products [12]. Despite its consumption there was no data and study on the safety of the plant. Therefore, this study was to investigate the safety of the plant extract in animal model.
Materials and Methods
Plant material collection and extraction
The fresh leaves of T. serrulatus were collected from north eastern Ethiopia around Debre-Sina, about 200 km far from Addis Ababa on March 2014. The plant material was authenticated by a botanist in the Ethiopian Public Health Institute and a voucher number HH-001 was deposited in the herbarium for future reference.
The fresh leaves were cleaned from extraneous materials, dried under shade at room temperature, and grinded by manual crusher to obtain powder. About 320 g of powdered leaves were macerated with distilled water for 2 hours with intermittent agitation by orbital shaker. Then, the supernatant part of agitated materials were decanted and filtered with 0.1 mm2 mesh gauze from the undissolved portion of the powder. The filtrates were freeze-dried at lower temperature and reduced pressure, and then lyophilized to obtain 47.73 g (14.9% w/w) aqueous extract. Then it was kept in a desiccator at room temperature until used.
Preparation of experimental animals
For this study healthy male and female Swiss albino mice of 8 to 12 weeks of age weighing 20-25 g obtained from the animal breeding unit of Ethiopian Public Health Institute were employed. Females were nulliparous and non-pregnant. Animals of the same sex were grouped into experimental and control groups in a standard cages with six animals per group (n=6) and were kept under standard conditions (at a temperature of 20°C (± 2°C)), with natural 12 hours light/12hours dark cycle). For feeding, conventional rodent laboratory diets was used with an unlimited supply of drinking water. The animals were acclimatized to laboratory conditions for one week prior to the experiment to alleviate any non- specific stress [19].
Method of extract administration
Each group of animal was given different doses of aqueous leaf extracts orally using intragastric catheter. For acute toxicity study, the extracts were given once after the animals were fasted of food for 18 hours with a free access for water. After the period of fasting, the animals were weighed and the dose was calculated according to the body weight, then the test substance was administered accordingly [20]. For the chronic toxicity study, the animals were given the aqueous leaf extract of T. serrulatus for 90 consecutive days, whereas the animals in the control group were given distilled water. All equipment used were cleaned and placed in an oven after each administration to prevent any contamination.
Acute toxicity study
The lethal dose for fifty percent (LD50) for aqueous leaf extracts of T. serrulatus was determined using Swiss albino mice [19]. Total of five animals (n=5) all females were used for each dose level investigated. The time interval between dosing of each animal at each level was determined by the onset, duration, and severity of toxic signs. A period of 24 hours was allowed between the dosing of each animal. All animals of each level were observed for 14 days. All experimental animals were observed closely for any acute toxicity responses. Treatment of animals at the next dose was given after the previously dosed animals survive. A period of 3 days was allowed between dosing at each dose. The starting dose was selected from the fixed dose levels of 5, 50, 300 and 2000 mg/kg as a dose expected to produce evident toxicity. In this study the starting dose was 300 mg/kg, because of no available evidences of the toxicity of the plant. The following doses, 300 mg/kg for group-1, 2000mg/kg for group-2, 5000 mg/kg for group-3, and 10,000 mg/kg for group-4 were given for the experimental animals. The animals in the control group were received distilled water.
Chronic toxicity study
The study was carried out by using 30 Swiss albino mice rats (15 male and 15 female). Out of the 30 rats, 20 were randomly assigned to 2 experimental groups of 10 mice per each group. The remaining 10 mice were assigned randomly as control group. Animals of different sexes were placed in the separate cages.
The animals in the experimental group were treated with the aqueous leaf extract at doses of 200 mg/kg, and 600 mg/kg with the intervals of 24 hours for 90 days. All animals have a free access to determined standard pellet and tap water. All groups were closely observed for any physical, food intake, behavioural alterations and sings of abnormalities throughout the study.
Body weight measurement
Body weight of all experimental animals was taken by using digital electronic balance before commencing the first oral administration and then weekly till last day of oral administration of the extract.
Cage side observation
For the acute toxicity study animals were observed individually at least once during the first 30 minutes after dosing and periodically during the first 24 hours (with special attention given during the first 4 hours [19]. Whereas, for sub-chronic toxic study, animals were observed daily in group immediately before and after administration of extract.
Blood collection
At the end of the experiment, all experimental animals were fasted overnight, cervically dislocated, and blood samples were collected by cardiac puncture into tube with anticoagulant ethylene di-amine tetraacetic acid (EDTA) for hematology and into a tube without anticoagulant for blood chemistry. Blood samples in test tubes containing EDTA were immediately processed for hematological parameters using Automated Hematological Analyzer, SYSMEX XT-1800i (SYSMEX CORPORATION, Japan). White blood cell count (WBC), red blood cell count (RBC), the hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count (PLC) were determined. For biochemical analysis, the blood samples in the plain test tubes were allowed to stand for 3 hours for complete clotting and then centrifuged at 5000 rpm for 15 minutes using a bench top centrifuge (HUMAX-K, HUMAN- GmbH, and Germany). The plasma was withdrawn and transferred into other clean vials. The sera were kept at -20°C until analysis for clinical biochemistry measurements. The concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, albumin and creatinine were automatically determined using COBAS INTEGRA 400 plus Analyzer (ROCHE DIAGNOSTICS, Japan).
Organs weight measurements and tissue sample
All experimental animals were sacrificed at the end of 90 days treatment after body weight had been measured. Then the animals were dissected and the target organs in the study were removed. After the organs has been removed from the body they were kept in 1% normal saline for a few minutes to clean off any extraneous tissues, further the organs were weighted with the precision balance; and tissue samples were taken from liver and kidney. Sample tissues were placed in a test tube containing 10% buffered formalin for 24 hours and thoroughly rinsed over running tap water overnight. Then the fixed tissues were dehydrated and cleared in a graded series of ethanol and xylene respectively. Then the tissues were infiltrated with molten paraffin wax and embedded in paraffin blocks. The blocks were sectioned at thickness of 5-6 μm using Leica rotary microtome (LEICA RM 2125 RT, China, checked in Germany). Ribbons of the tissue sections were gently collected using a forceps and laid onto the surface of a water bath heated at 30-400°C. After the sections were thoroughly spread on the water bath, they were placed over tissue glass slides. The slides were then arranged in slide racks and placed in an oven at a temperature of 20-400°C overnight to facilitate the fixation of the specimens onto the glass slides. The thin sections then were undergoes through different stages of xylene and alcohol, being stained with heamatoxylin and eosin [21].
Light microscopy and photomicrography
Stained tissue sections of the liver and kidney were carefully examined under binocular compound light microscope (OLYMPUS CX41, Japan). Tissue sections from the treated groups were examined for any evidence of histopathologic changes with respect to those of the controls. After examination, photomicrograph of selected slides from both the treated and control groups were taken under a magnification of X40 and X20 objective by using (EVOS XI, China) automated builtin digital photo camera.
Data processing and analysis
All data which are represented by numbers were packed and analyzed by SPSS statistical software. All values of parameters were expressed in mean±SEM (standard error of mean). Treatment over time were compared between control and treated groups by using oneway analysis of variance (ANOVA), followed by Dunnett’s t-test to determine their level of significance. Differences at p<0.05 were considered statistically significant.
Ethical consideration
The study was conducted after the ethical and clearance letter was obtained from Ethiopian Public Health Institute. Animals those were used in this study were kept from any unnecessary painful and terrifying situations and handled humanely throughout the study period [19].
Results
Acute toxicity
Intragastric administration of both extracts at different doses 300, 2000, 5000, and 10,000 mg/kg did not produce any sign of morbidity and mortality in female animals during the period of experiment for acute toxicity. This result indicates that the LD50 were above 10,000 mg/kg for the aqueous leaf extracts of T.serrulatus .
Chronic study
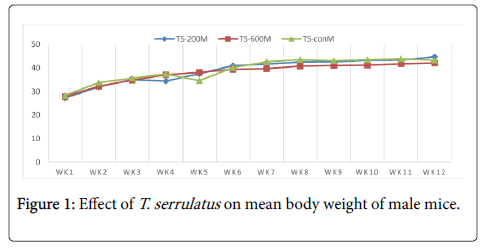
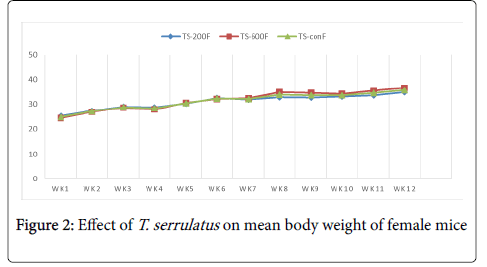
Effects of extract on the body weight: The chronic effect of aqueous leaf extract of T. serrulatus on the general body weight of the mice is illustrated in table 1. Both treated and control groups of mice showed overall increment in body weight with time over the period of the experiment.
| Groups | Body weight of male mice (g) | Body weight of female mice (g) | ||||
|---|---|---|---|---|---|---|
| Initial | Final | Change | Initial | Final | Change | |
| TS-200 | 27.24 ± 1.79 | 44.7 ± 1.30 | 17.46 | 25.2 ± 0.56 | 34.8 ± 1.23 | 9.6 |
| TS-600 | 27.85 ± 1.55 | 42.12 ± 1.05 | 14.27 | 24.3 ± 1.13 | 36.5 ± 1.37 | 12.3 |
| TS-con | 28.3 ± 1.12 | 43.26 ± 1.92 | 14.96 | 25.8 ± 1.43 | 37.4 ± 2.72 | 11.6 |
Values are expressed as mean ± SEM *=p<0.05
Table 1: The mean body weight of mice treated with T. serrulatus at different weeks and different doses.
Effects of extract on Hematological and Biochemical parameters
Regarding the effect of T. serrulatus on the hematological parameters of female and male mice the changes are displayed in tables 2. In the female and male mice the extract did not bring any significant effect on hematological parameters, except for the counts of white blood cell (WBC) which were increased significantly in groups that received the aqueous leadf extract at dose of 200 mg/kg (7.11 ± 0.06 WBC) compared to the control group (3.36 ± 0.79 WBC) (Table 2).
| Hematological | Female mice group | Male mice group | ||||
|---|---|---|---|---|---|---|
| Parameters | TS-200 | TS-600 | TS-conF | TS-200 | TS-600 | TS-conF |
| WBC (x103/µµL) | 7.1 ± 0.06* | 4.9 ± 0.08 | 3.4 ± 0.79 | 3.5 ± 1.44 | 2.6 ± 1.43 | 6.5 ± 0.74 |
| RBC (x106/µL) | 8.1 ± 1.07 | 8.7 ± 1.21 | 9.5 ± 0.53 | 8.7 ± 0.98 | 9.2 ± 0.67 | 9.8 ± 0.09 |
| HGB (g/dL) | 15.8 ± 1.08 | 15.1 ± 0.52 | 16.1 ± 0.12 | 13.6 ± 1.16 | 13.9 ±0.58 | 15.3 ± 0.44 |
| HCT (%) | 43.0 ± 1.93 | 42.6 ± 4.37 | 50.4 ± 0.81 | 44.4 ± 2.49 | 45.1 ± 2.00 | 45.2 ± 4.37 |
| MCV (fL) | 53.3 ± 4.82 | 49.9 ± 2.26 | 53.1 ± 2.31 | 52.4 ± 3.62 | 49.2 ± 1.60 | 48.5 ± 0.31 |
| MCH (pg) | 15.9 ± 0.73 | 18.4 ± 3.68 | 16.9 ± 1.08 | 15.8 ± 0.42 | 15.2 ± 0.53 | 17.0 ± 1.16 |
| MCHC (g/dL) | 30.6 ± 1.77 | 36.5 ± 5.48 | 31.8 ± 0.63 | 32.4 ± 1.09* | 30.9 ± 0.34 | 36.7±2.67 |
| PLT (X103/µL) | 1137.0 ± 225.02 | 676.3 ± 196.48 | 601.7 ± 33.79 | 547.8 ± 230.04 | 459.3 ±307.93 | 741.0 ± 141.26 |
Values are expressed as mean ± SEM *=p<0.05
Table 2: The chronic effect of aqueous leaf extract of T. serrulatus on hematological parameters in mice.
TSc-200: mice that received extract at dose of 200 mg/kg; TSc-600: mice that received extract at dose of 600 mg/kg; TSc-con: mice that received distilled water (control group)
The chronic effects of aqueous leaf extract of T.serrulatus on serum biochemical parameters of female mice is displayed in table 3. Regarding the concentration of Urea/BUN its level decreased significantly at doses of 200 mg/kg (46.66 ± 2.08) and 600 mg/kg (34.6 ± 3.05) compared with the control groups (53.33 ± 3.05). However, the plant extract did not produce any significant change on the levels of albumin, creatinine, ALP, and ALT (Table 3).
| Parameters | ||||||
|---|---|---|---|---|---|---|
| Groups | Creatinine (mg/dl) | Albumin (g/dl) | ALP (U/ L) | ALT (U/ L) | AST (U/L) | Urea (mg/dl) |
| TS-200 | 0.56±0.07 | 3.51±0.27 | 87.1±7.09 | 50.33 ± 2.51 | 201.00 ± 12.52 | 46.66± 2.08* |
| TS-600 | 0.47±0.06 | 3.12±0.20 | 92.1± 2.51 | 40.66±4.725* | 182.67 ± 10.01 | 34.60± 3.05* |
| TS-con | 0.34±0.15 | 3.27±0.31 | 91.67± 5.68 | 51.33 ± 2.52 | 195.00 ±6.56 | 53.33 ± 3.06 |
Values are expressed as mean±SEM; *=p<0.05.
Table 3: The chronic effect of aqueous extract of T. serrulatus on some serum biochemical parameters of female mice.
| Parameters | ||||||
|---|---|---|---|---|---|---|
| Groups | Creatinine (mg/dl) | Albumin (g/dl) | ALP (U/ L) | ALT (U/ L) | AST (U/L) | Urea (mg/dl) |
| TS-200 | 0.46 ± 0.22 | 3.71 ± 0.55 | 81.66 ± 97.77 | 47.00 ± 5.56 | 227.66 ± 118.72 | 47.33 ± 8.96 |
| TS-600 | 0.41± 0.03 | 3.21 ± .18 | 32.33 ± 5.51 | 58.33 ±3.51 | 228.00 ± 20.88 | 34.33 ± 3.51 |
| TS-con | 0.18 ± 0.05 | 3.42 ± 0.26 | 72.00 ±16.17 | 58.00 ± 7.02 | 234.00 ± 24.56 | 40.00 ± 3.00 |
Values are expressed as mean±SEM; *=p<0.05.
Table 4: The chronic effect of aqueous extract of T. serrulatus on some serum biochemical parameters of male mice.
Effects of extract on the weights of liver and kidney
Table 5 show the effect of aqueous leaf extract of T. serrulatus on the weight of liver and kidneys of male and female mice. As indicated in the tables the values did not reveal any significant change both in male and female groups treated with doses of 200 mg/kg and 600 mg/kg compared to the control groups.
| Groups | Male organ weight (g) | Female organ weight (g) | ||
|---|---|---|---|---|
| Liver | Kidney | Liver | Kidney | |
| TS-200 | 2.2 ± 0.13 | 0.274 ± 0.02 | 1.76 ± 0.15 (0.88) | 0.2 ± 0.01 (0.36) |
| TS-600 | 2.35 ± 0.064 | 0.24 ± 0.03 | 1.65 ± 0.06 (0.99) | 0.18 ± 0.004 (0.32) |
| TS-con | 2.26 ± 0.14 | 0.21 ± 0.01 | 1.66 ± 0.17 | 0.19 ± 0.01 |
| Values are expressed as mean ± SEM; *=p<0.05. | ||||
Table 5: The chronic effect of aqueous extract of T. serrulatus on the weight of liver and kidneys of mice.
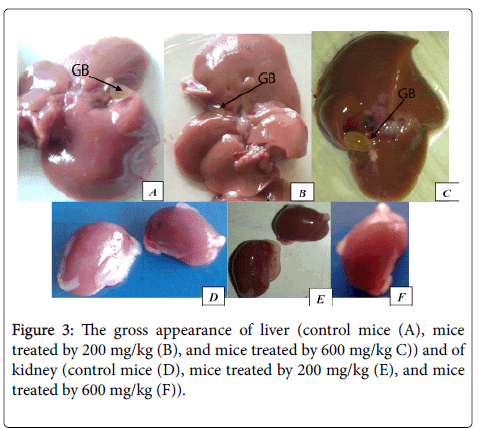
Gross pathologic observations
On the examination of the gross appearance of internal organs of treated rat and mice (Figure 1 A-F), such as liver and kidney did not show any abnormal changes in texture, shape, size or color compared to the control. There was no sign of necrosis or lesion was appreciated on the organs listed above of all groups.
Light microscopy of liver
The microscopic examination of liver sections from control mice (Figure 2) showed the normal architecture of structural units of the liver, the hepatic lobules, formed by cords of hepatocytes separated by hepatic sinusoids.
The chronic effects of aqueous leaf extract of T. serrulatus on serum biochemical parameters of male mice is displayed in table 4. There was no significant change in any of the levels of serum biochemical parameters.
There was no gross significant histopathologic alteration observed between the groups treated with vehicle and plant extract at both doses. The liver appeared normal with preserved hepatic architecture, hepatocytes arranged as radial plates, with eosinophilic cytoplasm and basophilic central nuclei. No cytoplasmic inclusions were seen and no portal inflammation (Figures 2). However, localized mononuclear lymphocytic infiltration was observed in the mice liver at the dose of 600mg/kg (Figure 2).
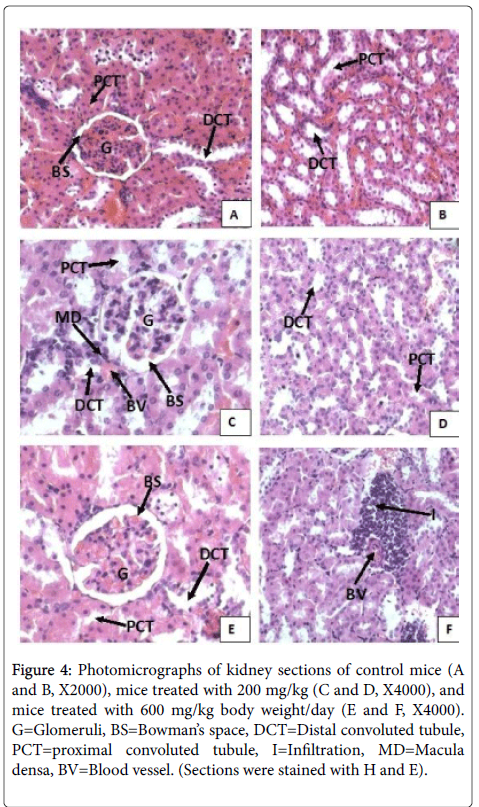
Light microscopy of kidney
The microscopic examination of kidney sections of both mice there were no significant histopathologic alteration observed in all the treatment groups. Normocellular glomerular tufts were displayed. Examination of kidney sections of mice treated with the aqueous extract of T. serrulatus (Figure 3, for mice) at both 200 mg/kg and 600 mg/kg doses indicated no structural changing as compared to the control groups (Figure 3 mice). The microscopic architecture of sections of kidney in treated groups had a similar appearance to that of the controls in which renal corpuscles maintaining their normal size of urinary space and normal tubular structures are examined. However, focal perivascular lymphocytic infiltrations were observed in small areas of kidney sections of mice treated with 600 mg/kg body weight dose (Figure 3). No necrosis was observed.
Figure 4: Photomicrographs of liver sections from control mice (A and B), treated mice at doses of 200 mg/kg (C and D), and 600 mg/kg (E and F). Figures A, C, and E shows the central vein regions, while figures B, D, and F shows the portal area of classic lobule. Note lymphocytic infiltrations (E and inset over figure F), and blood congestion in central vein (E) of liver section of mice treated at dose 600 mg/kg. CV=Central vein, E=Endothelial cells, PV=Portal vein, BD=Bile duct, H=Hepatocytes, S=Sinusoids, HA=Hepatic artery, I=Infiltration. (Sections were stained with H and E, X4000).
Discussion
Worldwide, various medicinal plants and botanical drugs have been widely adapted as primary therapeutic agents or supplements for treating various human illnesses. Based on the findings that herbal medicines are abused, there is a great need to look into their acute chronic toxicity effects. Toxicity tests are not designed to reveal the safety of a chemical, but to characterize the toxic effects a chemical can produce [22]. This is accomplished by the implementation of general preclinical toxicity experiments to uncover potential toxic effects of any drug in animals [23]. They are essentially performed on either mice or rats partly because of their physiologic and genomic similarities, but also because of their developments [24], availability, low cost, and their manageable laboratory size than other higher laboratory animals. Toxicity screening models provide important preliminary data to help select natural remedies with potential health beneficial properties for future work [25].
The first toxicity test performed on the formulation is the evaluation of acute toxicity as determined from the administration of a single exposure. It is conducted to determine the safety margin of the formulation [22]. Accordingly, in the present study, the aqueous leaf extracts of T. serrulatus did not induce lethality in mice when administered orally up to doses of 10,000 mg/kg. This result suggests that the oral LD50 of the extract to be greater than 10,000 mg/kg. In agreement with this result, one of previous study found that the LD50 were determined to be 1322 ± 1.5 mg for aqueous extract of T. serrulatus [26]. Hence, the plant extract can be assumed to be safe when administered orally according to the assertion that a substance that does not produce lethality up to 10,000 mg/kg orally is relatively non-toxic [27]. Visible signs of acute or delayed toxicity were not observed in the present acute toxicity study. This result goes in line with the previous studies done [28]. Observations by the naked eyes on the internal organs including the liver and kidneys of the treated animals after 2 weeks did not show any gross pathological change in color, organ swelling, texture and atrophy or hypertrophy after single administration of the extract compared with the control group.
The chronic toxicity study was carried out after 90 days administration of the aqueous leaf extracts of T. serrulatus at doses of 200 mg/kg and 600 mg/kg on liver and kidney, hematological, and biochemical parameters. Daily treatment with both doses of the aqueous leaf extracts did not show any toxicity related mortalities in male and female Swiss albino mice. Body weight is one of the sensitive indices in assessment of toxicity after exposure to toxic substances [29]. In this study, treatment with the aqueous leaf extracts of T. serrulatus did not produce statistically significant difference in body weight changes compared to the controls. There was a progressive increase in general body weight of the treated and control animals. Increment in body weight determines the positive health status of the animals [30]. Therefore, the overall weight gain in both treated and control mice and rats indicates a good health status of the animals. In all groups these results of the sub-chronic toxicity study go in line with other previous study [31] in which the body weights of rabbits treated with dietary supplementation of T. vulgaris were not significantly changed compared to the control.
Hematological parameters were also evaluated to obtain further toxicity related information. Studies on haematological parameters reveals abnormalities in body metabolic processes, and the blood profile usually provides important information on the response of the body to injury or lesion, deprivation and stress [32]. Therefore, the extent of toxic effect of drugs and or plant extracts can be determined by assessment of hematological parameters. In the study of clinical pathology, the core hematological tests recommended include total WBC count, RBC count, platelet count, hemoglobin concentration, hematocrit (or packed cell volume), mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration [33].
Red blood cell indices such as the mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) are the most useful indicators in the diagnosis of anemia in most animals [34-35].The effect of the aqueous extract of both plants studied on MCV, MCH, and MCHC were not significant in both treated mice and rats as compared to the controls. These observations demonstrate that the aqueous extract of the leaves in this study did not cause significant toxic effect on the levels of calculated red blood cell indices at both doses. Hence, chronic treatment with the aqueous leaf extract of T. serrulatus has no or minimal effect on the size of red blood cells and in hemoglobin weight per RBC in both mice and rats. This result suggests that the aqueous extracts of the leaves does not cause macrocytic and microcytic anemia [36]. This present result is in agreement with other findings in which the values of the various RBC parameters of rabbits treated with diets containing T.vulgaris were found to be comparable with those of the control group [31]. The changes in RBC count, average hemoglobin (Hgb) and hematocrit (HCT) levels of treated animals were also insignificant as compared with that of vehicle received mice and rats.
In this study hematological analysis of white blood cell (WBC) count was also performed. An increased WBC count indicates that the body is fighting an infection or intoxications from chemical substances. In herbal toxicity studies, increase in WBC may indicate the impact of plant extracts in inducing the immune response of treated animals [37]. On the other hand, significant decrease in the WBC of the blood indicates a decline in the production of leukocytes and the body is less able to fight off infections. The hematological analysis in this study demonstrated that most of the estimated total WBC count after sub-chronic administration of the aqueous leaf extract was not significantly changed in response to the administered 600 mg/kg compared to the control. This result may indicate that the aqueous leaf extract in this study does not possess chemicals capable of inducing leukocytosis or in suppression of normal production of WBC [35]. Similarly, one of previous study found that no changes in the WBC count following administration of diets containing T. vulgaris to rabbits [31]. However, the counts of WBC, and monocytes which were increased significantly, in female mice groups that received the aqueous leaf extract at dose of 200 mg/kg compared to the control group. This result may suggest that the animals in this group were fighting an infection [37].
Regarding platelet count, thrombocytopenia is a condition of abnormally low number of platelets in the circulation, which may result from decreased production or increased destruction of platelets [37]. Some drugs may provoke antibodies against platelet and cause platelet destruction, resulting in thrombocytopenia [35]. On the other hand, thrombocytosis is an abnormal increase in the number of circulating platelets [38]. However, in this study the change in the platelets count compared to the control group is insignificant in extract treated mice. This result, therefore, suggests that the aqueous leaf extracts of aqueous leaf extract at both doses in this study has no effect in inducing neither thrombocytopenia nor thrombocytosis.
The evaluation of serum biochemical parameters have significant importance to evaluate toxicological changes produced by toxicants. This is because of the body’s response to clinical signs and symptoms. To assess the possible toxic effects of drugs, evaluation of hepatic and renal function is primarily preferred as these organs are functionally predisposed. Elevated serum levels of enzymes produced by the liver or nitrogenous wastes to be excreted by the kidney might be indications to their spillage into the blood stream as a result of necrosis of the tissues [39-41].
Because of the liver’s strategic location between intestinal tract and the rest of the body it is the first organ to encounter ingested nutrients, vitamins, metals, drugs, and environmental toxicants as well as waste products of bacteria that enter portal blood [22]. As the result, liver is a primary destination for any toxic substance that entered to the body, especially through gastrointestinal route and suffers first. Because of its wide range of functions, any abnormal change in liver will definitely affect complete metabolism of an animal [39]. The most commonly used serum liver chemistry tests include serum transaminases (alanine aminotransferase (ALT), aspartate aminotransferase (AST)), serum alkaline phosphatase (ALP), Gamma-glutamyl transpeptidase (GGT), bilirubin and albumin. The major intracellular enzymes of the liver are alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Injuries of liver cells (hepatocytes) allowing for escape of these enzymes into the bloodstream raises their levels in the blood [42]. The levels of ALP and GGT in the serum are important parameters for evaluation of hepatobiliary route. For hepatocellular evaluation, measurement of a minimum of two scientifically appropriate blood tests is recommended, e.g. ALT, AST, sorbitol dehydrogenase, glutamate dehydrogenase, or total bile acids [33].
In the present liver function test, there were no significant changes in the serum level of ALT and AST in most animal groups treated with both doses of the aqueous leaf extracts in comparison to the controls. Such non-significant change of these enzymes between the control and treated group of animals after 12 weeks of administration indicates that the aqueous leaf extract does not cause significant toxic effect or hepatic damage on the liver. These results may signify that Thymus species does not cause liver damage. However, there was significant decrement in level AST was observed in female mice treated with 600 mg/kg and male rats treated at both doses compared with the controls. Regardless of the mechanism involved, one of the study concluded that decreased serum AST have not been shown to correlate with toxicologically significant effects on the liver [43].
Kidney is a sensitive organ, whose function is known to be aected by a number of factors such as drugs including phytochemicals that ultimately lead to renal failure [44]. The kidney performs many vital functions including regulating the body’s water balance, removing metabolic wastes from the bloodstream and reabsorb certain chemicals from the blood to maintain constancy of the normal composition of the body’s fluid. Many conditions can affect the ability of the kidney to carry out their vital functions. Both acute and chronic renal function decline can result in a buildup of toxic waste substances in the blood. Therefore, the measurement of concentrations of various chemicals in the blood normally regulated by the kidneys can help determine the cause and extent of kidney dysfunction. Kidney function test is a collective term for a variety of individual tests and procedures that can be done to evaluate how well the kidneys are functioning.
Accordingly, renal function can be assessed by measuring the levels of plasma creatinine, urea and uric acid concentrations [44]. Assessment of possible renal damage due to aqueous leaf extract of plants in this study was made by measuring plasma urea and creatinine levels. Creatinine, a waste product of muscle energy metabolism, is produced at a constant rate that is proportional to the individual’s muscle mass. Because the body does not recycle it, creatinine is filtered by the kidneys in a given amount of time and excreted in the urine. The rise in serum level of this chemical indicates a decline or failure in renal function to filter waste products from the blood and excrete them in the urine [45]. In the present study no significant alteration in the BUN and creatinine levels due to T. serrulatus treatment was observed. These, therefore suggest that the plant extracts do not affect the kidneys.
Histopathological examinations provide information to strengthen the findings on biochemical and heamatological parameters [46]. The present histological examination indicated that liver sections of mice treated with the aqueous leaf extract of T. serrulatus treated with 200 mg/kg and 600 mg/kg did not show focal necrosis and pyknosis. However, there were minor mononuclear leukocytic cell infiltration near central vein and bile ductin liver sections of mice treated with the aqueous leaf extracts of T. serrulatus only at 600 mg/kg body weight dose.
Focal perivascular lymphocytic infiltrations were also observed in kidney sections of mice treated with the aqueous extracts of T. serrulatus only at 600 mg/kg body weight dose. The observed infiltrations may be a response to parenchymal cell death with causes ranging from infectious agents, exposure to toxicants, generation of toxic metabolites, and tissue anoxia [47]. However, the general histological architecture and its functions were not affected in any of the treated mice and rats compared to the controls. It is reasonable to deduce, therefore, that T. serrulatus do not induce any damage to the liver or kidneys at both doses of the present study in mice. No dierence was observed in the weight and structure of the liver and kidney between the control and the treated groups. In agreement with these results, the finding of another study has demonstrated no change in liver weight in broiler chickens fed 1 g/kg thyme powder. Altogether, the sub-chronic study indicates that T. serrulatus ingestion do not induce detrimental changes and morphological alterations in liver and kidney of rats and mice at the studied doses.
Conclusion
In Swiss albino mice treated with both 200 mg/kg and 600 mg/kg doses of the aqueous leaf extracts of T. serrulatus , there was no significant change in the evaluated hematological and biochemical parameters as well the gross structures of liver and kidneys. In addition, the general behaviour, organ and body weights of treated mice and rats were not significantly affected compared to the control. This indicates that the aqueous leaf extracts of T. serrulatus did not significantly damage these organs and hence, did not compromise the hepato-renal function. However, at higher doses might induce focal mononuclear lymphocytic infiltration in microscopic structures of the liver and kidney sections of mice, although this change appears to be non-significant with regard to the liver and kidney function parameters.
Findings in the acute toxicity test suggest that the aqueous leaf extract of T. serrulatus are practically non-toxic to liver and kidney when administered orally. Chronic toxicity study indicated that the extract is relatively safe when administered orally for an extended period at doses of 200 mg/kg and 600 mg/kg. Therefore, it can be concluded from the findings of the present study that, the aqueous leaf extract of T. serrulatus is relatively safe in mice.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgements
The authors are grateful for financial support of this study which was provided by Ministry of Finance and Economic Development (OBN6.34/2006) through Ethiopian Public Health Institute and the School of Graduate Studies of Addis Ababa University. We also like to appreciate technical assistance of Mr. Abiy Abebe during laboratory work. Staffs of the Traditional and Modern Medicine Research Directorate, and Finance, plan & monitoring sincerely appreciated for their direct and indirect contribution during the work.
References
- Rao MR, Palada MC, Becker BN (2004) Medicinal and aromatic plants in agro-forestry systems.Agroforestry Systems 61: 107-122.
- AdewunmiCO,OjewoleJAO(2004)SafetyofTraditionalmedicines,ComplementaryandAlternative Medicines in Africa. Africa Journal of Traditional, Complementary and AlternativeMedicine1: 1-3.
- Veiga JWF, Pinto AC, Maciel MAM (2005) PlantasMedicinais: curasegura? Quim Nova 28: 519-528.
- Ogbe RJ, Yahaya AA, Anefu E (2012) Aqueous Ethanolic Extract of Mangiferaindica Stem BarkEffect on the Biochemical and Haematological Parameters of Albino Rats. Archives of AppliedScience Research 4: 1618-1622
- Abdel-Daim MM, Abuzead SM, Halawa SM (2013) Protective role of Spirulinaplatensis against acute deltamethrin-induced toxicity in rats. PLoS One 8:e72991.
- Abdel-Daim MM, AbdEldaim MA, Hassan AG (2015) Trigonellafoenum-graecum amelioratesacrylamide-induced toxicity in rats: Roles of oxidative stress, proinflammatory cytokines, and DNAdamage. Biochemistry and Cell Biology 93:192-198.
- Al-Sayed E, Martiskainen O, Seif el-Din SH, Sabra ANA, Hammam OA, et al.(2014) Hepatoprotective and Antioxidant Effect of Bauhinia Hookeri Extract againstCarbonTetrachloride-InducedHepatotoxicityinMiceandCharacterizationofItsBioactiveCompounds by HPLC-PDA-ESI-MS/MS. BioMed Research International 245171: 1-9.
- Azab S, Abdel-Daim M, Eldahshan O (2013) Phytochemical, cytotoxic, hepatoprotective and antioxidant properties of Delonixregia leaves extract. Medicinal Chemistry Research 22: 4269-4277.
- Eldahshan OA, Abdel-Daim MM (2015) Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnosnux-vomica. Cytotechnology 67:831-44.
- Al-SayedE,Abdel-DaimMM(2014)ProtectiveRoleofCupressuflavonefromCupressusmacrocarpaagainstCarbonTetrachloride-InducedHepato-andNephrotoxicityinMice.Plantamedica 80(18): 1665-1671.
- WHO (2002) Traditional medicine strategy 2002-2005, Geneva; accessed on 10thJune, 2014
- Dagne E, Hailu S, Bisrat D, Worku T (1998) Constituents of the essential oil of Thymus schimperi.Bull ChemSoc Ethiop 12: 79-82.
- Parve N, Yadav S (2010) Ethnopharmacology of single herbal preparations of medicinal plants inAsendabo district, Jimma, Ethiopia. Indian Journal of Traditional Knowledge 9: 727-728.
- Geleta B, Eyasu M, Kebamo S, Debella A, Makonnen E, et al.(2015) In vitro vasodilatory effect of aqueous leaf extract of Thymus serrulatus on thoracic aorta of Guinea pigs. Asian Pacific Journal of Tropical Biomedicine 5: 15-18.
- DweckAC(2009)TheToxicityofessentialoilreviewed;accessedon15thJune,2014
- NzeakoBC,Al-Kharousi ZSN,Al-Mahrooqui Z (2006)AntimicrobialActivitiesof CloveandThyme Extracts. Sultan QaboosUniv Med J 6: 33-39.
- Panizzi L, Flamini G, Cioni PL, Morelli I (1993) Composition and antimicrobial properties ofessentialoilsoffourMediterraneanLamiaceae.JournalofEthnopharmacology39:167-170.
- OECD (2008) Guidelines for the testing of new chemicals revised draft guideline 425. acute oral toxicity.
- Maxwell DLP, John AC, Noel WN, Steven GS, Naanlep TM (2007) The histopathologic effects ofSecuridacalongepedunculataonheart,liver,kidneyandlungsofrats.AfricanJournalofBiotechnology 6: 591-595.
- Forysth A (2005) Tissue processing: from the cut-up to the H and E slide.Accessed on 25thJune 2014
- Curtis(2007)Casarett andDoull’s Toxicology:Thebasicscience ofpoisons,(7thedit.), TheMcGraw-Hill Companies p18-40.
- Farzamfar B, Abdollahi M, Ka’abinejaian S, Heshmat R, Shahhosseiny MH, et al. (2008) Sub chronic toxicity study of a novel herbal-based formulation (Semelil) on dogs. DARU J Pharmaceutical Science 16: 15-19.
- Rosenthal N, Brown S (2007) The mouse ascending: perspectives for human-disease models. NatCell Biol 9: 993-999.
- ClarkeML,ClarkeEGC(1967)VeterinaryToxicology.BailliereTindall,London.CasselandCollier Macmillian Publishers p 268-277.
- Pour BM, Latha LY, Sasidharan S (2011) Cytotoxicity and oral acute toxicity studies of Lantana camara leaf extract. Molecules 16: 3663-3674.
- Bekele, Makonnen E, Debella A, Kinfu Y, Ergete W (2006) Evaluation of thespasmolytic activityandsafetyofT. serrulatusinlaboratoryanimals.Accessedon21stJune, 2014
- Heywood, Balls M, Riddell RJ, WordenAN (1983) Long term toxicity:In Animals and alternatives in toxicity testing, London: Academic Press p79-89.
- Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, Carvalho MDB, Cunha JM, et al. (2012) Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the In?ammatory Response. Evidence-Based Complementary and Alternative Medicine 657026: 1-10.
- Vahalia MK, Thakur KS, Nadkarni s, sangle VD (2011) Chronic toxicity study for TamraBhasma(A generic ayurvedic mineral formulation) in laboratory animals.Recent Res in Scien and Tech3:76-79.
- Tousson E, El-Moghazy M, El-Atrsh E (2011) The possible effect of diets containing Nigella sativaand Thymus vulgaris on blood parameters and some organs structure in rabbit.ToxicolInd Health 27:107-16.
- Raza M, Al-Shabanah OA, Al-Hadiya TM, Al-Majed A (2002) Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. PharmaceScien 70: 135-145.
- Rogers (2011) Blood: Physiology and Circulation (The Human Body), (1steditn.), EncyclopaediaBritannica 42: 121-225.
- Weingard K, Brown G, Hall R, Davies D, Gossett K, et al. (1996) Harmonization of animal clinical pathology testing in toxicity and safety studies. Fundamental and ApplToxicol 29:198-201.
- Akpamu U, Nwaopara AO, Izunya AM, Oaikhena GA, Okhiai O, et al. (2011) A comparative study on the acute and chronic effect of oral administration of Yaji (a complex Nigerian meat sauce) on some hematological parameters. British J Pharmaco and Toxico2: 108-112.
- Adeneye AA, Adeyemi OO, Agbaje EO, Banjo AAF (2010) Evaluation of the Toxicity and Reversibility Profile of the Aqueous Seed Extract of HunteriaUmbellata (K. Schum.) Hallier F. in Rodents. Afr J Tradit Complement Altern Med7: 350-369.
- Aajibade TO, Olayemia FO, Arowolo ROA (2012) The haematological and biochemical effects of methanol extract of the seeds of Moringaoleifera in rats. J Medic Plants Res6:615-621.
- Vitiritti M (2010) Thrombocytosis. BMC Geriatrics 10: 74.
- Prakash SEL, Manavalan R (2011) Acute toxicity studies of Andrographolide. Res J PharmaceBiol and ChemScien 2: 547-552.
- Paliwal A, Gurjar RK, Sharma HN (2009) Analysis of liver enzymes in albino rat under stress of λ- cyhalothrin and nuvan toxicity. Biol and Medic 2: 70-73.
- Rahman MF, Siddiqui MKJ, Jamil K (2001) Effects of vepacide (Azadirachtaindica) on aspartate and alanine aminotransferase profiles in a sub chronic study with rats. Human and ExperiToxicol20: 243-249.
- Thapa, Walia (2007) Liver function test and their interpretation. Indian J Pediatrics 74: 663-671.
- Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, et al. (2012) Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes-conclusions from the 3rd International ESTP ExpertWorkshop. ToxicolPathol 40:971-994.
- Stark JL (1980) BUN/creatinine: your keys to kidney function. Nursing 10: 33-38.
- PaulaAF,MarkAB(2011)Kidneyfunctiontest.p35-43.AccessedonJune9th,2014
- El-hilaly J, Israili ZH, Youssi B (2004) Acute and chronic toxicological studies of Ajugaiva in experimental animals. JEthnopharmacol 91: 43-50.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14308
- [From(publication date):
December-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13327
- PDF downloads : 981