Review Article Open Access
Histone Deacetylase Inhibitor for Neurodegenerative Diseases: A Possible Medicinal Strategy by Prevention of ER Stress-Mediated Apoptosis and Induction of Neurite Elongation
| Koji Shimoke1,2*, Takuma Tomioka2, Kouta Okamoto2, Daichi Fujiki2, Shinichi Uesato1,2, Hitoshi Nakayama3 and Toshihiko Ikeuchi1,2 | |
| 1Department of Life Science and Biotechnology, Faculty of Chemistry, Materials and Bioengineering, Kansai University, Japan | |
| 2Graduate School of Science and Engineering, Kansai University, Japan | |
| 3Department of Pharmacology, Nara Medical University, School of Medicine, Japan | |
| Corresponding Author : | Shimoke K Laboratory of Neurobiology Faculty of Chemistry, Materials and Bioengineering Kansai University, 3-3-35 Yamate-cho Suita, Osaka 564-8680, Japan Tel: +81-6-6368-0853 Fax: +81-6-6330-3770 E-mail: shimoke@kansai-u.ac.jp |
| Received February 06, 2013; Accepted March 02, 2013; Published March 04, 2013 | |
| Citation: Shimoke K, Tomioka T, Okamoto K, Fujiki D, Uesato S, Nakayama H, et al. (2013) Histone Deacetylase Inhibitor for Neurodegenerative Diseases: A Possible Medicinal Strategy by Prevention of ER Stress-Mediated Apoptosis and Induction of Neurite Elongation. Clin Pharmacol Biopharm S1:006. doi:10.4172/2167-065X.S1-006 | |
| Copyright: © 2013 Shimoke K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Neurite outgrowth is primarily necessary step to construct a neuronal network. If this step is collapsed, neurons are died and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, which are known to induce endoplasmic reticulum (ER) stress-mediated apoptosis, are occurred.
It has been elucidating that histone deacetylase (HDAC) plays a crucial role in the silencing of gene expression by the specific mechanisms. Thus, HDAC inhibitors have been shown to induce specific genes. We reported the upregulation of the nur77 gene, followed by histone modification via the protein kinase A signaling pathway or HDAC inhibitor-mediated molecular mechanisms. Then, we also focused on neurite outgrowth as a functional neuronal marker, and then described molecular targets and progressive pharmaceutical care for neurodegenerative disorders by using K-350. We propose that this kind of the candidate compound might contribute to build the therapeutic strategy for neurodegenerative diseases.
| Keywords |
| HDAC; Chromatin; Inhibitor; PC12 cell; Neurite outgrowth |
| Introduction |
| Neurons in the brain possess a number of dendrites and one axon that form neuronal networks. Recent research has shown that a specific network may be important for vertebrates to express physiological effects on phenotypes (including emotions, behavior, and memory) [1-3]. Thus, studies on the maintenance of homeostasis in the brain including neuronal networks, basically involving neuronal survival and neurite outgrowth are necessary for elucidation of the physiological maintenance mechanisms based on molecular neuronal functions. Since neurite outgrowth occurs only from viable neurons, appropriate interventions for maintaining neuron survival may be important in the treatment of neurodegenerative diseases, such as Alzheimer's and Parkinson's diseases, which are known to occur due to endoplasmic reticulum (ER) stress-mediated apoptosis. Thus, cell survival mechanisms have been investigated and include the Akt-mediated signaling pathway, which is activated by certain growth factors that may also promote cancer, and this pathway leads to an increase in the viability of neurons [4,5]. This evidence indicates that other mechanisms can be elucidated in order to develop new and effective medicines if we focus on neurodegenerative diseases. |
| Histone deacetylase (HDAC) plays a crucial role in the silencing of gene expression. Thus, HDAC inhibitors have been shown to induce specific genes. Previously, we reported the up-regulation of the nur77 gene, followed by histone modification via the protein kinase A signaling pathway or HDAC inhibitor-mediated molecular mechanisms, which in turn extended the neurites of PC12 cells [6,7]. These results emphasized the importance of a control mechanism for neurite outgrowth because this step is involved in the molecular mechanism following cell survival via the transduction of an action potential and release of a neurotransmitter if severe cell damage does not occur [8]. Thus, we also focused on neurite outgrowth as a functional neuronal marker, and then described molecular targets and progressive pharmaceutical care for neurodegenerative disorders. These foundational findings should contribute to the development of a therapeutic strategy for neurodegenerative diseases. |
| Neuronal Networks Transducing Centripetal or Centrifugal Stimulation via a Synaptic Connection |
| We are all sensitive to feel some stimuli in the brain when physical or chemical inputs are integrated into our body. The detailed mechanisms of this remain unclear; however, a specific neuronal network in the brain has been reported to be important for these sensing mechanisms [1-3]. We are all sensitive to feel some stimuli in the brain when physical or chemical inputs are integrated into our body. The detailed mechanisms of this remain unclear; however, a specific neuronal network in the brain has been reported to be important for these sensing mechanisms [9,10]. It has also been suggested that action potentials that release neurotransmitters keep neurons alive [11]. Therefore, cell survival leading to precise and static neurite formation, induced by neuronal plasticity, is important for the maintenance of homeostasis. |
| ER Stress-mediated Apoptosis in the Neuronal Networks |
| Apoptosis is an important process in vertebrate development. Twice as many neurons are produced during the development of the central nervous system (CNS) as that during the post-natal stage; however, more than 50% of these die before birth via apoptosis [12]. This process is considered to be a fundamental mechanism during the formation of correct synaptic connections. On the other hand, apoptosis is also closely associated with neurodegenerative diseases, and it is known to affect specific neurons in patients with such disorders [13,14]. Thus, neuronal networks are destroyed and severe dysfunction may occur if neuron death is caused by apoptosis. During major apoptotic pathways in most cells, a cascade of several caspases, which are cysteine proteases, is activated following mitochondrial dysfunction [15]. This involves the release of cytochrome c from injured mitochondria and the formation of a complex known as an apoptosome, which is comprised of pro-caspase-9, dATP, and Apaf-1. Subsequently, apoptosomes activate caspase-9 (initiator caspase), which in turn activates caspase-3 (effector caspase). This pro-apoptotic cascade eventually activates caspase-activated DNase (CAD) through the degradation of an inhibitor protein (ICAD), followed by the fragmentation of chromosomal DNA and condensation of the nucleus, which result in apoptotic cell death [16]. Pro-apoptotic Bcl-2 family proteins have been shown to be involved in these mainstream apoptotic pathways [17]. It was initially postulated that the neurons of patients with neurodegenerative diseases died as a result of conventional apoptosis; however, subsequent analysis has now revealed that other apoptotic pathways exist that are responsible for this loss of neurons. |
| Neuronal apoptosis has been shown to differ from the normal programmed cell death (ordinary apoptosis) mechanisms described above and is caused by both genetic and environmental factors. Unfolded proteins were shown to accumulate in the endoplasmic reticulum (ER) of neurons following certain stimuli. This is known as ER stress and also causes the apoptosis of neurons, which we have referred to as ER stress-mediated apoptosis. In cultured cells, ER stress is readily induced by the addition of inhibitors of protein glycosylation in the ER [18,19], inhibitors of the Ca2+-ATPase in the ER [20], or by inhibitors of glucose metabolism [21]. During ER stressmediated apoptosis in rat or mouse neurons, caspase-12 was shown to be specifically activated in the ER with condensed chromatin [19,22]. Similarly, caspase-4, also located in the ER, was also activated during ER stress-induced apoptosis in human neurons [23]. However, it is still unclear what kinds of caspases or substrates are involved in the cascade downstream of this protease in ER stress-mediated apoptosis. In addition, the types of pro-apoptotic Bcl-2 family proteins responsible for ER stress-mediated apoptosis and their mechanisms of action have been analyzed [24,25]. We observed similar apoptotic signaling mechanisms during ER stress-induced apoptosis in previous analyses from our laboratory in cultured cerebral cortical neurons and PC12 cells. Hence, two apoptotic pathways, ordinary and ER stress-induced apoptotic signaling, exist and both involve caspases and pro-apoptotic Bcl-2 family proteins, leading to destruction of the neuronal networks. |
| We have reported that fragments of caspase-12 were translocated to the nucleus due to thromboembolic infarction in the rat, suggesting that caspase-12 fragments may have a pro-apoptotic role via their transcriptional activity to induce ER stress-mediated apoptosis [26]. |
| Taken together, these findings question the importance of truncated caspase-12; however, these fragments may play an important role in the progression of neurodegenerative diseases caused by ER stress-mediated apoptosis and neuronal networks are collapsed by its mechanism of action. |
| ER Stress-mediated Apoptosis and Neurodegenerative Diseases |
| The initial causes of neurodegenerative diseases are known to be genetic and environmental factors, and the intracellular signaling mechanisms by which specific neurons die through ER stress-mediated apoptosis have gradually been elucidated. Along with these findings, specific genes responsible for neurodegenerative diseases have also been identified. In the case of Alzheimer’s disease, hippocampal neurons die and basal forebrain cholinergic neurons projecting to the hippocampus subsequently die, which leads to dysfunctions in learning, memory, and behavior. The genes responsible include amyloid precursor protein (APP) [27,28], presenilin-1 (PS1) [29], and presenilin-2 (PS2) [30]. It has been postulated that senile plaques are formed by the unnatural cleavage of APP via PS1 and PS2, which induce ER stress-mediated apoptosis. In addition, the apolipoprotein E (apoE) gene has been identified as a risk factor for Alzheimer’s disease [31]. In the case of Parkinson’s disease, dopaminergic neurons of the substantia nigra undergo ER stress-mediated apoptosis, resulting in a loss of motion control because these neurons project to the striatum. Parkin, which has been recognized as an anti-cell death protein, and Chip have also been identified as the genes responsible for this disease [32]. In Huntington’s disease, neurons of the striatum undergo ER stress-mediated apoptosis, resulting in the loss of both motion and behavioral control, with the Huntingtin gene being the causative factor [33]. Therefore, ER stress-mediated apoptosis has been recognized as the central mechanism in almost all neurodegenerative diseases. Through the above specific apoptosisinducing step, the somas bearing neurites in the brain are destroyed during neurodegenerative diseases. |
| Epigenetic Gene Expression in Neurodegenerative Disease |
| Gene expression is controlled by DNA methylation or histone modification, which is also referred to as epigenetics. When we focus on DNA methylation, gene silencing occurs if GpC islets are involved in the upstream sequence of the gene. In addition to DNA methylation, histone modifications, including methylation (mono-, di-, or tri-), phosphorylation, and acetylation, occur to control gene expression in the histone tail on a specific amino acid residue. These two mechanisms, which can be in the modified or unmodified form in genomic DNA or the histone tail, lead to epigenetic gene regulation. However, the mechanisms by which DNA and histone modifications occur have not been clearly understood. Specific gene expression has been reported to be controlled by a multiple mechanisms in patients with neurodegenerative disorders. For example, PS1 or APP for Alzheimer's disease [34,35], Parkin for autosomal recessive Parkinson’s disease [35], and Huntingtin for Huntington’s disease [36], have been discovered and the mechanisms of epigenetic gene expression have been reported. In addition, the ubiquitin-proteasome system may be involved in epigenetic gene regulation [37]. |
| Induced Expression of the Nur77 gene by an HDAC Inhibitor, K-350, for Medical Purposes |
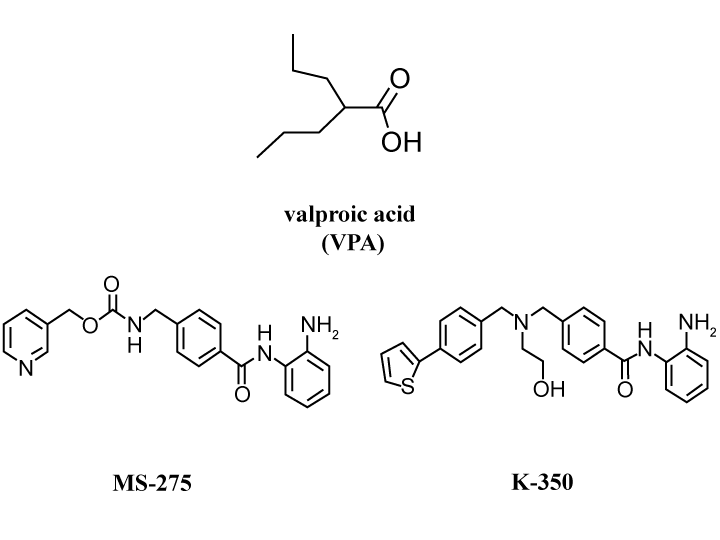
| GGene expression can be achieved by histone deacetylation (HDAC) inhibitors. They consist of eleven subtypes and are divided into at least three groups: type I, type II, and type IV. The wellknown medical compound, valproic acid (val), has been used to treat epilepsy, even though its HDAC activity was not initially expected. The specificity of the effect of val on HDAC groups is quite broad. MS-275 has been developed as an anti-cancer drug and it generally shows specificity to type II. The ED50 of val is known to be very high and controlling its dosage is necessary for the treatment of disorders including epilepsy. Although MS-275 is effective against cancer cells, normal cells are also killed. We previously reported that nur77 gene expression via the PKA-mediated signaling pathway promoted neurite outgrowth in PC12 cells [15]. Recently, we also proposed that K-350, which is one of the 2-aminobenzamide-type HDAC inhibitors, may promote neurite outgrowth with histone acetylation using PC12 cells (Figure 1). The synthesis methods that Prof. Uesato et al. developed are described in a previous report [38]. From the data regarding neurite outgrowth obtained by val and MS-275, it is suggested that the specificity is similar to that of MS-275. We also showed that the nur77 gene, which is a transcription factor gene, was up-regulated by K-350, which suggests that K-350 may be a candidate drug not only for cancer but also for neurodegenerative disorders via the promoted-expression of the nur77 gene [7]. |
| Cytoskeletal genes can also be expressed for neurite outgrowth with this drug. We found one possible target for neurodegenerative disorders; however, we have to investigate mechanisms that may involve Rho-related gene expression for the promotion of neurite outgrowth. Moreover, we have to construct an in vitro estimation system to reflect the in vivo moiety, such as a rat or mouse brain slice culture system including glial cells before an animal study for analyses of the pharmacokinetics, stability, and efficacy. |
| Moreover, a novel screening system should be constructed, such as modified high content screening (mHCS). We have already applied for a patent on a novel method of mHCS. It clearly seems that a novel method of mHCS will be critical in the creation and development of effective medicine for neuronal diseases. |
| Acknowledgements |
| This research was supported, in part, by SENRYAKU (2008-2012) and by KAKENHI (grant number 25340104) from MEXT (Ministry of Education, Culture, Sports, Science, and Technology of Japan). This research was also financially supported, in part, by the Kansai University Research Grants: Grant-in-Aid for Joint Research, 2011-2012. |
| References |
References
- Domínguez-Borràs J, Saj A, Armony JL, Vuilleumier P (2012) Emotional processing and its impact on unilateral neglect and extinction. Neuropsychologia 50: 1054-1071.
- Strata P, Scelfo B, Sacchetti B (2011) Involvement of cerebellum in emotional behavior. Physiol Res 60 Suppl 1: S39-48.
- Orpwood RD (2010) Perceptual qualia and local network behavior in the cerebral cortex. J Integr Neurosci 9: 123-152.
- Kaplan DR, Miller FD (2000) Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10: 381-391.
- Shimoke K, Sasaya H, Ikeuchi T (2011) Analysis of the role of nerve growth factor in promoting cell survival during endoplasmic reticulum stress in PC12 cells. Methods Enzymol 490: 53-70.
- Maruoka H, Sasaya H, Shimamura Y, Nakatani Y, Shimoke K, et al. (2010) Dibutyryl-cAMP up-regulates nur77 expression via histone modification during neurite outgrowth in PC12 cells. J Biochem 148: 93-101.
- Maruoka H, Hosokawa R, Hirata Y, Kawa H, Okamoto K, et al. (2012) New Orally Bioavailable 2-aminobenzamide-type Histone Deacetyase Inhibitor Promotes Neurite Outgrowth via Histone H3 Modification in PC12 cells: a Possible Therapeutic Candidate for Neuronal Diseases. J Bioengineer & Biomedical Sci S5: 001.
- Takei N, Numakawa T, Kozaki S, Sakai N, Endo Y, et al. (1998) Brain-derived neurotrophic factor induces rapid and transient release of glutamate through the non-exocytotic pathway from cortical neurons. J Biol Chem 273: 27620-27624.
- Domínguez-López S, Howell R, Gobbi G (2012) Characterization of serotonin neurotransmission in knockout mice: implications for major depression. Rev Neurosci 23: 429-443.
- Szot P (2012) Common factors among Alzheimer's disease, Parkinson's disease, and epilepsy: possible role of the noradrenergic nervous system. Epilepsia 53 Suppl 1: 61-66.
- Shimoke K, Kubo T, Numakawa T, Abiru Y, Enokido Y, et al. (1997) Involvement of phosphatidylinositol-3 kinase in prevention of low K(+)-induced apoptosis of cerebellar granule neurons. Brain Res Dev Brain Res 101: 197-206.
- Oppenheim RW (1991) Cell death during development of the nervous system. Annu Rev Neurosci 14: 453-501.
- Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110: 1389-1398.
- Rao RV, Ellerby HM, Bredesen DE (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11: 372-380.
- Adams JM, Cory S (2002) Apoptosomes: engines for caspase activation. Curr Opin Cell Biol 14: 715-720.
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, et al. (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43-50.
- Wang HG, Reed JC (1998) Mechanisms of Bcl-2 protein function. Histol Histopathol 13: 521-530.
- Shimoke K, Utsumi T, Kishi S, Nishimura M, Sasaya H, et al. (2004) Prevention of endoplasmic reticulum stress-induced cell death by brain-derived neurotrophic factor in cultured cerebral cortical neurons. Brain Res 1028: 105-111.
- Shimoke K, Amano H, Kishi S, Uchida H, Kudo M, et al. (2004) Nerve growth factor attenuates endoplasmic reticulum stress-mediated apoptosis via suppression of caspase-12 activity. J Biochem 135: 439-446.
- Shimoke K, Kishi S, Utsumi T, Shimamura Y, Sasaya H, et al. (2005) NGF-induced phosphatidylinositol 3-kinase signaling pathway prevents thapsigargin-triggered ER stress-mediated apoptosis in PC12 cells. Neurosci Lett 389: 124-128.
- Kishi S, Shimoke K, Nakatani Y, Shimada T, Okumura N, et al. (2010) Nerve growth factor attenuates 2-deoxy-d-glucose-triggered endoplasmic reticulum stress-mediated apoptosis via enhanced expression of GRP78. Neurosci Res 66: 14-21.
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, et al. (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98-103.
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, et al. (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol 165: 347-356.
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, et al. (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300: 135-139.
- Rodriguez DA, Zamorano S, Lisbona F, Rojas-Rivera D, Urra H, et al. (2012) BH3-only proteins are part of a regulatory network that control the sustained signalling of the unfolded protein response sensor IRE1α. EMBO J 31: 2322-2335.
- Shimoke K, Matsuki Y, Fukunaga K, Matsumura Y, Fujita E, et al. (2011) Appearance of nuclear-sorted caspase-12 fragments in cerebral cortical and hippocampal neurons in rats damaged by autologous blood clot embolic brain infarctions. Cell Mol Neurobiol 31: 795-802.
- van Duijn CM, Hendriks L, Cruts M, Hardy JA, Hofman A, et al. (1991) Amyloid precursor protein gene mutation in early-onset Alzheimer's disease. Lancet 337: 978.
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, et al. (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349: 704-706.
- Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, et al. (1999) Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol 1: 479-485.
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, et al. (1995) Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 269: 973-977.
- Weiner MF, Vega G, Risser RC, Honig LS, Cullum CM, et al. (1999) Apolipoprotein E epsilon 4, other risk factors, and course of Alzheimer's disease. Biol Psychiatry 45: 633-638.
- Kitada T, Asakawa S, Matsumine H, Hattori N, Shimura H, et al. (2000) Progress in the clinical and molecular genetics of familial parkinsonism. Neurogenetics 2: 207-218.
- Rubinsztein DC, Barton DE, Davison BC, Ferguson-Smith MA (1993) Analysis of the huntingtin gene reveals a trinucleotide-length polymorphism in the region of the gene that contains two CCG-rich stretches and a correlation between decreased age of onset of Huntington's disease and CAG repeat number. Hum Mol Genet 2: 1713-1715.
- Francis YI, Fà M, Ashraf H, Zhang H, Staniszewski A, et al. (2009) Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer's disease. J Alzheimers Dis 18: 131-139.
- Kwok JB (2010) Role of epigenetics in Alzheimer's and Parkinson's disease. Epigenomics 2: 671-682.
- Sadri-Vakili G, Cha JH (2006) Mechanisms of disease: Histone modifications in Huntington's disease. Nat Clin Pract Neurol 2: 330-338.
- Love KR, Catic A, Schlieker C, Ploegh HL (2007) Mechanisms, biology and inhibitors of deubiquitinating enzymes. Nat Chem Biol 3: 697-705.
- Kiyokawa S, Hirata Y, Nagaoka Y, Shibano M, Taniguchi M, et al. (2010) New orally bioavailable 2-aminobenzamide-type histone deacetylase inhibitor possessing a (2-hydroxyethyl)(4-(thiophen-2-yl)benzyl)amino group. Bioorg Med Chem 18: 3925-3933.
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 13598
- [From(publication date):
specialissue-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9065
- PDF downloads : 4533
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
