Histamine: The Missing Link in the Pathogenesis of Some Brain Disorders
Received: 18-Jan-2016 / Accepted Date: 01-Mar-2016 / Published Date: 07-Mar-2016
5267Historical Background
Histamine (HA) is a rebellious neurotransmitter with pleiotropic activity. Among the classics (adrenaline, noradrenaline, acetylcholine, serotonin), HA has been the last to reach a status of recognition as a neurotransmitter with defined neuromodulatory functions. According to data obtained from the PubMed database, during the period 1918-2016, 83,801 papers on HA have been published (134,040 papers on adrenaline (1914-2016); 116,182 papers on noradrenaline (1946-2016); 131,361 papers on serotonin (1946-2016); and 86,369 papers on acetylcholine (1934-2016). These numbers reflect the weak consideration of the scientific community with respect to HA as compared with the other classical neurotransmitters.
HA (β-aminoethylimidazole) was synthesized by Windaus and Vogt in 1907. In 1910, Dale and Laidlaw discovered the role of HA in anaphylactic shock; in 1927, Best and co-workers found HA (also known at that time as Lewis’ Substance H) in several tissues. Between 1948 and 1955, Folkow and Furghgott postulated the existence of two different HA receptors. In 1966, Ash and Schild identified HA H1 receptors, and in 1972 Black and colleagues did the same with H2 receptors. In 1955, Pepys postulated that HA could affect immune response. For one decade (1955-1965) the interest in HA was focused on allergy, bronco-pulmonary function, and gastric secretion (Sir James White Black was awarded the Nobel Prize for Medicine in 1988 for work leading to the development of cimetidine). In the early 1960’s, Carlini and Green studied the subcellular distribution of HA in the brain, and in 1973 Baudry and co-workers studied the distribution of histidine decarboxylase in neural tissue. It was in 1970 when Douglas and Green proposed that HA was a neurotransmitter, and the idea was validated by Snyder and Taylor in 1972, by Browstein in 1974, and by Schwartz in 1975. This was the beginning of a new era in HA research, and several groups started an exciting race to map HA in the brain [1]. In 1982, Wilcox and Seybold demonstrated the presence of intraneuronal HA using anti-HA antibodies; in 1982-1984, Wada’s and Watanabe’s group in Japan generated specific histidine decarboxylase antibodies [2] and were able, together with Tohyama’s group at Osaka University Medical School, to map brain HA using histidine decarboxylase as a marker [3,4]. At the same time, Steinbusch and Mulder in The Netherlands [5] and Panula et al. [6], in the USA, also mapped HA in the posterior hypothalamus by using specific anti-HA antibodies [1]. Since then, brain HA became a fashion for a decade and thereafter brain HA research declined again to a non-enthusiastic level. In 1985, Ganellin and Schwartz [7] published a book, as a tribute to Heinz Schild, with contributions from the most relevant authors of the time, including Haas, Pollard, Hough, Arrang, Saeki, Green, Weinreich, Watanabe, and Richelson [7]. Another important volume was published by Watanabe and Wada in 1991 with chapters by Fukui, Yamatodani, Schwartz, Tohyama, Panula, Steinbusch, Nagy, Tuomisto, Haas, Green, Kupfermann, Cacabelos, Richelson, Sakata, Takeda,Philippu, Saeki, Nowak, Wada and Watanabe, reporting the most relevant findings in brain HA function [8].
Brain Histamine
HA is in a privileged position to display multiple pleiotropic functions in peripheral tissues and in the CNS. HA is synthesized by histidine decarboxylase (HDC; EC 4.1.1.22) from L-histidine in different cellular compartments (mast cells, basophils, glial cells, endothelial cells, neurons). HA is metabolized by histamine N-methyltransferase (HNMT) which inactivates HA, transferring a methyl group from S-adenosyl-l-methionine to HA. HA acts through different types of HA receptors (H1R, H2R, H3R, H4R). H1R and H2R are widely distributed in most cells and tissues; however, H3Rs are mainly expressed in the CNS, and H4Rs are expressed in hematopoietic cells, indicating their function in neurotransmission and immunomodulation, respectively. H3R is a recognized drug target for neuronal diseases, such as cognitive impairment, schizophrenia, sleep/wake disorders, epilepsy and neuropathic pain [9]. The presence of H4Rs in the CNS is still a controversial matter [10,11].
The organization of the brain histaminergic system shows a strategic disposition, with HA neurons located in the posterior hypothalamus, from where ascending pathways (to the anterior hypothalamus, limbic structures (hippocampus), neocortex, and subcortical structures) and descending pathways (to the brain stem and spinal cord) are organized [3-6]. Recent studies by Moriwaki et al. [12] distinguish five neuronal clusters (E1-E5) in the hypothalamus at the postnatal stage. Neuronal HA clusters are localized in the ventrolateral part of the most posterior hypothalamus (E1), ventrolateral part of the posterior hypothalamus (E2), ventromedial part from the medial to the posterior hypothalamus (E3), periventricular part from the anterior to the medial hypothalamus (E4), and diffusely extended part of the more dorsal and almost entire hypothalamus (E5).
Mast Cells
Brain HA is distributed in several compartments (neuronal, glial, endothelial, mast cells). Mast cells are connective tissue cells, discovered by Ehrlich in 1887, which are rich in metachromatic granules containing HA, heparin and serotonin. Riley, West and coworkers were the first to establish an association between mast cells and HA in 1953, and Benditt and colleagues showed, in 1955, that mast cells also contained 5-hydroxytryptamine (5-HT; serotonin). In 1953, Riley demonstrated that injection of HA releasers was followed by damage to mast cells. Examples of HA releasers include compound 48/80, mast cell-degranulating peptide (MCD), polylysine, polymyxin B, dextran, phosphatidylserine, mellitin, F-Met-tripeptides, C5a/C3a anaphylotoxins, C4a, calcium ionophore A23187, and many cytokines and growth factors. Many, if not all, mast cells are innervated by varicose axons in different body structures, and even an “axon reflex” pathway involving mast cells was proposed in the skin by Kiernan in 1965 [13]. Mast cell secretory granules contain a great variety of mediators, such as histamine, serotonin, tryptase, chymase, and antizyme inhibitor 2 (AZIN2), an activator of polyamine biosynthesis whose catalyzing enzyme is ornithine decarboxylase [14]. Mast cells are resident in the brain and contain numerous mediators, including neurotransmitters, cytokines, and chemokines, that are released in response to a variety of natural and pharmacological triggers. Brain mast cells are of two types, namely, metachromatic type I cells and normochromatic type II cells, or neurolipomastocytoid cells. In mice devoid of mast cells (W/Wv mice), the administration of α- fluoromethyhistidine (FMH), a suicide inhibitor of HDC, reduces the concentration of brain HA to nearly zero, suggesting that approximately 50% of the content of HA in the CNS belongs to the non-neuronal pool [15,16]. The presence of mast cells in meninges and perivascular locations on the brain side of the BBB, especially in thalamic and hippocampal regions, may indicate that this cell type is relevant in neurovascular responses. The number of mast cells in the brain fluctuates with stress and various behavioral and endocrine states. Mast cells migrate to critical regions influencing neuronal activity, repairing tissue damage or aggravating inflammatory processes within the CNS. These properties suggest that mast cells are poised to influence neural systems underlying behavior. Using genetic and pharmacological loss-of-function models, Nautiyal et al. [17] performed a behavioral screen for arousal responses including emotionality, locomotor, and sensory components and found that mast cell-deficient KitW-sh/W-sh (sash-/-) mice had a greater anxiety-like phenotype than WT and heterozygote littermate control animals in the open field arena and elevated plus maze. Blockade of brain, but not peripheral, mast cell activation increased anxiety-like behavior. Brain mast cells might be implicated in the modulation of anxiety-like behavior and provide evidence for the behavioral importance of neuroimmune links [17].
Microglia
Microglia can constitutively express HA receptors (H1R, H2R, H3R, H4R), and the expression of H1R and H4R can be selectively upregulated by HA in microglial cells in a dose-dependent manner. HA can also stimulate microglia activation and production of proinflammatory cytokines. HA induces TNF-α and IL-6 release from activated microglia via H1R and H4R-MAPK and the PI3K/AKT-NFkappa B signaling pathway [18]. Microglia express H2R, H3R, histidine decarboxylase, and histamine N-methyltransferase. Both forskolin-induced cAMP accumulation and ATP-induced intracellular Ca2+ transients are reduced by the H3R agonist imetit but not by the H2R agonist amthamine. H3Rs can regulate various microglial functions. HA and imetit inhibit microglial chemotaxis, phagocytosis, and lipopolysaccharide (LPS)-induced cytokine production [19]. HA and substance P can trigger microglial activation and release of proinflammatory factors from microglia, thus contributing to the development of microglia-mediated inflammation in the brain [20].
Brain Functions
The interplay of brain HA in neurons, endothelial cells, glia, and mast cells is fundamental for the regulation of diverse physiological functions (neuroendocrine system, circadian rhythms, sleepwakefulness cycle, psychomotor activity, mood, learning, cognition, appetite and eating behavior); and alterations in multicompartment brain HA are involved in several pathological conditions [21-24].
HA is implicated in the control of arousal state, exerting a potent phase-shifting effect on the circadian clock in the suprachiasmatic nucleus (SCN). To reset the circadian clock, HA increases [Ca2+]i in SCN neurons by activating CaV 1.3 channels through H1Rs, and secondarily by causing Ca2+-induced Ca2+ release from RyR-mediated internal stores [25]. Light has direct effects on sleep and wakefulness causing arousal in diurnal animals and sleep in nocturnal animals. Histaminergic neurotransmission attenuates the light-induced sleep response during the dark period [26]. Using knockout (KO) mice lacking HDC, Parmentier et al. [27] demonstrated the importance of histaminergic neurons in maintaining wakefulness under behavioral challenges. H1-receptor KO (H1-/-) mice share several characteristics with HDC KO mice, including a decrease in wakefulness after lightsoff despite its normal baseline daily amount, a decreased EEG slow wave sleep (SWS)/W power ratio, and inability to maintain wakefulness in response to behavioral challenges. Most of these effects are mediated by central H1Rs [27]. Yu et al. [28] reported that GABA and HA are involved in the mechanisms of wakefulness. HA neurons in the tuberomammilary nucleus (TMN) of the hypothalamus form a widely projecting, wake-active network that sustains arousal. HA neurons contain GABA. Selective siRNA knockdown of the vesicular GABA transporter (vgat, SLC32A1) in HA neurons produces hyperactive mice with an exceptional amount of sustained wakefulness. Ablation of the vgat gene throughout the TMN further sharpens this phenotype. Optogenetic stimulation in the caudateputamen and neocortex of HA axonal projections from the TMN evokes tonic, extrasynaptic GABAA receptor Cl- currents onto medium spiny neurons and pyramidal neurons. These currents are abolished following vgat gene removal from the TMN area. Thus, wake-active HA neurons may generate a paracrine GABAergic signal that serves to provide a brake on over activation of HA, and to increase the precision of neocortical processing. Yu et al. [29] studied the contribution of one putative local clock in mouse histaminergic neurons in the tuberomamillary nucleus to the regulation of the sleepwake cycle. Histaminergic neurons are silent during sleep, and start firing after wake onset. HA enhances wakefulness. HDC gene expression varies with time of day. Deletion of the Bmal1 (Arntl/ Mop3) clock gene from HA cells removes this variation, producing higher HDC expression and brain HA levels during the day. The consequences include more fragmented sleep, prolonged wakefulness at night, shallower sleep depth (lower nonrapid eye movement (NREM) δ power), increased NREM-to-REM transitions, hindered recovery sleep after sleep deprivation, and impaired memory. Removing BMAL1 from histaminergic neurons does not, however, affect circadian rhythms.
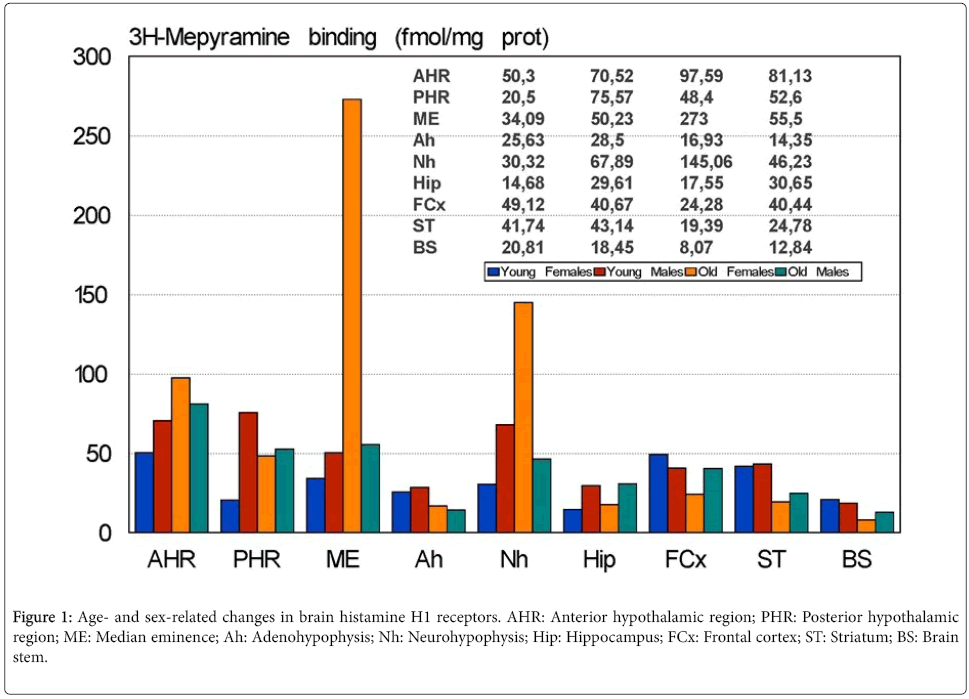
There are important age- and sex-related changes in the concentration of HA and H1Rs in different regions of the CNS [30] (Figure 1). Dramatic changes in HA and H1Rs have been reported after surgical manipulation of the neuroendocrine system (e.g. castration, adrenalectomy), clearly indicating the relevant effect that changes in the hypothalamic-pituitary-gonadal axis and hypothalamicpituitary- adrenergic axis exert on brain HA [15,30]. Similarly, direct interactions between the somatotropinergic system (GRH/SS-GHIGF1) and HA [31] and the vasopressinergic system and HA have been extensively demonstrated [32]. Interaction of HA with dopamine, noradrenaline, serotonin and other neurotransmitters may contribute to the regulation of different neuroendocrine functions [33].
HA is a powerful regulator of neuroimmune function [13]. LHistidine and HA induce a time- and dose-dependent decrease in hypothalamic IL-1β, and this effect is not affected by mepyramine (H1R antagonist), famotidine (H2R antagonist) or thioperamide (H3R antagonist) [34]. The abolishment of neuronal HA by α- fluoromethylhistidine, a suicide inhibitor of HDC, causes a significant decrease in the hypothalamic concentration of TNF-α [35].
Brain Pathology
Important changes in the peripheral and central levels of HA occur in patients with different CNS disorders. HA has been studied in anxiety, depression, psychosis, stroke, dementia (Alzheimer’s disease, vascular dementia), Parkinson’s disease, attention-deficit/hyperactivity disorder, migraine, sleep disorders, epilepsy, mental retardation, cerebrovascular encephalopathy, multiple sclerosis, brain tumors, cranial nerve neuropathies, and post-traumatic brain injury [22,36]. Although the peripheral levels of HA exhibit a great variability, high levels of HA are currently seen in cases with psychosis, stroke, attention-deficit/hyperactivity disorder, Parkinson’s disease, and brain tumors; however, HA changes tend to be genotype-related and the quantification of peripheral HA in individual cases is of poor value, except in those patients with a clear immunologic/allergic condition [36].
Altered HA neurotransmission has been reported in an animal model of Lesch-Nyhan syndrome, an X-linked chromosomal disorder with congenital deficiency of hypoxanthine-guanine phosphoribosyltransferase. These animals show a decrease in dopamine and 3-methoxytyramine concentrations in the brain, together with reduced 1-methylhistamine and 1-methylimidazole-4- acetic acid concentrations in the brain and medulla [37]. Heidari et al. [38] identified two homozygous HNMT alterations, p.Gly60Asp and p.Leu208Pro, in patients affected with nonsyndromic autosomal recessive intellectual disability from two unrelated consanguineous families of Turkish and Kurdish ancestry, respectively. p.Gly60Asp disrupts the enzymatic activity of the protein, and p.Leu208Pro reduces protein stability, resulting in decreased HA inactivation.
Brain HA alterations have also been reported in other CNS and neurometabolic disorders such as anxiety [39], amyotrophic lateral sclerosis [40], multiple sclerosis [41], Parkinson’s disease [42], LDOPA- induced dyskinesia [43], migraine [44], psychosis [42], Tourette syndrome [45,46], attention-deficit/hyperactivity disorder [47], narcolepsy [48], eating disorders, obesity and metabolic syndrome [49]. Severe head trauma is associated with a marked loss (41%) of HA neurons. Reduced histamine signaling may contribute to increased sleep need, and therapies that enhance histaminergic tone may improve arousal after head trauma or other conditions [50]. Furthermore, HA H1 antagonists have hypnotic, appetite-promoting, and sedative side effects, and second-generation antipsychotics (olanzapine, quetiapine) have potent antagonistic effects on H1Rs [51]. Studies in animal models (HDC-deficient mice, α-FMH-treated mice) show that selective serotonin reuptake inhibitors require the integrity of the brain histamine system to display their pharmacodynamic effects [52]. Results of clinical trials with H3 antagonists such as ABT-288 [53] or GSK239512 have recently been reported in dementia [54]. GSK239512, a brain-penetrant H3R antagonist, was also investigated as a potential cognitive enhancer in patients with schizophrenia [55]. However, no breakthroughs have been achieved with novel antihistaminics in brain disorders.
H3Rs are expressed in various tumors and correlated with malignancy and tumor proliferation. H3R mRNA and protein levels are up-regulated in glioblastoma (GBM) and glioma cell lines compared to normal brain tissue and astrocytes. In the U87MG cell line, inhibition of H3R by siRNA or the antagonist ciproxifan suppresses proliferation, invasiveness, and the expression of epithelialmesenchymal transition (EMT) activators. Inhibition of H3R by siRNA or ciproxifan inactivates the PI3K/Akt and MEK/ERK signaling pathways, while inhibition of Akt or ERK activity with antagonists or siRNAs suppresses H3R agonist (R)-(α)-(-)- methylhistamine dihydrobromide-mediated invasion and reorganization of cadherinhousehold. These data reported by Lin et al. [56] suggest that overexpression of H3Rs is associated with glioma progression and that inhibition of H3Rs leads to suppressed invasion and EMT of GBM by inactivating the PI3K/Akt and MEK/ERK pathways in gliomas.
Alzheimer’s Disease
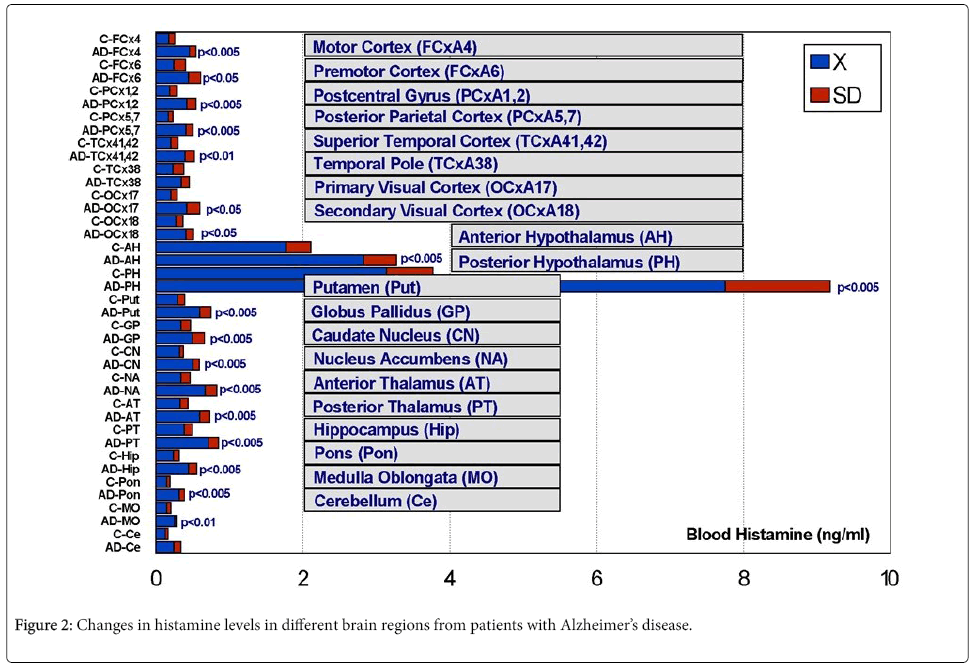
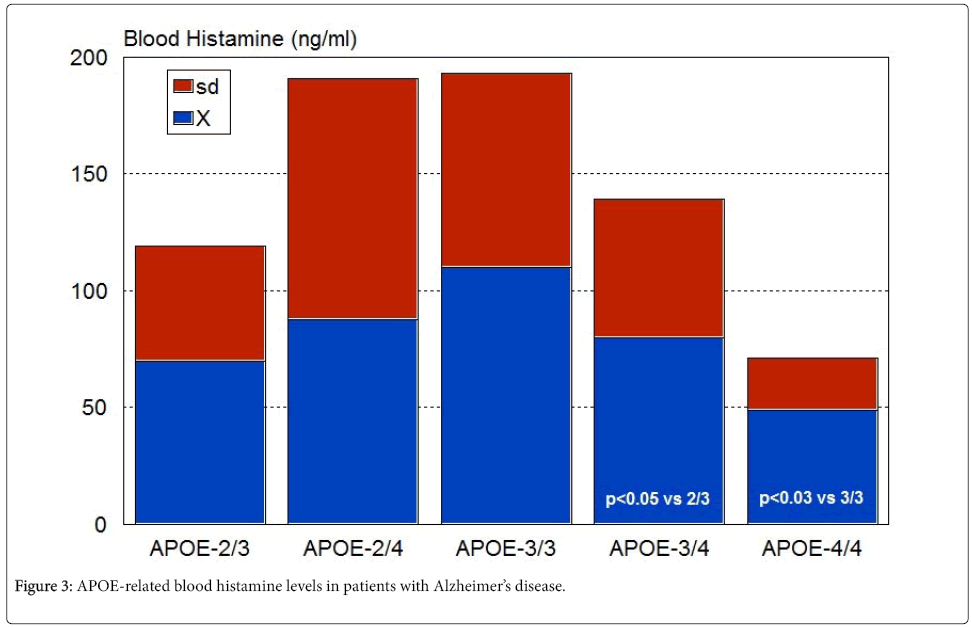
In 1991, a profound histaminergic dysregulation was observed in patients with Alzheimer’s disease (AD), where HA levels are significantly increased in most CNS regions [57] (Figure 2). HA, TNF- α and IL-1β regulate each other in the hypothalamus. Changes in HA, TNF-α [58], and IL-1β [58,59] in AD correlate with cerebrovascular dysfunction and cognitive decline [21,60]. Furthermore, improvement in cognitive function with neuroprotectants (e.g. CDL-choline) [61-63] or vegetal neurotrophins (e.g. anapsos) [64,65] can reverse alterations in HA, TNF-α and IL-1β levels in AD and in animal models of dementia [13,34,64-67]. AD-related neurodegeneration exhibits a pathological phenotype compatible with a reactive neuroinflammatory process in which HA, TNF-α and IL-1β, among many other immune effectors, are involved. There is also a correlation between microglia activation and APOE genotypes [68,69]; and peripheral HA levels are also APOE-related, with the lowest levels present in patients harboring the APOE-4 allele [70] (Figure 3). An association between APOE-4 and increased expression of CD95 on T cells has also been reported, suggesting that hyperexpression of Fas mRNA and surface Fas receptor on CD45RO+ T lymphocytes may explain the occurrence of inflammatory cellular infiltrates in AD brain tissue [71]. Recent data also indicate that mast cells are close to amyloid plaques in AD. Mast cells drastically accumulate in cortical and hippocampal regions prior to the consolidation of amyloid plaques in brain slices of APPswe/ PS1dE9 mice, a murine model of AD. This may explain the high levels of HA found in AD brains, probably indicating that mast cells act as early sensors of amyloid peptide and recruit other cells to the neuroinflammatory response, thus playing a critical role in the onset and progression of AD [72-74].
This brief summary on brain HA data illustrates the potential role of HA as a fundamental player in AD-related neuroinflammation and in other neuropsychiatric disorders [24,36].
Neuroimmunology
Over the past 30 years, growing evidence has revealed the dense interplay of neurotransmitters, neuropeptides, hormones, and immune effectors in the regulation of complex behaviors. Since the pioneering work of George Freeman Solomon, and the excellent synopses of papers collected by Cotman et al. [75], Goetzl and Spector [76], Ader et al. [77], Baxter and Ross [78], Husband [79], and Thèze [80], among many others, abundant literature in the field reflects the potential role of particular neuroimmune events in the pathogenesis and phenotypic expression of complex disorders of the CNS [36].
Diverse data provide support to a multidimensional crosstalk among neural, endocrine, and immune signals probably contributing to global homeostasis, constant surveillance to maintain control against exogenous and/or endogenous stressors, brain maturation and development, neuroprotection, and fine-tuning of brain functions associated with higher activities of the CNS. Neuroimmune dysfunction may be part of the pathogenesis of different CNS disorders, including mental disorders (schizophrenia, depression, anxiety, post-traumatic stress disorder), neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, Prion disease), brain infections, stroke, brain tumors, and demyelinating disorders. It is likely that the neuroimmune system acts as a regulatory, defense system which reacts against noxious stimuli that endanger brain function stability and optimum performance.
In this context, neuroimmune activation in CNS pathology might be a two-sided reactive phenomenon: (i) neuroprotective, when the stimulus activating the neuroimmune cascade is able to neutralize tissue damage and/or brain dysfunction; and (ii) neurotoxic, when the reactive neuroinflammatory event is persistent and becomes an autoaggression which magnifies the damage. This Sword of Damocles (dual role) hanging over the CNS and other peripheral tissues under central control must be exquisitely balanced to avoid dangerous decompensation (dominance of neurotoxic effects over neuroprotective effects). This homeostatic equilibrium can be maintained by the interplay of different cytokines, chemokines, neurotransmitters, neuropeptides, neurohormones, and pleiotropic substances such as histamine. This biogenic amine, acting on different receptors or emanating from different sources (neuronal histamine vs. non-neuronal histamine) can exert dual effects (neurotrophic vs. neurotoxic) on neuronal targets inducing either protection or damage. Therefore, a revival of scientific interest for brain HA would probably be highly beneficial for the good health of neuroscience and neuroimmunology, and also for the better understanding of the pathogenesis of several CNS disorders.
References
- Cacabelos R (1980) Histaminergic modulation of the neuroendocrine system. Santiago de Compostela University Medical School, Santiago de Compostela.
- Taguchi Y, Watanabe T, Kubota H, Hayashi H, Wada H (1984) Purification of histidine decarboxylase from the liver of fetal rats and its immunochemical and immunohistochemical characterization. J BiolChem 259: 5214-5221.
- Watanabe T, Taguchi Y, Hayashi H, Wada H, Kubota H, et al. (1983) Evidence for the presence of histaminergic nervous system in the brain: an immunochemical analysis. NeurosciLett 39: 249-254.
- Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, et al. (1984) Distribution of the histaminergic neuron system in the central nervous system of rats: A fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res 295: 13-25.
- Steinbusch HWM, Mulder AH (1984) Immunohistochemical localization of histamine in neurons and mast cells in the rat brain. In: Handbook of Chemical Neuroanatomypp: 126-140.
- Panula P, Yang HY, Costa E (1984) Histamine-containing neurons in the rat hypothalamus. ProcNatlAcadSci U S A 81: 2572-2576.
- Ganellin CR, Schawartz JC (1985) Frontiers in Histamine Research. A tribute to Heinz Schild. Advances in Biosciences Vol 51, Pergamon Press, England.
- Watanabe T, Wada H (1991) Histaminergic neurons: morphology and function. CRC Press, Boca Raton.
- Tiligada E, Zampeli E, Sander K, Stark H (2009) Histamine H3 and H4 receptors as novel drug targets. Expert OpinInvestig Drugs 18: 1519-1531.
- Schneider EH, Seifert R (2015) The histamine H4-receptor and the central and peripheral nervous system: A critical analysis of the literature. Neuropharmacology 15: 185-189.
- Feliszek M, Speckmann V, Schacht D, von Lehe M, Stark H, et al. (2015) A search for functional histamine H4 receptors in the human, guinea pig and mouse brain. NaunynSchmiedebergs Arch Pharmacol 388: 11-17.
- Moriwaki C, Chiba S, Wei H, Aosa T, Kitamura H, et al. (2015) Distribution of histaminergic neuronal cluster in the rat and mouse hypothalamus. J ChemNeuroanat 68: 1-13.
- Cacabelos R, Fernández-Novoa L, Franco-Maside A, Ãlvarez XA (1992) Neuroimmune function of brain histamine: implications for neurotrophic activity and neurotoxicity. Ann Psychiat 3: 147-200.
- Kanerva K, Lappalainen J, Mäkitie LT, Virolainen S, Kovanen PT, et al. (2009) Expression of antizyme inhibitor 2 in mast cells and role of polyamines as selective regulators of serotonin secretion. PLoS One 4: e6858.
- Cacabelos R, Yamatodani A, Fukui H, Watanabe T, Hariguchi S, et al. (1985) Nature of histaminergicneuromodulation of the corticotropinergic system. Biogenic Amines 3: 9-19.
- Cacabelos R (1990) Histaminergic system: neuroendocrine function of brain histamine. Methods Find ExpClinPharmacol 12: 341-376.
- Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R (2008) Brain mast cells link the immune system to anxiety-like behavior. ProcNatlAcadSci U S A 105: 18053-18057.
- Dong H, Zhang W, Zeng X, Hu G, Zhang H, et al. (2014) Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. MolNeurobiol 49: 1487-1500.
- Iida T, Yoshikawa T, Matsuzawa T, Naganuma F, Nakamura T, et al. (2015) Histamine H3 receptor in primary mouse microglia inhibits chemotaxis, phagocytosis, and cytokine secretion. Glia 63: 1213-1225.
- Zhu J, Qu C, Lu X, Zhang S (2014) Activation of microglia by histamine and substance P. Cell PhysiolBiochem 34: 768-780.
- Fernández-Novoa L, Cacabelos R (2001) Histamine function in brain disorders. Behav Brain Res 124: 213-233.
- Baronio D, Gonchoroski T, Castro K, Zanatta G, Gottfried C, et al. (2014) Histaminergic system in brain disorders: lessons from the translational approach and future perspectives. Ann Gen Psychiatry 13: 34.
- Afrin LB, Pöhlau D, Raithel M, Haenisch B, Dumoulin FL, et al. (2015) Mast cell activation disease: An underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain BehavImmun 50: 314-321.
- Shan L, Bao AM, Swaab DF (2015) The human histaminergic system in neuropsychiatric disorders. Trends Neurosci 38: 167-177.
- Kim YS, Kim YB, Kim WB, Yoon BE (2015) Histamine resets the circadian clock in the suprachiasmatic nucleus through the H1R-CaV 1.3-RyR pathway in the mouse. Eur J Neurosci 42: 2467-2477.
- Muindi F, Colas D, Ikeme J, Ruby NF, Heller HC (2015) Loss of Melanopsin Photoreception and Antagonism of the Histamine H3 Receptor by Ciproxifan Inhibit Light-Induced Sleep in Mice. PLoS One 10: e0128175.
- Parmentier R, Zhao Y, Perier M, Akaoka H (2015) Role of Histamine H1-receptor on Behavioral States and Wake Maintenance during Deficiency of a Brain Activating System: a Study using a Knockout Mouse Model. Neuropharmacology 15: 30210.
- Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, et al. (2015) Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron 87: 164-178.
- Yu X, Zecharia A, Zhang Z, Yang Q, Yustos R, et al. (2014) Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. CurrBiol 24: 2838-2844.
- Cacabelos R, Yamatodani A, Fukui H, Takeda M, Nishimura T, et al. (1986) Age- and sex-related changes in histamine and H-1 receptors in the central nervous system following a long-term castration. Bull JpnNeurochemSoc 25: 271-273.
- Cacabelos R, Yamatodani A, Fukui H, Niigawa H, Miyake A, et al. (1987) Time- and dose-dependent responses of brain histamine to intracerebroventricular and intraperitoneal administrations of growth hormone-releasing factor (GRF1-44). Tohoku J Exp Med 151: 429-442.
- Cacabelos R, Yamatodani A, Niigawa H, Hariguchi S, Nishimura T, et al. (1987) Histaminergicneuromodulation of the release of vasopressin. Neuroendocrinology 45: 368-375.
- Flik G, Folgering JH, Cremers TI, Westerink BH, Dremencov E (2015) Interaction Between Brain Histamine and Serotonin, Norepinephrine, and Dopamine Systems: In Vivo Microdialysis and Electrophysiology Study. J MolNeurosci 56: 320-328.
- Cacabelos R, Ãlvarez XA, Franco A, Fernández-Novoa L (1993) Dose-and time-dependent effects of histamine on hypothalamic levels of interleukin-1ß in rats. Agents Actions 38: 260-262.
- Fernández-Novoa L, Franco-Maside A, Alvarez XA, Cacabelos R (1995) Effects of histamine and alpha-fluoromethylhistidine on brain tumor necrosis factor levels in rats. Inflamm Res 44: 55-57.
- Cacabelos R, Torrellas C, Fernández-Novoa L, Aliev G (2016) Neuroimmune Crosstalk in CNS Disorders: The Histamine Connection. Curr Pharm Des 22: 819-848.
- Tschirner SK, Gutzki F, Kaever V, Seifert R, Schneider EH (2015) Altered histamine neurotransmission in HPRT-deficient mice. NeurosciLett 609: 74-80.
- Heidari A, Tongsook C, Najafipour R, Musante L, Vasli N, et al. (2015) Mutations in the histamine N-methyltransferase gene, HNMT, are associated with nonsyndromic autosomal recessive intellectual disability. Hum Mol Genet 24: 5697-5710.
- Yeung M, Treit D, Dickson CT (2015) Ventral hippocampal histamine increases the frequency of evoked theta rhythm but produces anxiolytic-like effects in the elevated plus maze. Neuropharmacology 15: 30116-30117.
- Volonté C, Parisi C, Apolloni S (2015) New kid on the block: does histamine get along with inflammation in amyotrophic lateral sclerosis? CNS NeurolDisord Drug Targets 14: 677-686.
- Kritas SK, Saggini A, Cerulli G, Caraffa A, Antinolfi P, et al. (2014) Impact of mast cells on multiple sclerosis: inhibitory effect of natalizumab. Int J ImmunopatholPharmacol 27: 331-335.
- Yang X, Liu C, Zhang J, Han H, Wang X, et al. (2015) Association of histamine N-methyltransferase Thr105Ile polymorphism with Parkinson's disease and schizophrenia in Han Chinese: a case-control study. PLoS One 10: e0119692.
- Lim SA, Xia R, Ding Y, Won L, Ray WJ, et al. (2015) Enhanced histamine H2 excitation of striatal cholinergic interneurons in L-DOPA-induced dyskinesia. Neurobiol Dis 76: 67-76.
- Noseda R, Kainz V, Borsook D, Burstein R (2014) Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One 9: e103929.
- Richards CA, Black KJ (1000) Tourette Syndrome research highlights 2014. Version 2 .
- Rapanelli M, Frick LR, Pogorelov V, Ota KT, Abbasi E, et al. (2014) Dysregulated intracellular signaling in the striatum in a pathophysiologically grounded model of Tourette syndrome. EurNeuropsychopharmacol 24: 1896-1906.
- Hutson PH, Heins MS, Folgering JH (2015) Effects of lisdexamfetamine alone and in combination with s-citalopram on acetylcholine and histamine efflux in the rat pre-frontal cortex and ventral hippocampus. J Neurochem 134: 693-703.
- Shan L, Dauvilliers Y, Siegel JM (2015) Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol 11: 401-413.
- Provensi G, Blandina P, Passani MB (2015) The histaminergic system as a target for the prevention of obesity and metabolic syndrome. Neuropharmacology .
- Valko PO, Gavrilov YV, Yamamoto M, Finn K, Reddy H, et al. (2015) Damage to histaminergictuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann Neurol 77: 177-182.
- Sato H, Ito C, Hiraoka K, Tashiro M, Shibuya K, et al. (2015) Histamine H1 receptor occupancy by the new-generation antipsychotics olanzapine and quetiapine: a positron emission tomography study in healthy volunteers. Psychopharmacology (Berl) 232: 3497-3505.
- Munari L, Provensi G, Passani MB, Galeotti N, Cassano T, et al. (2015) Brain Histamine Is Crucial for Selective Serotonin Reuptake Inhibitors' Behavioral and Neurochemical Effects. Int J Neuropsychopharmacol 18: pyv045.
- Haig GM, Pritchett Y, Meier A, Othman AA, Hall C, et al. (2014) A randomized study of H3 antagonist ABT-288 in mild-to-moderate Alzheimer's dementia. J Alzheimers Dis 42: 959-971.
- Grove RA, Harrington CM, Mahler A, Beresford I, Maruff P, et al. (2014) A randomized, double-blind, placebo-controlled, 16-week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild-to-moderate Alzheimer's disease. Curr Alzheimer Res 11: 47-58.
- Cacabelos R, Yamatodani A, Niigawa H, Hariguchi S, Tada K, et al. (1989) Brain histamine in Alzheimer's disease. Methods Find ExpClinPharmacol 11: 353-360.
- Jarskog LF, Lowy MT, Grove RA, Keefe RS, Horrigan JP, et al. (2015) A Phase II study of a histamine H3 receptor antagonist GSK239512 for cognitive impairment in stable schizophrenia subjects on antipsychotic therapy. Schizophr Res 164: 136-142.
- Lin JJ, Zhao TZ, Cai WK, Yang YX, Sun C, et al. (2015) Inhibition of histamine receptor 3 suppresses glioblastoma tumor growth, invasion, and epithelial-to-mesenchymal transition. Oncotarget 6: 17107-17120.
- Cacabelos R, Alvarez XA, Fernández-Novoa L, Franco A, Mangues R, et al. (1994) Brain interleukin-1 beta in Alzheimer's disease and vascular dementia. Methods Find ExpClinPharmacol 16: 141-151.
- Cacabelos R, Franco-Maside A, Alvarez XA (1991) Interleukin-1 in Alzheimer's disease and multi-infarct dementia: neuropsychological correlations. Methods Find ExpClinPharmacol 13: 703-708.
- Alvarez XA, Franco A, Fernández-Novoa L, Cacabelos R (1996) Blood levels of histamine, IL-1 beta, and TNF-alpha in patients with mild to moderate Alzheimer disease. MolChemNeuropathol 29: 237-252.
- Cacabelos R, Ãlvarez XA, Franco A, Fernández-Novoa L, Caamaño J, et al. (1992) Therapeutic effects of CDP- choline in Alzheimer´s disease and multi-infarct dementia: psychometric assessment and immune function. Ann Psychiat 3: 233-45.
- Cacabelos R, Alvarez XA, Franco-Maside A, Fernández-Novoa L, Caamaño J (1993) Effect of CDP-choline on cognition and immune function in Alzheimer's disease and multi-infarct dementia. Ann N Y AcadSci 695: 321-323.
- Fernández-Novoa L, Alvarez XA, Franco-Maside A, Caamaño J, Cacabelos R (1994) CDP-choline-induced blood histamine changes in Alzheimer's disease. Methods Find ExpClinPharmacol 16: 279-284.
- Ãlvarez XA, Franco A, Fernández-Novoa L, Cacabelos R (1992) Effects of anapsos on behavior and brain cytokines in rats. Ann Psychiat 3: 329-41.
- Ãlvarez XA, Miguel-Hidalgo JJ, Fernández-Novoa L, DÃaz J, Sempere JM, et al. (1997) Anapsos: Neuroimmunotrophic treatment in Alzheimer´s disease and neurodegenerative disorders. CNS Drug Rev 3: 181-206.
- Fernández-Novoa L, Ãlvarez XA, Franco A, Caamaño J, Cacabelos R (1993) Histamine-induced interleukin-1 changes in the rat hypothalamus. Meth Find ExpClinPharmacol 15: 735-41.
- Maneiro E, Lombardi VRM, Lagares R, Cacabelos R (1997) An experimental model to study the cytotoxic effects induced by ß-amyloid, histamine, LPS and serum from Alzheimer patients on rat cultured endothelial cells. Methods Find ExpClinPharmacol 19: 5-12.
- Lombardi VRM, Garcia M, Cacabelos R (1998) APOE-Induced microglial activation: An in vitro assay to screen sera from Alzheimer´s disease patients and novel therapeutic compounds. Methods Find ExpClinPharmacol 20: 377-386.
- Lombardi VR, GarcÃa M, Cacabelos R (1998) Microglial activation induced by factor(s) contained in sera from Alzheimer-related ApoE genotypes. J Neurosci Res 54: 539-553.
- Cacabelos R, Fernandez-Novoa L, Lombardi V, Kubota Y, Takeda M (2005) Molecular genetics of Alzheimer's disease and aging. Methods Find ExpClinPharmacol 27 Suppl A: 1-573.
- Lombardi VR, Fernández-Novoa L, Etcheverria I, Seoane S, Cacabelos R (2004) Association between APOE epsilon4 allele and increased expression of CD95 on T cells from patients with Alzheimer's disease. Methods Find ExpClinPharmacol 26: 523-529.
- Harcha PA, Vargas A, Yi C, Koulakoff AA, Giaume C, et al. (2015) Hemichannels Are Required for Amyloid β-Peptide-Induced Degranulation and Are Activated in Brain Mast Cells of APPswe/PS1dE9 Mice. J Neurosci 35: 9526-9538.
- Zlomuzica A, Dere D, Binder S, De Souza Silva MA, Huston JP, et al. (2015) Neuronal histamine and cognitive symptoms in Alzheimer's disease. Neuropharmacology .
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, et al. (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol 14: 388-405.
- Cotman CW, Brinton RE, Galaburda A, McEwen B, Schneider DM (1987) The Neuro-Immune-Endocrine Connection. New York: Raven Press.
- Goetzl EJ, Spector NH (1989) Neuroimmune Networks: Physiology and Diseases. New York: Alan R Liss.
- Ader R, Felten DL, Cohen N (1991) Psychoneuroimmunology. San Diego: Academic Press.
- Baxter A, Ross R (1991) Cytokine interactions and their control. Chichester: Wiley.
- Théze J (1999)The cytokine network and immune functions. Oxford: Oxford University Press.
Citation: Cacabelos R (2016) Histamine: The Missing Link in the Pathogenesis of Some Brain Disorders. J Clin Exp Neuroimmunol 1:103.
Copyright: © 2016 Cacabelos R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 15252
- [From(publication date): 3-2016 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 14157
- PDF downloads: 1095