Research Article Open Access
Histamine Modulates Isoproterenol Efficacy at the β2 Adrenoceptor: Inferences Regarding Allosteric Modulation by Imidazole-Containing Compounds
| Marvin A Soriano-Ursúa1*, Daniel McNaught-Flores2, José Correa-Basurto3,4, José A Arias-Montaño2 and José G Trujillo-Ferrara3,4 | |
| 1Departamento de Fisiología y Sección de Estudios de Posgrado e Investigación, Escuela Superior de Medicina, Instituto Politécnico Nacional, México | |
| 2Departamento de Fisiología, Biofísica y Neurociencias, Centro de Investigación y de Estudios Avanzados del IPN, Av. Instituto Politécnico Nacional, México | |
| 3Departamento de Bioquímica, Escuela Superior de Medicina, Instituto Politécnico Nacional, México | |
| 4Laboratorio de Modelado Molecular y Bioinformática de la Sección de Estudios de Posgrado e Investigación, Escuela Superior de Medicina, Instituto Politécnico Nacional, México | |
| *Corresponding Author : | Marvin A Soriano-Ursúa Departamento de Fisiología. Escuela Superior de Medicina Instituto Politécnico Nacional Plan de San Luis y Díaz Mirón, C.P. 11340, México D.F Tel: (052)57296000 Extn.62747 Fax: (052)57296000 Extn.62745 E-mail: msoriano@ipn.mx |
| Received July 19, 2013; Accepted August 16, 2013; Published August 19, 2013 | |
| Citation: Soriano-Ursúa MA, McNaught-Flores D, Correa-Basurto J, Arias-Montaño JA, Trujillo-Ferrara JG (2013) Histamine Modulates Isoproterenol Efficacy at the β2 Adrenoceptor: Inferences Regarding Allosteric Modulation by Imidazole-Containing Compounds. Biochem Physiol 2:114. doi:10.4172/2168-9652.1000114 | |
| Copyright: © 2013 Soriano-Ursúa MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
In the last few years, evidence has been mounting of the possible allosteric modulation of second messenger formation on β2-adrenoceptors (β2ARs) induced by non-arylethylamine compounds. We herein addressed this issue by carrying out functional and molecular modeling studies to explore the possibility that imidazole-containing compounds (ICCs) affect the formation and accumulation of cAMP by mediating β2AR activation. Results show that histamine (an ICC) had no effect on basal cAMP accumulation in COS-7 cells transfected with the human β2AR (10.4 ± 1.1 pmol/mg protein), but significantly augmented the receptor response to the agonist isoproterenol (137 ± 7% of controls, EC50 10.5 μM, pEC50 5.87 ± 0.06). Moreover, histamine (10 μM) did not affect the isoproterenol inhibition of 3H]-dihydroalprenolol binding to hβ2ARs in membranes of transfected COS-7 cells. Q-site Finder program and molecular docking studies identified possible sites of interaction for ICCs on the β2AR, but the estimated affinities were lower than those reported for well-known β2AR ligands on the orthosteric site. On the basis of the present experimental and theoretical data, we postulate that direct ICC-hβ2AR interactions can allosterically modulate agonist-induced receptor activation. Implications of these findings for drug design are discussed.
| Keywords |
| β2-adrenoceptor; Allosteric modulation; Histamine; Molecular modeling; Imidazole |
| Introduction |
| The superfamily of G-protein coupled receptors (GPCRs) includes α- and β-adrenoceptors [1]. These two adrenoceptor subtypes are activated by many types of ligands, including two neurotransmitters/ hormones— noradrenaline and adrenaline. Human β2AR (hβ2AR) is a major target of pharmaceutical research [2,3] and enormous efforts have been made to gain insight into the mechanisms of ligand recognition and receptor activation [3,4]. |
| The recognition site for some ligands on the hβ2AR is known [3-5], and recent discoveries from X-ray crystallography about the tridimensional structure of this receptor have led to important insights into its conformational changes and activation [6-15]. It is now known that the function of GPCRs can be influenced by allosteric modulators, which are compounds that bind to the receptor at sites other than the orthosteric site, and in so doing modulate conformational changes of the receptor that influence agonist binding [15]. Within the context of hβ2AR activation, theoretical studies have explored the orthosteric site and possible allosteric sites. Studies have suggested that the G-protein dependent pathway is triggered at the orthosteric site and the G-protein independent (kinase) pathway triggered at the allosteric site. However, few reports have sought to gain insight into the interconnection between G-protein dependent and independent pathways, or between orthosteric and allosteric modulation in the activation of the hβ2AR [2,15]. |
| One known allosteric modulator, the divalent cation Zn2+, has been shown to increase receptor affinity for agonists in membranal preparations and enhance agonist-induced cAMP accumulation in intact cells [16,17]. The activation of Adenylyl Cyclase (AC) and cyclic AMP (cAMP) formation is induced when adrenoceptors (βARs) couple to Gα proteins [1,2]. In this respect, some imidazole-containing compounds (ICCs), such as CGP20712A and oxymetazoline, can act as ligands for hβ2ARs [18]. It was demonstrated in a previous study that histamine had no effect of its own on basal [3H]-cAMP accumulation in DU-145 cells, derived from a human prostate cancer and endogenously expressing β2ARs. However, histamine augmented the β2AR-mediated response [19], an effect that was insensitive to antagonists/agonists at H1, H2 or H3/H4 receptors, and that was mimicked by a set of ICCs (clobenpropit, R-α-methyl-histamine and immepip) as well as by imidazole itself. This suggests that β2AR function can be allosterically modulated by the imidazole moiety of histamine. |
| Several studies have attempted to use computational tools to elucidate the key factors involved in the structure-dynamic phenomena on the hβ2AR, and some of these theoretical simulations have contributed to the clarification of hitherto unresolved experimental phenomena [6-8]. In this regard, we and others have reported data that strongly suggest a functional relation between hβ2AR-ligands that act at the orthosteric site and allosteric regions of the hβ2AR [15]. |
| Some drug design studies have added bulky moieties to the amine extreme of classic arylethylamine pharmacophore, and have suggested that these moieties interact with residues outside of the orthosteric site, at a binding region reached by endogenous ligands [15]. Several reports have recently been published regarding the inclusion of new chemical moieties in the classic arylethylamine pharmacophore of hβ2AR-ligands, including piperazine-, arylpiperazine-, indol- and boron-containing moieties [20-24]. Some of these drug design studies also included imidazole-related moieties, yielding ICC with activity on adrenoceptors [18,25-28], and suggesting that interactions at allosteric regions can be involved. |
| We herein report an apparent allosteric modulation of the hβ2AR in COS-7 cells transfected with these receptors. Histamine increased agonist-induced cAMP formation without affecting agonist binding. By using molecular modeling studies, we explored the possibility, suggested by the current in vitro experimental results with histamine and other ICCs, that these ligands bind to the hβ2AR at allosteric regions, and tried to identify specific residues involved in ICC recognition. |
| Materials and Methods |
| Materials |
| The following compounds were purchased from Sigma (St. Louis, MO): (±)-isoproterenol hydrochloride, histamine dihydrochloride, 3-isobutyl-1-methylxantine (IBMX), protein kinase A (PKA) regulatory subunit, ICI-118,551 ((±)-1-[2,3-(dihydro-7-methyl-1Hinden- 4-yl)oxy]3-[(1-methylethyl)amino]2-butanol hydrochloride) and CGP-20712A ((±)-2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl- 4-(trifluoromethyl)-1H-imidazole-2-il]phenoxy]propyl]amin] ethoxy] benzamide methanosulfonate). [Methyl-3H]-dihydroalprenolol (specific activity 111.8 Ci/mmol) was acquired from New England Nuclear (Boston, MA), and the plasmid pcDNA3.1-hβ2AR from Missouri S&T cDNA Resource Center (www.cdna.org). |
| Functional assays |
| Cell culture and transfection: COS-7 African green monkey kidney cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine fetal serum, penicillin (50 UI/ml) and streptomycin (0.1 mg/ml) under a humidified atmosphere (5% CO2) at 37°C. Cells were transiently transfected with the plasmid pcDNA3.1-hβ2AR (0.25 μg cDNA/106 cells) and DEAE-dextran. All assays were performed 48 h after transfection. |
| [3H]-Dihydroalprenolol ([3H]-DHA) binding to cell membranes: Transfected COS-7 cells, grown in Petri dishes, were lysed by incubation (20 min, 4°C) in hypo-osmotic buffer (10 mM Tris-HCl, 1 mM EGTA, pH 7.4). After centrifugation at 42,000xg for 20 min, pellets were resuspended (∼1 mg protein/ml) in incubation buffer (50 mM Tris-HCl, pH 8). [3H]-DHA binding assays were carried out by a previously reported method [19]. |
| Measurement of cAMP accumulation: Transfected cells, grown in 24-well plates, were washed twice with phosphate-buffered saline solution (PBS) before incubation (37°C) in 250 μl PBS containing 1 mM IBMX (3-isobutyl-1-methylxantine). After 15 min drugs were added in a 10μl volume and incubations continued for 30 min. The composition of the PBS solution was (mM): NaCl 137, KCl 2.7, Na2HPO4 10, KH2PO4 1.76, MgCl2 1, CaCl2 1, pH 7.4. Incubations were terminated with 25 μl ice-cold 1N HCl, samples were neutralized with 25 μl ice-cold 1N NaOH and 100 μl 1M Tris-HCl (pH 7.4). Endogenous cAMP was determined in 50 μl-samples by assaying the inhibition of [3H]-cAMP binding to the regulatory subunit of PKA, as described in a previously reported method [29]. |
| Molecular modeling |
| The hβ2AR model: For in silico assays we selected a refined β2AR model based on the structure reported by Cherezov et al. (PDB ID: 2rh1) [10]. This model, which we reported previously, was selected on the basis of the good correlation between experimental results and theoretical affinity prediction [30]. Before docking analysis, the protein was treated as reported previously [30,31]. |
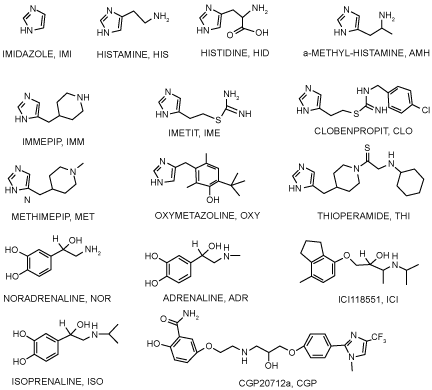
| Ligand retrieval: A group of well-known hβ2AR ligands and a set of ICCs that could potentially bind to the hβ2AR were tested (Scheme 1). For each ligand the 3-D structure was built and geometrically optimized at the B3LYP/6-31G* level with Gaussian 03 software [32]. |
| Docking methodology: In order to identify the hβ2AR recognition sites and determine ligand affinities, docking simulations were performed using 3-D ligand/receptor structures. To corroborate the availability of the putative binding site in the refined model, a binding site prediction was previously carried out with the Q-site Finder program [33]. |
| All rigid/flexible bonds, partial atomic charges, and non-merge hydrogens of the ligands were assigned. The Kollman partial charges were assigned for all atoms in the hβ2AR, and the non-merge hydrogens were added using AutoDock Tools 1.5.0, maintaining the rest of the parameters at the default setting [34]. Docking simulations were performed using a commonly used search algorithm (hybrid Lamarckian Genetic) implemented on AutoDock 4.0.1 [35]. The initial population was 100 randomly-placed individuals, and the maximum number of energy evaluations was 10 million. To search for all potential binding sites on the hβ2AR, input initializations of the ligand structures and hβ2AR binding site definitions were carried out using a GRIDbased procedure [36]. In order to explore the interaction on the whole hβ2AR, a blind docking procedure using a box of 126×126×126 Å point grid with 0.375-Å spacing was used, centered automatically on the protein by AutoDock Tools 1.5.0 program. Docked orientations within a root-mean square deviation of 0.5 Å were clustered together and the lowest free-energy cluster returned for each compound was used for further analysis using AutoDock Tools 1.5.0. Docking results (hβ2ARligand complexes) were visualized with VMD 1.9 [37]. |
| Results and discussion |
| Binding and functional characteristics of hβ2ARs expressed in COS-7 cells |
| Specific [3H]-DHA binding to membranes from transfected COS- 7 cells yielded a maximum binding (Bmax) of 10.4 ± 1.1 pmol/mg of protein and an equilibrium dissociation constant (Kd) of 0.70 ± 0.17 nM (based on four experiments). [3H]-DHA binding was inhibited by the βAR antagonists ICI-118,551 and CGP-20712A. Estimates for pKi (-log10 Ki) were consistent with values reported for the h2AR (Supplementary Table 1). |
| The AR agonist isoproterenol stimulated cAMP accumulation in a concentration-dependent manner (Supplementary Figure 1A) with an EC50 of 143 nM (pEC50 6.73 ± 0.19; based on three experiments) and a maximum response of 14.1 ± 3.5 times the basal value. The accumulation of cAMP induced by 300 nM isoproterenol was inhibited by the selective β2AR antagonist ICI-118,551 (1 μM; Supplementary Figure 1B), and the extent of inhibition (94%, n=4) was close to the theoretical value (99% inhibition) for an action mediated by the hβ2AR, assuming the EC50 value for isoproterenol-induced cAMP accumulation as the agonist Kd. |
| Effect of histamine on isoproterenol stimulation of cAMP accumulation and inhibition of [3H]-DHA binding |
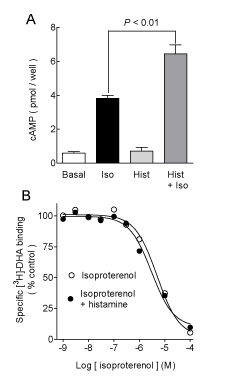
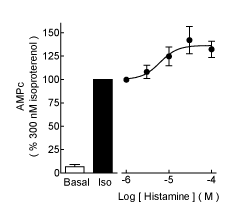
| Histamine had no significant effect on basal cAMP accumulation (Figure 1A) at 30 μM (93 ± 16% of basal, n=4, P>0.05, ANOVA and post-hoc Student-Newman-Keuls test), but significantly enhanced the stimulatory action of 300 nM isoproterenol (153 ± 15% of the response to the agonist alone, P<0.01). For these series of determinations (n=4), the effect of histamine was concentration-dependent (Figure 2), with an EC50 of 6.2 μM (pEC50 5.21 ± 0.24) and a maximum stimulation of 137 ± 7% of that induced by isoproterenol alone. Figure 1B shows that histamine (10 μM) had no effect on the isoproterenol inhibition of [3H]-DHA binding to membranes from transfected COS-7 cells, with pKi values of 5.99 ± 0.21 and 5.95 ± 0.14 in the absence and presence of histamine, respectively (n=4, P>0.05, Student t test). |
| COS-7 cells have been reported to express low levels of histamine H1 receptors [38]. However, the effect of histamine on hβ2ARmediated cAMP accumulation was insensitive to mepyramine, a selective antagonist for H1 receptors (data not shown). This supports our previous findings with endogenous receptors, in which histamine enhanced β2AR-mediated cAMP accumulation independently of the activation of known metabotropic histamine. Partial and full agonists for hβ2AR stabilize several possible receptor conformations, which are linked to differential activation of the signaling cascades [5,15,39]. Therefore, allosteric modulation could influence receptor activation by stabilizing new intramolecular interactions [8,15,40]. |
| Isoproterenol, known to act as a full agonist on the hβ2AR [41], is often used as the compound with the maximum intrinsic activity on this receptor [41,42]. However, the increased activity in COS-7 cells induced by histamine in the present study suggests that isoproterenol behaves as a superagonist. That is, the agonist induced a supra-physiological effect, judging from its action on cAMP accumulation [43]. The mechanism for this effect has been related with the interaction of ligands (a peptide or small molecules) with extracellular domains of the corresponding GPCR [15,44-46]. |
| The hβ2AR binding sites for ICCs and relationship between in silico and in vitro data |
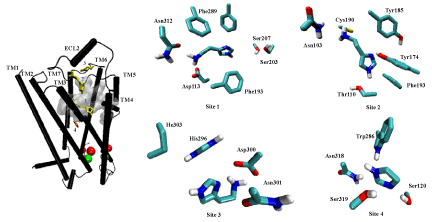
| Analysis with the Q-site Finder program identified ten putative binding sites on the hβ2AR model, although only three sites are accessible from the extracellular side of the receptor. These sites were found in similar dimensions at all the available crystals of β2AR [9-14] (not shown) Site 1 (747 Å3 in volume) is located near the extracellular region of the core-crevice formed by TM3 to TM7. This site was similar to that described by previous theoretical and crystallography assays, and coincides with the reported orthosteric site (or binding pocket) for β2AR ligands [9-14,47]. The two other sites suggested by the present analysis are located below the extracellular loops (with 367 Å3 and 240 Å3 in volume) of the hβ2AR. |
| From docking simulations using well-known or ICC ligands (Figure 3) on the refined hβ2AR structure, a binding site was found that is common to all tested ligands. Specific interactions for each ligand were identified at this binding pocket, which is similar to site 1 and was predicted by Q-site Finder. Additionally, three alternative sites were identified as being common to most ICCs, but not to the well-known hβ2AR ligands. The latter fit only in two regions —at site 1 and near the region previously described by ourselves and others [9-15,30,47]. |
| Hence, docking simulations led to the identification of four binding sites on the hβ2AR for ICC ligands (Figure 4), one of them being the orthosteric site (site 1). Two other sites are located on the extracellular domains: site 2 (constituted by side chains of residues Asn103, Glu107, Cys108, Tyr174, Arg175, Tyr185 and Cys190), which is located just below the disulfide bonds present in the second extracellular loop, and site 3 (constituted by side chains of residues His296, Asp300, Asn301 and Ile303), which is below the third extracellular loop. Finally, site 4 is constituted by transmembrane domains 3 to 7 (side chains of residues Asp119, Ser120, Trp286, Gly315, Asn318 and Ser 319), and is deeper than site 1. No other sites were visualized with the analysis of the one hundred complexes of greatest affinity shown by AutoDock Tools 1.5.0 software [35]. |
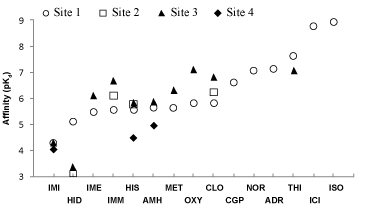
| Not all ICCs interact with each of the four sites identified. For instance, immepip, methimepip and oxymetazoline bind only to site 1 (orthosteric) and site 3. It is noteworthy that several ICCs show greater affinity for site 3 than for the orthosteric site (Figure 5). |
| On the basis of data presented herein and from a previous study that included known hβ2AR agonists [30], the calculated ligand affinities (Figure 5) are in line with the affinities calculated from experimental data. For example, the theoretical pKi value for ICI-118,551 indicates nanomolar affinity, as was observed in vitro [10,48], while other hβ2AR ligands showed micromolar affinity, also in accordance with experimental data. Furthermore, the calculated affinities for ICI-118,551 and CGP-20712A match those obtained experimentally in the present study in assays on the inhibition of [3H]-DHA binding to membranes from COS-7 cells transfected with the hβ2AR (Supplementary Table 1), as well as in assays on cAMP formation in DU-145 cells [19]. |
| Overall, these results indicate that the theoretical methods used in this study allow for reliable approximation to the affinity of the hβ2AR for ICCs, with values from ~1000 μM for histidine to ~0.1 μM for oxymetazoline. The estimated affinities are also in accordance with the ICC concentrations shown to modulate isoproterenol-induced cAMP formation in DU-145 cells (immepip 1 μM, clobenpropit 1 μM, imidazole 10 μM, R-α-methyl-histamine 10 μM and histamine 30 μM) [19]. The relationship appears stronger if the ICC affinity for site 3 (below the third extracellular loop) is considered, with the rank order: immepip ≈ clobenpropit>R-α-methyl-histamine ≈ histamine>imidazole. |
| Histamine binding on the hβ2AR: Feasibility of allosteric modulation by ICCs |
| The current results raise the possibility that histamine and other ICCs bind to an extracellular site of the hβ2AR (Figure 4), and in this way increase the receptor signaling effica cyI.n this sense, it has already been shown that Zn2+ acts as an allosteric modulator of the hβ2AR, increasing the affinity for agonists in membranal preparations and enhancing agonist-induced cAMP accumulation in intact cells [16,17]. While the effect of Zn2+ on agonist affinity involves amino acids located on the third intracellular loop (Glu225, Cys265 and His269), the enhancement of cAMP accumulation seems to result from an interaction at the extracellular domains of the receptor [16,17]. Increasing evidence suggests that in the ligand-receptor interaction, extracellular loops of several GPCRs are involved in allosteric modulation of signaling pathways [15,45,49-52]. |
| Regarding imidazole, the effects in biological systems (including on smooth muscle tissue) are well-known [25-28,53], although the mechanism of action for this and other structurally related compounds is unclear. Some possible receptors for imidazoline have been proposed [53]. Also, ligands containing imidazole moieties have been developed and some of these have shown a selective effect on adrenoceptor subtypes. Hence, some ICCs act on α-adrenoceptors [26,27] and a few on β adrenoceptors, such as the aforementioned CGP-20712A and oxymetazoline [18,25,28]. |
| We have herein generated preliminary data about the binding of ICCs on adrenoceptors. On the basis of our in vitro results, the orthosteric site of the hβ2AR does not appear to play a relevant role in ICC-hβ2AR interactions, evidenced by the fact that histamine increased hβ2AR signaling when the receptor was stimulated by an agonist, but histamine alone induced no changes in cAMP levels. Hence, we suggest that ICCs can act as allosteric modulators of the hβ2AR during its interaction with some well-known ligands. The two sites identified by our docking simulations, sites 2 and 3 (located below the second and third extracellular loops, respectively), have been previously reported by our work group and others as potential binding regions for allosteric modulation, possibly influencing the entrance of ligands to the orthosteric binding site [15,24,54]. For example, in a previous work we suggested that the residues forming site 2 could be involved in the modulation by phenylboronic acid of cAMP production, an effect induced by salbutamol acting on the hβ2AR [24]. |
| Interestingly, histamine and other ICCs showed a greater affinity for the binding sites in the extracellular domains of the receptor than for the orthosteric binding site. We therefore propose that the binding of histamine and other ICCs to an allosteric site located on the extracellular face of the hβ2AR modulates agonist-induced responses by stabilizing a receptor conformational state related to increased efficacy by agonists. This could explain the enhancement of cAMP accumulation that occurred without affecting the agonist binding properties. |
| In this respect, our simulations showed the feasibility of an interaction by histamine at site3, involving micromolar affinity of this compound with residues His296 (a likely component of the Zn2+ binding site [17]), Asp300, Asn301 and Ile303. Because these residues are located on the third extracellular loop, the resulting interactions could lead to conformational changes in TM6 and TM7, known to participate (along with essential changes in TM3 and the TM5-ICL3- TM6 region) in receptor activation [55]. In addition, such interactions could favor the accessibility of other ligands (e.g., isoproterenol) to the orthosteric binding site. |
| A similar effect was found in regard to the binding of ligands to site 2, which increased the accessibility of agonists to the orthosteric site. In this case, the second extracellular loop apparently has a filtering role, affecting the entrance of ligands to the orthosteric binding pocket [8,30], in accordance with ligand-specific changes on the extracellular surface suggested recently [56]. |
| Finally, the site 4 appears not to be available for all ICCs, probably due to the hindrance effect of residues found deeper in the binding pocket than the orthosteric site. It seems that only the smaller ligands (imidazole, histamine and α-methyl-histamine) fit into this site, which reduces its possible usefulness for allosteric modulation. |
| Some limitations of the present study should be considered: a) the use of a rigid hβ2AR model, which impedes the study of dynamic receptor behavior in the presence of allosteric and orthosteric ligands [8]; b) the study of only one of several possible signaling pathways [2]; c) the possibility of ICCs acting as phosphodiesterase inhibitors, as previously suggested [28]; and d) the lack of in vitro evaluation of multiple ICCs in order to further clarify the role of this moiety in allosteric modulation of ligand affinity. Future studies are needed to expand the scope of the current contribution by taking these points into consideration. |
| In spite of these limitations, the current data suggests that ICCs can modulate hβ2AR activity. The study of the effects of histamine on hβ2ARs bears relevance to medicinal chemistry, because the histaminergic system is often involved in the normal and pathological function of the hβ2AR [2,8]. Thus, it is important to design new compounds that could affect hβ2AR function, taking into account that allosteric regions probably modify the potency and efficacy of agonists acting on the orthosteric site of this receptor [45,57,58]. |
| Conclusions |
| Histamine significantly augmented the response of the hβ2AR to the agonist isoproterenol. At the same time, it showed no effect on the basal cAMP accumulation in COS-7 cells transfected with the hβ2AR, nor did it produce any change in the isoproterenol inhibition of [3H]- dihydroalprenolol binding to this receptor. We therefore propose that histamine and probably other ICCs can allosterically modulate hβ2AR, and in this way influence agonist-induced receptor activation, a possibility supported by in vitro assays as well as by molecular docking simulations that identified hβ2AR regions capable of binding to imidazole-containing drugs. Further experimental and theoretical studies are required to deepen insights into the mechanism(s) involved in ICC modulation of β2AR-mediated signaling, as well as to explore the possibility that the addition of imidazole moieties to some compounds targeting β2AR could yield more potent and efficient drugs that act simultaneously on both the orthosteric and allosteric sites of the receptor. |
| Acknowledgements |
| We would like to express our gratitude to the colleagues who reviewed this manuscript. We thank Bruce Allan Larsen for reviewing the use of English in the manuscript. This work has been supported by CONACYT (grants CB-168116, 204908 and 128205), CINVESTAV, COFAA and SIP-IPN. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 14176
- [From(publication date):
November-2013 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 9604
- PDF downloads : 4572
