Highly Neurovirulent Viruses Invade and Spread in the Brain Using the Host Reactions Triggered by Infection
Received: 27-Feb-2017 / Accepted Date: 10-Mar-2017 / Published Date: 19-Mar-2017 DOI: 10.4172/2476-2024.1000124
Abstract
Coronaviruses (CoVs) have relatively high mutation and RNA recombination rates and undergo rapid crossspecies transmission events in vitro and in vivo. The viruses have evolved diverse strategies to evade, blocking host immune responses, and resulting in an emergence of highly virulent CoVs to cause fatal diseases in human, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Among CoVs used for experimental studies, the cl-2 virus (cl-2), isolated from a neuropathogenic JHM viral strain (JHMV) of mouse hepatitis virus (MHV), shows extremely high virulence. All mice infected with cl-2 die within 3 days post-inoculation (dpi). Less virulent but still highly neurovirulent mutant viruses, soluble receptor resistant mutant virus (srr7) and Mu-3 virus (Mu-3), have been isolated from cl-2 and srr7, respectively. Although these viruses cause different pictures of neuropathologies with different distribution of viral antigens after infection, they use the same pathway to enter the brain before inducing lesions in the brain parenchyma. The pathway is formed as an astrocytic reticular network (ARN) after infection. The ARN functions as a conduit system in a similar way to a fibroblastic reticular network (FRN) in lymphoid organs. In addition, the viruses induce an immunosuppressive state in infected mice, mimicking endotoxin tolerance (ET), or lipopolysaccharide (LPS) tolerance. The immunosuppressive states are detected by; 1) pathological findings as 1a); a very low -level inflammatory response, 1b); the shrunken spleen, 1c); the refractory state to LPS challenge, and 1d); the local production of anti-inflammatory cytokines by neurons, and by 2) immunological studies, which showed 2a); the systemic production of anti-inflammatory cytokines by eliciting CD11b+Gra-1+ suppressor monocytes/macrophages (Mo/Mas).In addition, cultured splenic cells and Mo/Mas after infection exhibit 2b); low-level responses to stimulation of toll-like receptors (TLRs) 4 and 7, and 2c); the induction of Lewis X carbohydrate structure (Lex), which can trigger the C-type lectin-like receptor that initiates an immunosuppressive pathway, respectively.
Introduction
CoVs exhibit high mutation rate, leading to an emergence of life-threatening virulent viruses [1]. However, the mechanisms of the high-rate mutation and the emergence of highly virulent viruses have not been elucidated. In the experimental model, many types of CoVs, ranging from highly virulent to attenuated viral clones, have been isolated from mouse hepatitis virus (MHV), which would provide an opportunity to investigate the mechanisms more efficiently compared with those of human origin [2]. MHV is an enveloped virus with single-stranded, positive-sense genomic RNA that is about 30 kilobases long. The envelop of virion has projections of glycoprotein, Spike (S) protein, which is responsible for binding to a receptor and also for the cell entry mechanism of MHV [2]. Mutation frequently occurs in the MHV genes [3,4], and several mutant clones have been isolated from JHMV [2], such as cl-2 [5], srr7 [6], and Mu-3 [7,8]. Although many other highly virulent clones have been isolated from MHV, the immunological background of high virulence has been mainly explained that CoVs, in general, block interferon (IFN)-I signaling and counter the action of downstream effector molecules [8], until recent studies revealed that cl-2 and its progenies cause immunosuppressive states in the infected animals by triggering the production of anti-inflammatory cytokines after infection [9,10].
In addition to triggering the suppressive immune responses, the cl-2 exhibits exremely high neurovirulence by infecting neurons, which do not express carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM 1), a major MHV receptor (MHVR), and the infected mice die within 3 days post-inoculation (dpi). The lesions caused by cl-2 infection in an MHVR-independent manner are mainly distributed in the grey matter of the brain, while srr7 infection induces lesions in the white matter of the brain without infecting neurons, and mice infected with srr7 survive more than 7 dpi up to 10 dpi [8,11,12]. These differences in the neuropathogenesis and dependency on MHVR between srr7 and cl-2 are attributed to a single mutation in the S2 gene [13] in the S protein-encoding region. The mutant virus isolated from srr7, Mu-3, induces apoptosis of the pyramidal neurons in the hippocampus, especially in CA2 and CA3 subregions after infection. This unique neuropathology is not observed after infection with srr7 or cl-2 [7]. Mu-3 has a single amino acid mutation at position 596 (Ans to Lys) of the S2 subunit of the S protein relative to srr7, and exhibits higher virulence than srr7 and lower virulence than cl-2. Mu-3 induces mixed neuropathological pictures induced by srr7 and cl-2 infection, causing the lesions in the brain distributed both in the white and grey matter [7].
In spite of these differences, the three viruses spread in the brain in the same manner during the early phase of infection. The viral antigens are initially detected in the CD11b-positive monocytes/macrophages (Mo/Mas) exuded in the meninges at 12 h post-inoculation (hpi) [8,11,12,4]. Before 48 hpi, infected Mo/Mas appear in the ventricle, and the viral antigens are detected in the ependymal cells and GFAP positive cells or nestin-positive progenitor cells that take up positions in the subventricular zone (SVZ) [12]. The infected GFAP-positive cells in the SVZ are found to project cell processes to the ependymal cell layer, which are referred to as type B cells [14]. Type B cells develop as the primary neuronal precursors besides contributing to the niche environment in the SVZ of adult mice [14]. The ventricular wall is also the target site during the initial phase of infection with other viruses, including herpes simplex virus [15] and lymphocytic choriomeningitis virus [16].
In addition to the viral spreading manner during the early phase of infection, the three viruses induce the same pathological state, that is an immunosuppressive state [12,4]. Although the viruses cause widespread pathological change in the brain after infection, the lesions in the brain parenchyma are accompanied by very low-level inflammatory cell infiltration. This type of host response after viral infection is typically observed in retroviral infection [17]. In addition, mice infected with the viruses show a shrunken spleen with reduced cell population [18,7]. Therefore, the host responses after viral infection that facilitate viral entry into the brain by forming a passage, and the immune responses that allow viral replication and spread have been investigated.
Astrocytic Reticular Network (ARN)
Fibroblastic reticular network and ARN
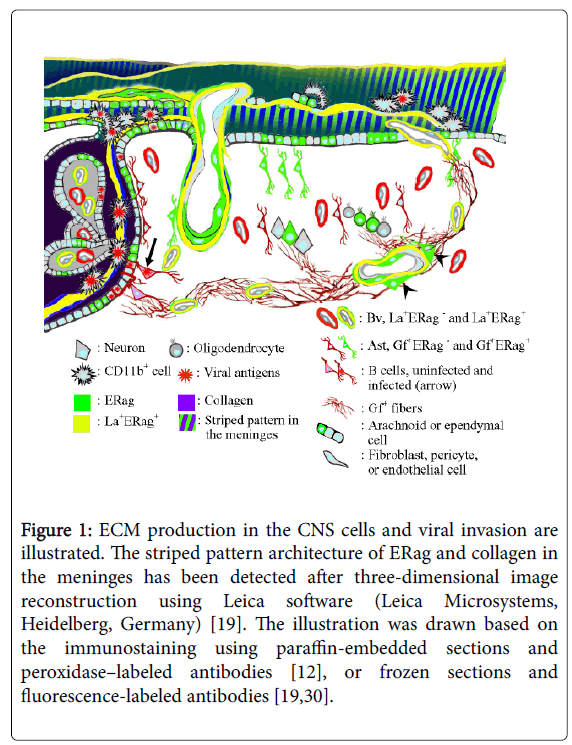
A unique passage used for viral entry between the meninges, the initial site of infection, and the ventricle is formed after infection with cl-2. The passage is found around the foramen of Luschka at the cerebellopontine angle [19]. In the normal state, the foramen of Luschka is used for cerebrospinal fluid (CSF) to flow from the ventricle into the , and there is a barrier called blood-CSF barrier, with a unique structure, the choroid plexus [20]. The passage is composed of fibrous extracellular matrix (ECM), which includes an antigen recognized by Erasmus University Rotterdam-thymic reticulum antibody 7 (ER-TR7) [21]. The antigen detected by ER-TR7 (ERag) in the passage is associated with collagen and laminin. The ERag-positive fibers (ERfibs) have been recognized as a hallmark of the fibroblastic reticular fibers that form the fibroblastic reticular network (FRN) in the lymph nodes [22,23] and spleen [24,25]. The FRN in lymphoid organs functions as a conduit, facilitating the transfer of foreign antigens or immuno-mediating cytokines to their proper sites, and guiding immuno-competent cells for their homing [25]. The fibers of FRN in the lymphoid organs contain collagen fibers as a core, which is wrapped by an ERag-positive ECM, and further by a basement membrane enriched with laminin [24]. The fibers are finally wrapped by the cytoplasm of fibroblastic reticular cells (FRC), which produce major components of the fibers and nurse the FRN in the lymphoid organs.
In the brain, ERag already appear at 12 hpi in the astrocytes facing the border of peripheral nerve and the central nervous system (CNS) in the root of trigeminal nerve and those facing the arachnoid membrane, as well as in the fibrous structure in the meninges [19]. The finding of ERag-positive fibrous structure in the meninges is obtained through two-dimensional observation, a usual microscopic finding. However, three-dimensional reconstruction of the ERag-positive structure in the meninges revealed that the appearance of ERfibs are the sections of a stack of thin sheets with a striped pattern architecture of ERag and collagen (Figure 1). Each of the sheets was approximately 0.7-1 μm in thickness, which corresponds to the diameter of a ERag-positive reticular fiber serving as a unit of the conduit system in the lymphoid organs [26]. Furthermore, the cells exuded in the meninges, which are initial targets of infection, are put between the sheets [19]. The meningo-brain barrier (MBB) has been considered to be constructed by the thin arachnoid membrane and astrocytes facing the membrane. However, these observations indicate that the MBB is formed immediately after infection by producing a pile of ERag-positive membranous sheets, and the production of ERag is one of the host defense mechanisms against infection.
Figure 1: ECM production in the CNS cells and viral invasion are illustrated. The striped pattern architecture of ERag and collagen in the meninges has been detected after three-dimensional image reconstruction using Leica software (Leica Microsystems, Heidelberg, Germany) [19]. The illustration was drawn based on the immunostaining using paraffin-embedded sections and peroxidase–labeled antibodies [12], or frozen sections and fluorescence-labeled antibodies [19,30].
After 24 hpi, ERfibs or ERag become apparent in the ventricles, ependymal cells, and ventricular wall including SVZ [19]. Along with ERfibs formed between the meninges and ventricle, infected Mo/Mas enter the ventricle from the meninges. Usually, extracellularly released viral particles are not detectable by immunofluorescence, but concentrated viral antigens in the ERag-positive fibrous structure could be observed. Such colocalization of viral antigens or viral particles with ECM is detectable after infection with lymphocytic choriomeningitis virus (LCMV) in the spleen [27]. Similar phenomena have been reported on infection with extremely virulent viruses, such as Ebola [28], Marburg, and Lassa [29] viruses in the lymphoid organs. It has been considered that the intimate interaction of the viral particles with ECM facilitate the spread of the viruses. Because close contact of cl-2 to ERfibs is observed, the viral antigens expressed on the surface of infected MoMas possibly interact with newly formed ERfibs, which guide the cells to enter the ventricle.
ERag-producing cells in the CNS
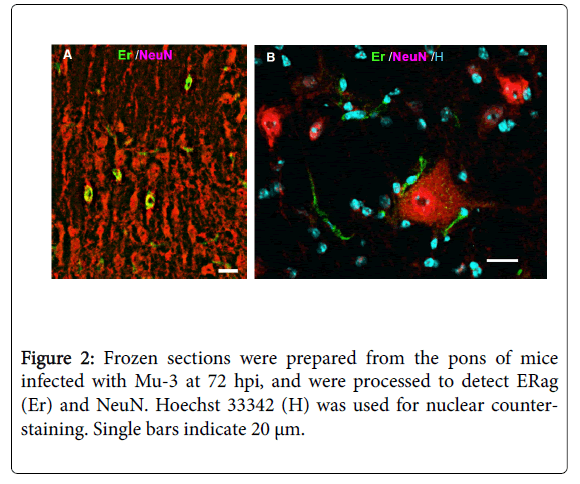
By in vivo studies using immunofluorescence, ERag is detected at 48 hpi in almost all cell types that constitute the CNS, including arachnoid cells, ependymal cells, pericytes, oligodendroglia, astrocytes, and neurons. However, CD11b-positive cells including microglia do not produce ERag. Using the brain primary culture, most of the observation on ERag-producing cells were confirmed, except for neurons [30]. Reexamination of the in vivo brain infected with an attenuated viral clone, Mu-3, using serial sections raised the possibility that ERag-positive satellite cells attached to neurons (Figure 2) show a falls-positive appearance of ERag-positive neurons. Satellitosis has been recognized as a response of the brain to a variety of infections, and it is considered to be an increase in oligodendrocytes as satellites to neurons [31]. Therefore, we should wait for the results of electron microscopic studies to see whether neurons produce ERag after infection.
Although many cell types in the CNS produce ERag after infection, the most prominent ERag-producing cells in the brain parenchyma are astrocytes, which start producing ERag at 12 hpi. Furthermore, astrocytes in in vivo and in vitro produce ECM other than ERag after infection, such as collagen type I, type III, and laminin [30], which are main components of ERag-positive reticular fibers in the lymphoid organs. Finding of close interaction of GFAP-positive astrocytic fibers with fine ERfibs indicates that astrocytes play a role in producing ERag-positive reticular network in the brain parenchyma in a way FRCs do in the lymphoid organs.
Immunosuppression
Induction of immunosuppressive Mo/Mas
Mice infected with srr7 show a low response to bacterial endotoxin/ lipopolysaccharide (LPS) stimulation [32], mimicking the state in endotoxin tolerance (ET), or LPS tolerance, which has been thought to be a protective system to regulate the over-production of proinflammatory cytokines. Experimentally, ET is induced by prior exposure of Mo/Mas to LPS, which causes them to be refractory to further LPS stimulation [33]. Activated macrophages that play a key role in ET have been recognized as M2 macrophages or CD11b +Gra-1+ suppressor Mo/Mas, an immunosuppressive subset of macrophages, which secrete the anti-inflammatory cytokines IL-10 and TGF-β, appearing in the blood stream [34] and lymphoid organs [35]. M2 macrophages also appear in local inflammatory sites as exuded cells, such as peritoneal exudate cells (PECs) in the ET state [36]. Mu-3-infection in the peritoneal cavity induces a large CD11b +Gra-1+ population among PECs. Furthermore, cultured PECs secrete IL-10 and TGF-β after infection with Mu-3 [10]. In the brain, the pyramidal neurons in the hippocampus produce IL-10 and TGF-β after infection with Mu-3 [9]. Thus, Mu-3-infection causes systemic and local immunosuppressive states.
Involvement of Lewis X carbohydrate structure
Using Fut9-/- mice [37], which lack α1,3-fucosyltransferase 9 and are unable to synthesize the Lewis X carbohydrate structure (Lex). It has been shown that the expression of Lex is involved in the immunosuppressive state induced by infection with cl-2 and its progeny. The ET-mimicking immunosuppression observed in wild-type mice after infection does not occur in Fut9-/- mice, which respond to LPS stimulation equally with or without infection [32]. Furthermore, stimulation of toll-like receptor (TLR) 4, TLR7, and TLR9 with ligands LPS, single-stranded RNA (ssRNA), and cytosine-phosphodiester- guanine oligonucleotides (CpG) revealed that the splenic cells after infection with srr7 failed to trigger not only TLR4- mediated cascade by LPS but also TLR7-mediated cascade by ssRNA, as summarized in (Figure 3). The splenic cells derived from both wild-type and Fut9-/- mice failed to produce interferon (IFN)-β after infection by stimulation of TLRs. It occurred because coronaviral infection causes direct interference in the cascades to produce IFN-β [1].
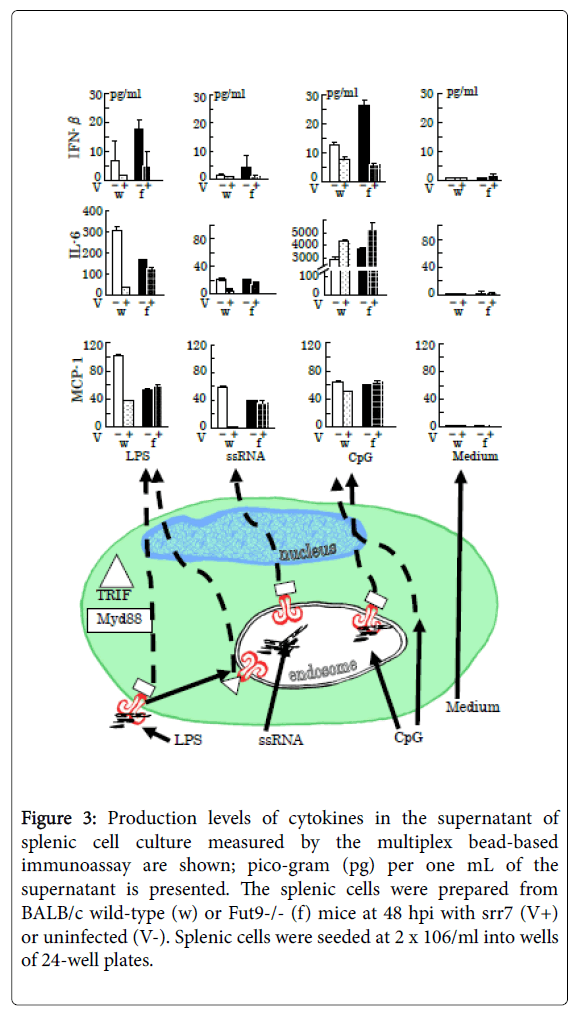
Figure 3: Production levels of cytokines in the supernatant of splenic cell culture measured by the multiplex bead-based immunoassay are shown; pico-gram (pg) per one mL of the supernatant is presented. The splenic cells were prepared from BALB/c wild-type (w) or Fut9-/- (f) mice at 48 hpi with srr7 (V+) or uninfected (V-). Splenic cells were seeded at 2 x 106/ml into wells of 24-well plates.
The interaction between carbohydrate structures and their binding partners, glycan-binding proteins, or lectins play varied roles in immunological responses [38]. Lex is a natural ligand of dendritic cell-specific intercellular-adhesion-molecule-grabbing nonintegrin (DC-SIGN), a C-type lectin-like receptor. DC-SIGN initiates an immunosuppressive pathway after interaction with Lex [39].
In the normal state, the expression of Lex is very low level and undetectable by immunofluorescence. Infection of cultured PECs with srr7 or Mu-3 induces detectable level of Lex expression in the PECs by immunofluorescence [40,10]. IL-10-producing PECs observed after infection are found to express Lex [10]. Thus, highly neurovirulent viruses proliferate and spread under the immunosuppressive condition induced in the host animals.
These studies would provide an opportunity to investigate the mechanisms of emergent coronaviruses that cause lethal diseases in humans, such as SARS and MARS, where neither ECM production that facilitate viral spread nor immunosuppressive states induced after infection have been studied.
Representative and simplified cascades for cytokine production after stimulation for 24 h of TLRs by LPS (5 μg/ml), ssRNA (1 μg/ml), and CpG (5 μM), with respect to adaptor protein myeloid differentiation factor 88 (MyD88) (square)-dependent and -independent signaling [41], are shown in the lower picture. The triangle indicates the adaptor protein Toll-interleukin 1 receptor (TIR) homology domain-containing adaptor-inducing interferon-β (TRIF). Vertical lines indicate SD.
The production levels of MCP-1 and IL-6 are shown because the most marked differences between infected wild-type and Fut9-/- mice were observed in the production of these two cytokines among several cytokines examined. The production level of INF-β, which is down-regulated through direct interference in the cascades by coronaviral infection [1], is reduced after infection showing no difference between wild-type and Fut9-/- mice.
The LPS initiates intracellular cascades via TLR4 in a MyD88- or TRIF-dependent manner using them as cytoplasmic signaling adaptor proteins, while ssRNA binds to TLR7 with MyD88-dependency to cause signal transduction [41]. The TLR9 also shows MyD88- dependency in signal transduction, but CpG induced similar levels of MCP-1 and IL-6 production in the culture of SpCs derived from infected and mock infected mice regardless of the mouse strains. CpG can initiate proinflammatory responses through a TLR9- and Myd88- independent mechanism [42].
References
- Vijay R1, Perlman S2 (2016) Middle East respiratory syndrome and severe acute respiratory syndrome. Curr Opin Virol 16: 70-76.
- Bender SJ1, Weiss SR (2010) Pathogenesis of murine coronavirus in the central nervous system. J Neuroimmune Pharmacol 5: 336-354.
- Baric, E. Sullivan, L. Hensley, B. Yount, W. Chen, et al. (1999) Persistent infection promotes cross-species transmissibility of mouse hepatitis virus. J Virol 73: 638-649.
- Tresnan, R Levis, KV Holmes (1996) Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J Virol 70: 8669-8674.
- Taguchi, F S G Siddell, H Wege, V ter Meulen (1985) Characterization of a variant virus selected in rat brains after infection by coronavirus mouse hepatitis virus JHM. J Virol 54: 429-435.
- Matsuyama S, R Watanabe, F Taguchi (2001) Neurovirulence in mice of soluble receptor-resistant (srr) mutants of mouse hepatitis virus: intensive apoptosis caused by less virulent srr mutant. Arch Virol 146: 1643-1654.
- Nomura R, H Kashiwazaki, M Kakizaki, S Matsuyama, F Taguchi, et al. (2011) Receptor-independent infection by mutant viruses newly isolated from the neuropathogenic mouse hepatitis virus srr7 detected through a combination of spinoculation and ultraviolet radiation. Jpn J Infect Dis 64: 499-505.
- Kakizaki M, H Kashiwazaki, R Watanabe (2014) Mutant murine hepatitis virus-induced apoptosis in the hippocampus. Jpn J Infect Dis 67: 9-16.
- Kakizaki M, R Watanabe (2017) IL-10 expression in pyramidal neurons after neuropathogenic coronaviral infection. Neuropathology in press.
- Kakizaki M, A Togayachi, R Watanabe (2017) Induction of anti-inflammatory cytokines in CD11b- and Gr-1-positive cells after neuropathogenic coronaviral infection. Jpn J Infect Dis in press.
- Takatsuki H, F Taguchi, R Nomura, H Kashiwazaki, M Watanabe, et al. (2010) Cytopathy of an infiltrating monocyte lineage during the early phase of infection with murinecoronavirus in the brain. Neuropathology 30: 361-371.
- Kashiwazaki H, R Nomura, S Matsuyama, F Taguchi, R Watanabe, et al. (2011) Spongiform degeneration induced by neuropathogenic murine coronavirus infection. Pathol Int 61: 184-191.
- Saeki K, N Ohtsuka, F Taguchi (1997) Identification of spike protein residues of murine coronavirus responsible for receptor-binding activity by use of soluble receptor-resistant mutants. J Virol 71: 9024-9031.
- Mirzadeh Z, FT Merkle, M Soriano-Navarro, JM Garcia-Verdugo, A Alvarez-Buylla, et al. (2008) Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3: 265-278.
- Kopp SJ, G Banisadr, K Glajch, UE Maurer, K Grunewald, et al. (2009) Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci U S A 106: 17916-17920.
- Nayak D, KR Johnson, S Heydari, TL Roth, BH Zinselmeyer, et al. (2013) Type I interferon programs innate myeloid dynamics and gene expression in the virally infected nervous system. PLoS Pathog 9: e1003395.
- Watanabe R, S Takase-Yoden (1995) Gene expression of neurotropic retrovirus in the CNS. Prog Brain Res 105: 255-262.
- Kashiwazaki H, F Taguchi, Y Ikehara, R Watanabe (2011) Characterization of splenic cells during the early phase of infection with neuropathogenic mouse hepatitis virus. Jpn J Infect Dis 64: 256-259.
- Watanabe R, M Kakizaki, Y Ikehara, A Togayachi (2016) Formation of fibroblastic reticular network in the brain after infection with neurovirulent murine coronavirus. Neuropathology 36: 513-526.
- Sakka L, G Coll, J Chazal (2011) Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128: 309-316.
- Van Vliet E, M Melis, JM Foidart, W Van Ewijk (1986) Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J Histochem Cytochem 34: 883-890.
- Gretz JE, AO Anderson, S Shaw (1997) Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev 156: 11-24.
- Sixt M, N Kanazawa, M Selg, T Samson, G Roos, et al. (2005) The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22: 19-29.
- Nolte MA, JAM Belien, I Schadee-Eestermans, W Jansen, WWJ Unger, et al. (2003) A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J Exp Med 198: 505-512.
- Bajenoff M, N Glaichenhaus, RN Germain (2008) Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol 181: 3947-3954.
- Malhotra D, AL Fletcher, J Astarita, V Lukacs-Kornek, P Tayalia, et al. (2012) Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol 13: 499-510.
- Mueller SN, KA Hosiawa-Meagher, BT Konieczny, BM Sullivan, MF Bachmann, et al. (2007) Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317: 670-674.
- Davis KJ, AO Anderson, TW Geisbert, KE Steele, JB Geisbert, et al. (1997) Pathology of experimental Ebola virus infection in African green monkeys. Involvement of fibroblastic reticular cells. Arch Pathol Lab Med 121: 805-819.
- Steele KE, AO Anderson, M Mohamadzadeh (2009) Fibroblastic reticular cells and their role in viral hemorrhagic fevers. Expert Rev Anti Infect Ther 7: 423-435.
- Watanabe R, M Kakizaki (2017) Extracellular matrix in the CNS induced by neuropathogenic viral infection. Neuropathology in press.
- Luse S (1968) Â Pathologic Alterations in Oligodendrocytes. In Pathology of the Nervous System. J. Minckler, ed. McGraw-Hill Education, New York. pg: 547-553.
- Kashiwazaki H, M Kakizaki, Y Ikehara, A Togayachi, H Narimatsu, et al. (2014) Mice lacking alpha1,3-fucosyltransferase 9 exhibit modulation of in vivo immune responses against pathogens. Pathol Int 64: 199-208.
- Biswas SK, E Lopez-Collazo (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30: 475-487.
- del Fresno C, F GarcÃa-Rio, V Gómez-Piña, A Soares-Schanoski, I Fernández-RuÃz, et al. (2009) Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol 182: 6494-6507.
- Delano MJ, PO Scumpia, J S Weinstein, D Coco, S Nagaraj, et al. (2007) MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204: 1463-1474.
- Xiong Y, AE Medvedev (2011) Induction of endotoxin tolerance in vivo inhibits activation of IRAK4 and increases negative regulators IRAK-M, SHIP-1, and A20. J Leukoc Biol 90: 1141-1148.
- Kudo T, M Kaneko, H Iwasaki, A Togayachi, S Nishihara, et al. (2004) Normal embryonic and germ cell development in mice lacking alpha 1,3-fucosyltransferase IX (Fut9) which show disappearance of stage-specific embryonic antigen 1. Mol Cell Biol 24: 4221-4228.
- Rabinovich GA, Y van Kooyk, BA Cobb (2012) Glycobiology of immune responses. Ann N Y Acad Sci 1253: 1-15.
- Gringhuis SI, J den Dunnen, M Litjens, M van der Vlist, T. B. Geijtenbeek, et al. (2009) Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol 10: 1081-1088.
- Kakizaki M, A Togayachi, H Narimatsu, R Watanabe (2016) Contribution of Lewis X Carbohydrate Structure to Neuropathogenic Murine Coronaviral Spread. Jpn J Infect Dis 69: 405-413.
- Kawai T, S Akira (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34: 637-650.
- Landrigan A, MT Wong, PJ Utz (2011) CpG and non-CpG oligodeoxynucleotides directly costimulate mouse and human CD4+ T cells through a TLR9- and MyD88-independent mechanism. J Immunol 187: 3033-3043.
Citation: Watanabe R, Kakizaki M (2017) Highly Neurovirulent Viruses Invade and Spread in the Brain Using the Host Reactions Triggered by Infection. Diagn Pathol Open 2: 124. DOI: 10.4172/2476-2024.1000124
Copyright: © 2017 Watanabe R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4386
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 3545
- PDF downloads: 841