High Volume Image Guided Injections with or without Steroid for Mid-Portion Achilles Tendinopathy: A Pilot Study
Received: 29-Sep-2017 / Accepted Date: 11-Oct-2017 / Published Date: 18-Oct-2017 DOI: 10.4172/2329-910X.1000249
Abstract
Objective: High volume image guided injections of local anaesthetic and saline plus steroid plus structured rehabilitation (HVIGI and SR) have been shown to improve outcomes in Achilles tendinopathy (AT) patients. Due to concerns about steroid safety, our aim was to determine whether omitting the steroid altered the clinical effects of HVIGI and SR.
Methods: 23 consecutively recruited patients with AT (26 symptomatic tendons) were treated with a HVIGI composed of 10mls of 0.5% Marcaine mixed with either 40 ml of normal saline including 25 mg of hydrocortisone or 40 ml of saline only. Treatment allocation was determined by patient selection. The VISA-A questionnaire and visual analogue scales (VAS) were administered at pre-injection; 2, 6 and 10 weeks; and 12-month follow-up.
Results: VISA-A scores improved significantly in both groups, from a pre-injection mean (± SD) of 42.0 (± 20.5) to 86.9 (± 11.8) (p<0.01) in the ‘with steroid’ group, and from 42.6 (± 9.1) to 83.3 (± 12.2) (p<0.01) in the non-steroid group at final follow-up. VAS scores improved significantly in both groups. There was no statistically significance difference between the groups at baseline or any follow-up, in either VISA-A and VAS changes. A stronger effect size in favour of no steroid was seen at 10 weeks.
Conclusion: Overall, HVIGI and SR without steroid yields similar effects on pain reduction and functional improvement in comparison to HVIGI and SR with steroid. These results suggest that the effects of the injection may be mechanical rather than chemical, and that the steroid can be omitted. A randomised controlled trial is required to confirm this.
Keywords: Injection; Ultrasound; Corticosteroid; Achilles tendinopathy; Victorianinstitute of sports assessment–Achilles; Visual analogue scale; High volume
Introduction
Achilles tendinopathy is the most common foot and ankle overuse injury in sport. Among runners, Achilles tendinopathy is the most frequent overuse injury, accounting for 6-17% of all running injuries [1-3]. It is also the most common foot and ankle overuse injury in activities such as rugby union, football, and gymnastics [1,4,5] It is also common in non-athletes, with an incidence of 1.85 per 1000 in general practice [6]. It most commonly occurs in the mid portion of the Achilles tendon, an area 2-6 cm above the calcaneal insertion [7-9]. The condition is difficult to manage, with only extra corporeal shockwave therapy and eccentric loading exercises shown to have strong evidence of efficacy [10].
The diagnosis is a clinical one, best confirmed by imaging. Common imaging signs include tendon thickening, disorganised collagen structure, and neovascularisation around the tendon [11]. It has been shown by various case series that high volume injections deep to the tendon can alleviate pain and improve performance, and have long term success when combined with structured rehabilitation (SR). These effects are hypothesised to be mediated by local mechanical effects including stretching, breaking or occlusion of neo-vessels with disruption of the associated neural ingrowth [12,13]. Other possibilities are a chemical effect of steroid or local anaesthetic, or a combination of mechanisms. A number of studies have evaluated High Volume Image Guided Injection and structured rehabilitation (HVIGI and SR), and consistently found significant improvement in short and medium-term pain and function as well as a reduced grade of neovascularisation and tendon thickness [12-16]. One recent study showed greater improvement in the short term for HVIGI and SR when compared to platelet-rich plasma (PRP) injections and structured rehabilitation [17].
Typically, HVIGI has included 25 mg of hydrocortisone acetate a steroid although one evaluation with similar results used Aprotinin, a broad-spectrum serine protease inhibitor [18]. An early case series suggests promising results of a HVIGI and SR without corticosteroid or aprotinin, but there was no comparison group [16]. The rationale of each chemical additive is to prevent an acute inflammatory reaction in response to the large amount of fluid injected and to inhibit scarring [13]. The mechanism of action, and indeed requirement for any chemical additive to the saline volume is worthy of investigation so as to minimise the chances of tendon degradation due to steroid administration. This is especially pertinent as steroid use in soft tissue injections is widely debated, due to reported risks such as tendon degeneration and rupture [19]. Furthermore, there have been reports of 8% of patients reporting pain, and <1% report rupture in patients receiving corticosteroid injections for tendinopathy [20]. The complication rates are not clear from the literature but concerns persist.
The aim of this pilot study was to determine whether omitting the steroid from the injectates altered the clinical effects of HVIGI and SR. This would increase understanding of the mechanism of action, and would help determine whether the steroid could be omitted from future preparations of the injection, potentially improving the safety profile without a loss of efficacy. Patient selection was allowed to enable patient choice.
Materials and Methods
Procedure
Ethical approval was granted by the QMUL Ethics of Research Committee (QMREC2011/07) and consent for the study to take place granted by the London Independent Hospital medical advisory committee. Patients were sequentially recruited directly from the Sports Medicine clinic at the London Independent Hospital having been referred to one single radiologist for Achillodynia. The clinical diagnosis was ultrasonographically confirmed, via tendon thickness measurement, pain provocation using the ultrasound probe, exclusion of subtle tears and degree of neovascularisation [21-23]. The same ultrasound scanner was used for all patients (Siemens Medical Systems Incorporation, high specification Sonoline Elegra scanner, with a 13 MHz linear high-resolution probe).
The patient was asked for consent to the injection. Written consent for follow-up measures was obtained and a pre-injection VISA-A questionnaire completed based on the week prior to the injection. Patients were counselled regarding potential adverse events regarding corticosteroid use in the procedure, balanced with our experience of minimal side-effects and published case series, and then asked to make an informed decision as to whether they would receive steroid as part of the injection mixture or not.
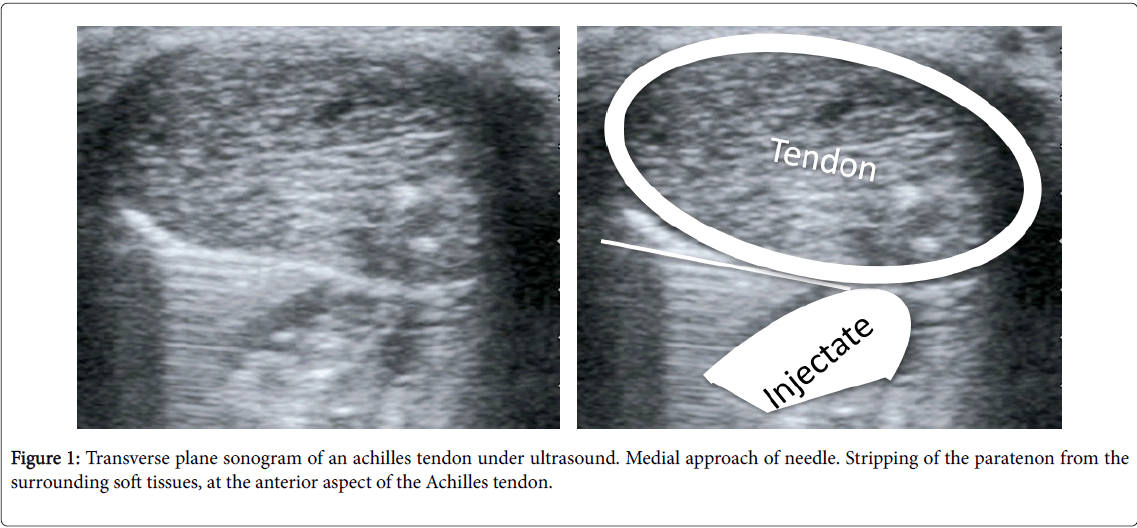
For the injection, the patient was positioned supine with the hip externally rotated, knees flexed to 45° and ankle plantargrade as per previous reports. Depending on the decision, patients were administered one or other injection using an aseptic technique, via a 21-gauge needle inserted with real time ultrasound guidance between the anterior aspect of the Achilles tendon and Kager’s fat pad, targeting the area of maximal neovascularisation (Figure 1) [12]. Only one injection was given per patient.
After the injection patients were advised to walk normally but advised to avoid any high impact activity for at least 72 hours. All patients were assessed by a single consultant physiotherapist, involved in the study but blind to patient group status following the injection, who prescribed structured rehabilitation with a focus on eccentric loading exercises to be completed twice daily, alongside any other antigravity muscle strengthening and sports-specific conditioning required. Further follow-up rehabilitation was in a place local to and convenient for the patient and followed clear guidance from the consultant physiotherapist.
Patients were emailed follow up VISA-A questionnaires at 2 weeks, 6 weeks and 10 weeks. The final follow up questionnaire at one year included a VAS scale to summarise improvement in pain since the injection.
Patients
23 consecutive patients with 26 tendinopathic tendons were injected (14 non-steroid, 12 steroids) and included in the study after meeting the inclusion and exclusion criteria outlined. Inclusion criteria were unilateral or bilateral mid-portion Achilles tendinopathy diagnosed clinically and confirmed on ultrasound with a neovascularisation grade of 3 or above, aged over 18 years and sportingly active [24]. Exclusion criteria were: previous Achilles rupture, previous surgery on the Achilles tendon, concurrent inflammatory disease, ultrasound detected tendon tear and an inability to complete questionnaires due to language barriers.
Patients had attempted numerous conservative management options prior to injection (eccentric loading exercises, massage therapy, orthotics, physiotherapy, acupuncture). As a minimum, they had to have attempted a trial of supervised progressive loading.
Outcome measures
The primary outcome measure in this study was the Victorian Institute of Sports Assessment–Achilles (VISA-A) questionnaire at 2- weeks, 6-weeks, 10-weeks and 12-months, a valid and reliable selfadministered tool in assessment of patients with Achilles Tendinopathy [25].
The questionnaire was developed specifically to assess function, pain and sporting activity in Achilles tendon pathology, and consists of 8 questions, yielding a measure on a 0-100 scale with 100 reflecting a perfect score [26].
The secondary outcome measure was a Visual Analogue scale (VAS) (0-100 mm) assessing pain and symptoms pre-and post-injection. The VAS consisted of a scale (0-100 mm) with 100 representing severe pain or severe symptoms, and 0 representing no pain or no symptoms.
Data analysis
Data was analysed using SPSS Statistical Package for Social Scientists, (version 20.0, IBM, UK). Significance was set at p<0.05. Shapiro Wilks tests were used to determine if outcome measures and baseline characteristics were normally distributed. Independent Student’s t tests were used to test for differences between groups for age, weight, duration of symptoms, sporting participation and VISA Scores at baseline.
The non-parametric Mann-Whitney test was used to assess for differences between groups in height. Pearson Chi-Square test was used to test for difference in gender between the two groups. Within group differences were tabulated and plotted to observe for change over time and enable comparison with previously published data.
A two-way (Time × Treatment Group) mixed analysis of variance (ANOVA) was used to identify differences in VISA-A scores between treatment conditions. The Greenhouse-Geisser correction was used for epsilon <0.75, while the Huynh-Feldt correction was used adopted for less severe asphericity. When significant F values were found, Bonferroni corrected P-values were used to determine the location of the variance. Independent t-tests were used to compare changes (preinjection to post-injection) in reported VAS pain and VAS symptoms between the treatment groups
Effect sizes (differences in change scores/pooled baseline [preinjection] SD) were also used to convey the magnitude of observed differences, and were interpreted with the following scale: <0.2 trivial; 0.2 to 0.6 small; 0.6 to 1.2 moderate; and >1.2 large [27].
Results
There were no significant differences between groups at baseline in age, gender, duration of symptoms, height, weight, duration of weekly sporting activity and pre-injection VISA-A scores (Table 1). No patients were lost to follow up at 10 weeks. 3 patients failed to complete the 12-month follow up questionnaire (2 steroids, 1 non-steroid).
| Baseline characteristics | Non-Steroid Group (Mean (± SD)) |
Steroid Group (Mean (± SD)) |
Significance |
|---|---|---|---|
| Age (years) | 48.2 (± 9.6) | 46.0 (± 13.0) | p=0.97 |
| Duration of symptoms (months) | 12.4 (± 10.1) | 12.6 (± 13.8) | p=0.83 |
| Height (cm) | 173.4 (± 9.6) | 171.7 (± 20.5) | p=0.34 |
| Weight (kg) | 80.3 (± 17.5) | 80.4 (± 18.8) | p=0.98 |
| Weekly sporting activity (hours) | 7.6 (± 4.1) | 5.2 (± 3.2) | p=0.14 |
| Pre-injection VISA A scores | 42.0 (± 20.5) | 42.6 (± 9.1) | p=0.93 |
| Gender | 8 males, 4 females | 7 males, 3 female |
Table 1: Baseline characteristics of patients included in study.
The two-way mixed ANOVA showed a significant effect of Time (p<0.001), but no significant effect for Treatment Group (p=0.53) or for the interactive effect of Time × Treatment Group (p=0.07).
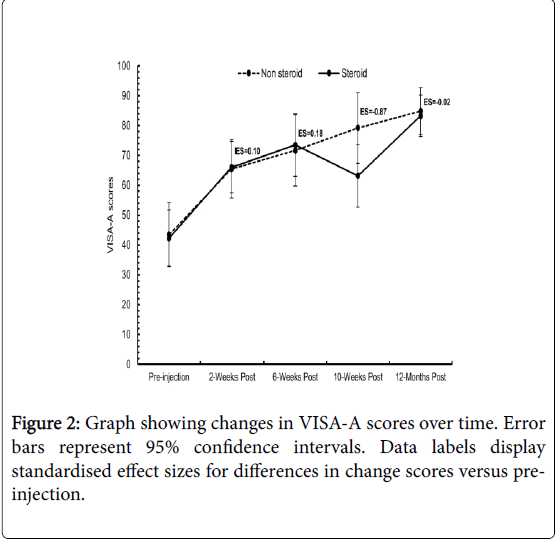
Effect sizes (ES) for the differences in change scores between treatment groups were all trivial (<0.20), except for the 10-weeks postinjection time point, which displayed a moderate (ES=-0.87) effect size in favour of the non-steroid group (Figure 2). Individual patient data are shown in Table 2.
| Patient | Group | Return to Sport | Exercise Compliant | % Effectiveness | Complications |
|---|---|---|---|---|---|
| 1 | Steroid | Yes–At Same Level | Yes | 100, then 50 after return to sport | None |
| 2 | Non-Steroid | Yes–At Reduced Level | Yes | 80 | None |
| 3 | Non-Steroid | Yes-At Reduced Level | Yes | 20 | None |
| 4 | Non-Steroid | Yes–At Same Level | Yes | 50 | None |
| 5 | Non-Steroid | Yes–At Same Level | Yes | 60 | None |
| 6 | Non-Steroid | Yes–At Reduced Level | No | 30 | None |
| 7 | Non-Steroid | Yes–At Reduced Level | Yes | 90 | Post injection soreness lasting a week |
| 8 | Non-Steroid | Yes–At Same Level | Yes | 50 | None |
| 9 | Non-Steroid | Yes–At Same Level | Yes | 85 | None |
| 10 | Non-Steroid | Yes–At Improved Level | Yes | 75 | None |
| 11 | Steroid | Yes–At Reduced Level | No | 70 | None |
| 12 | Non-Steroid | Yes–At Same Level | Yes | 10 | None |
| 13 | Steroid | Yes–At Improved Level | Yes | 90 | None |
| 14 | Non-Steroid | Yes–At same Level | Yes | 70 | None |
| 15 | Steroid | No | Yes | 80 | None |
| 16 | Steroid | Yes–At same Level | Yes | 90 | None |
| 17 | Steroid | Yes–At Reduced Level | Yes | 70 | None |
| 18 | Non-Steroid | Yes–At Same Level | Yes | 100 | None |
| 19 | Steroid | No | No | 20 | None |
| 20 | Steroid | Yes–At Same Level | Yes | 100 | None |
| 21 | Non-Steroid | Yes–At Same Level | Yes | 80 | None |
| 22 | Steroid | Yes–At Same Level | Yes | 90 | None |
| 23 | Steroid | No | Yes | 20 | None |
Table 2: Responses regarding return to sport, compliance, injection satisfaction and complications.
VISA-A
Patients showed significant improvements from pre-injection to all follow ups in VISA-A scores in both the steroid and non-steroid groups.
VAS
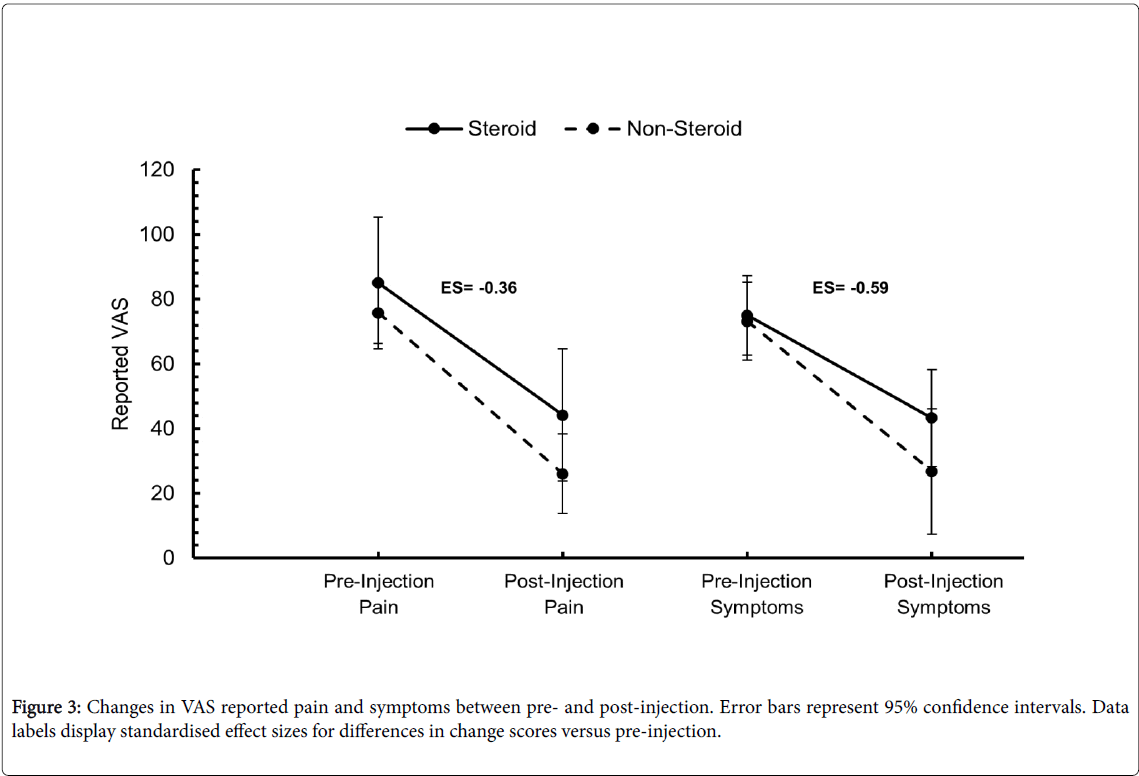
There was no statistically significant difference in the VAS changes for pain (p=0.42) or symptoms (p=0.23) between the two treatment groups. Effect sizes for the difference in change scores between treatment groups were small (ES=-0.36) and large (ES=-0.59) for pain and symptoms, respectively, with both suggesting a larger improvement in the non-steroid group in comparison to the steroid group (Figure 3).
In the steroid cohort, the pre-injection VAS scores improved by 40.8 mm for pain (p=0.01), and 31.7 mm (p=0.05) for symptoms post injection. An improvement of 49.7 mm for pain (p<0.01), and 46.3 mm for symptoms (p<0.01) was seen in the non-steroid cohort.
Discussion
This prospective pilot study showed encouraging short-term outcomes in patients with chronic Achilles tendinopathy receiving a high-volume injection of normal saline with anaesthetic, with similar impressive results after treatment to patients receiving a high-volume injection with the traditional steroid component. HVIGI and SR both with and without steroid improved self-reported pain, function and resulted in a return to sporting participation in the majority of patients. This is similar to the recent findings by Boesen et al [17]. Short-term post injection soreness was reported by a patient in the non-steroid group; Resteghini et al. also reported this side effect in patients receiving a HVIGI and SR with steroid [14]. There was no significant difference between groups at any time stage although the effects of the injection without steroid were more marked at 10 weeks.
Injections for Achilles tendinopathy have become commonplace within the Sports Medicine fields, with numerous substances used by clinicians. Research on the use of Platelet Rich Plasma, sclerosing injections, corticosteroid and Aprotinin have shown less than promising long-term results [18,26,28,29]. A number of studies examining high volume injections as an alternative to other injection treatments for Achilles tendinopathy have shown improvements in both sonographic and clinical outcomes [12-15]. Chan et al. hypothesised that the high volume of fluid being injected would cause a local mechanical effect causing neovessels to stretch, occlude or break [12,13]. The possibility of removing the steroid from the injectates has been postulated for some time, with patients declining corticosteroid administration providing a unique control group in which to compare the effects of each technique. A single case series has been performed demonstrating positive outcomes for a HVIGI and SR without steroid or aprotinin [16]. This was the first to directly compare outcomes with a group of patients receiving a HVIGI with steroid. The results suggest that the injection effect is the local mechanical effect of the high volume of fluid with the drug effects being negligible. However, we cannot yet judge the extent of the role of the local anesthetic in the therapeutic effect of the injections.
The strengths of this study were the presence of two treatment groups, the prospective nature of the study, the use of an independent assessor of outcome blinded to the radiographic assessment and use of steroid, the use of a validated outcome measure (VISA-A questionnaire), the presence of multiple follow ups including a longterm measure, a structured rehabilitation programme and a single radiologist assessing and injecting all patients. The main weakness is that patients were allowed to choose their treatment, although within the health service patient choice is deemed exceptionally important. Expectation theory suggests that ‘people make decisions among alternate plans of behaviour based on their perceptions of the degree to which a given behaviour will lead to desired outcomes’ [30]. The idea behind this theory is that an individual will be inclined to believe their decision will lead to the desired outcome [31]. In this study, the most common desired outcome was to reduce pain and increase activity. The choice of steroid or non-steroid could determine the approach of the patient to the outcome, adherence to the structured loading programme, and could therefore lead to positive outcomes by different mechanisms than those induced by the injection and rehabilitation alone. The potential for clinician induced bias due to injection selection was controlled for by standardising the rhetoric to all patients in order to facilitate clinician and patient equipoise. However, as a pilot study this does demonstrate that further research is required and a randomised trial is now warranted to confirm the results.
A limitation is that the study did not assess any imaging outcomes, limiting possible exploration of differential effects on tendon structure or neovascularity, and therefore the mode of action of the injection without steroid. While the length of final follow-up gives a long-term indication of the clinical outcome of the injection, the presence of an imaging follow-up assessing changes in neovascularisation and maximal tendon thickness would add to the validity and clinical applicability of the findings. All patients were recruited from the same centre, a private hospital. However, the location of the hospital in the multicultural and diverse district of East London adds to the applicability of the findings, as does the mixed nature of professional, recreational and non-sporting participants that are referred to the clinic.
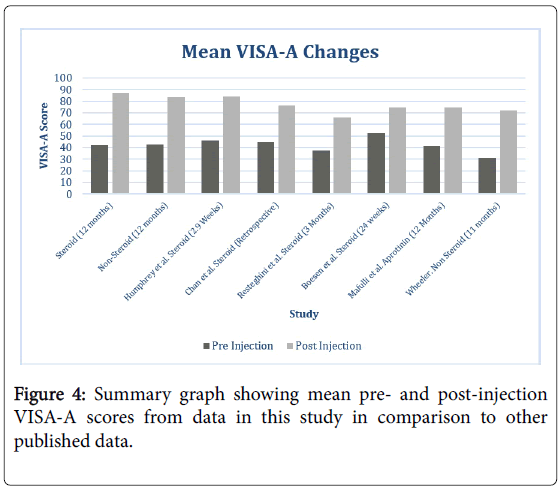
The positive improvements seen in this study are broadly in line with previous research conducted on HVIGI and SR (Figure 4) [12-16]. Whilst at the 2 week follow up the improvement seen in the VISA score is less than the numbers reported by Humphrey et al. and Chan et al., at the final follow up the mean change in VISA-A score seen in the non-steroid group is higher [12,15]. Resteghini et al. used the same HVIGI and SR procedure and found comparable results at 3 months while the one year follow up VISA-A is higher in each group of the current study compared to the study using aprotinin, no longer licensed for the purpose of HVIGI and SR [14]. The findings of a similar outcome between the two groups in this study suggest a HVIGI and SR without steroid could be the ideal treatment initially, as it can be considered as theoretically safer without the potential risks of the steroid component, more appropriate for competing athletes and marginally cheaper.
Conclusion
HVIGI and SR is likely an effective treatment in patients with Achilles tendinopathy struggling with conservative management options. This pilot prospective cohort study showed HVIGI and SR with just saline is just as effective in improving clinical outcome in patients with Achilles tendinopathy, and could be the better management option. The necessity of further research is highlighted, such as including imaging outcome measures, a larger sample size, actively seeking adverse event notifications, randomisation and blinding of participants, but the findings from this initial study encourage reconsideration of injection contents and increase understanding of the mechanisms underlying observed effects. Full scale clinical trials are warranted.
Funding
Dr Morrissey is part funded by the NIHR/HEE Senior Clinical Lecturer scheme. This report presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Sobhani S, Dekker R, Postema K, Dijkstra PU (2013) Epidemiology of ankle and foot overuse injuries in sports: A systematic review. Scand J Med Sci Sports 23: 669-686.
- Longo UG, Rittweger J, Garau G, Radonic B, Gutwasser C, et al. (2009) No influence of age, gender, weight, height, and impact profile in achilles tendinopathy in masters track and field athletes. Am J Sports Med 37: 1400-1405.
- Knobloch K, Yoon U, Vogt PM (2008) Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int 29: 671-676.
- Sankey RA, Brooks JH, Kemp SP, Haddad FS (2008) The epidemiology of ankle injuries in professional rugby union players. Am J Sports Med 36: 2415-2424.
- Walls RJ, Brennan SA, Hodnett P, Byrne JM, Eustace SJ, et al. (2010) Overuse ankle injuries in professional Irish dancers. Foot Ankle Surg 16: 45-49.
- de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, et al. (2011) Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med 45: 1026-1028.
- Papa JA (2012) Conservative management of Achilles Tendinopathy: A case report. J Can Chiropr Assoc 56: 216-224.
- Zanetti M, Metzdorf A, Kundert HP, Zollinger H, Vienne P, et al. (2003) Achilles tendons: Clinical relevance of neovascularization diagnosed with power Doppler US. Radiology 227: 556-560.
- Rompe JD, Furia JP, Maffulli N (2008) Mid-portion Achilles tendinopathy--current options for treatment. Disabil Rehabil 30: 1666-1676.
- Rompe JD, Furia JP, Maffulli N (2008) Mid-portion Achilles tendinopathy--current options for treatment. Disabil Rehabil 30: 1666-1676.
- Maffulli N (1999) Tendon Problems: A basic science primer. J Sports Traumatol 21: 3-10.
- Rowe V, Hemmings S, Barton C, Malliaras P, Maffulli N, et al. (2012) Conservative management of midportion Achilles tendinopathy: a mixed methods study, integrating systematic review and clinical reasoning. Sports Med 42: 941-967.
- Chan O, O'Dowd D, Padhiar N, Morrisse D, King J, et al (2008) High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil 30: 1697-1708.
- Resteghini P, Yeoh J (2012) High Volume Injection in the management of recalcitrant mid-portion Achilles Tendinopathy: A prospective case series assessing the influence of neovascularity and outcome. Int Musculosk Med 34: 100-192.
- Maffulli N, Spiezia F, Longo UG, Denaro V, Maffulli GD (2013) High volume image guided injections for the management of chronic tendinopathy of the main body of the Achilles tendon. Physical Ther Sport 14: 163-167.
- Resteghini P, Yeoh J (2012) High Volume Injection in the management of recalcitrant mid-portion Achilles Tendinopathy: A prospective case series assessing the influence of neovascularity and outcome. Int Musculosk Med 34: 100-192.
- Humphrey J, Chan O, Crisp T, Padhiar N, Morrissey D, et al. (2010) The short-term effects of high volume image guided injections in resistant non-insertional Achilles tendinopathy. J Sci Med Sport 13: 295-298.
- Wheeler P (2014) The use of high volume image guided injections (HVIGI) for Achilles Tendinopathy-A case series and pilot study. Int Musculosk Med 36: 96-103.
- Shrier I, Matheson GO, Kohl HW 3rd (1996) Achilles tendonitis: Are corticosteroid injections useful or harmful? Clin J Sports Med 6: 245-250.
- Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H (2017) Effect of high-volume injection, platelet-rich plasma plasma, and sham treatment in chronic midportion achilles tendinopathy: A randomized double-blinded prospective study. Am J Sports Med 45: 2034-2043.
- Brown R, Orchard J, Kinchington M, Hooper A, Nalder G (2006) Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. Brit J Sports Med 40: 275-279.
- Webborn N, Morrissey D, Sarvananthan K, Chan O (2015) Tears of the fascia cruris at the attachment to the achilles tendon. Br J Sports Med 49: 1398-1403.
- Coombes BK, Bisset L, Vicenzino B (2010) Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: A systematic review of randomised controlled trials. Lancet 376: 1751-1767.
- Chan O MS, Pritchard M, Parkes T, Malliaras P, et al. (2017) Intratendinous tears of the Achilles tendon: A new pathology? Analysis of a large 4-year cohort. Muscles Ligaments Tendons J 7: 53-61.
- Robinson JM, Cook JL, Purdam C, Visentini PJ, Ross J, ET AL. (2001) The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med 35: 335-41.
- Webborn N, Morrissey D, Sarvananthan K, Chan O (2015) Tears of the fascia cruris at the attachment to the achilles tendon. Br J Sports Med 49: 1398-1403.
- Morton S, Chan O, Webborn N, Pritchard M, Morrissey D (2016) Tears of the fascia cruris demonstrate characteristic sonographic features: a case series analysis. Muscles Ligaments Tendons J 5: 299-304.
- Ohberg L, Lorentzon R, Alfredson H (2001) Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc 9: 233-238.
- Robinson JM, Cook JL, Purdam C, Visentini PJ, Ross J, ET AL. (2001) The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med 35: 335-41.
- de Jonge S, de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, et al. (2011) One-year follow-up of platelet-rich plasma treatment in chronic achilles tendinopathy a double-blind randomized placebo-controlled trial. Am J Sports Med 39: 1623-1629.
- http://www.psu.edu/search/gss?query=PSYCH484/Expectancy%2BCase%2BStudy.
Citation: Abdulhussein H, Chan O, Morton S, Kelly S, Padhiar N, et al. (2017) High Volume Image Guided Injections with or without Steroid for Mid-Portion Achilles Tendinopathy: A Pilot Study. Clin Res Foot Ankle 5: 249. DOI: 10.4172/2329-910X.1000249
Copyright: © 2017 Abdulhussein H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6852
- [From(publication date): 0-2017 - Dec 06, 2025]
- Breakdown by view type
- HTML page views: 5807
- PDF downloads: 1045