Review Article Open Access
High Throughput Detection Methods for Multiplex Mycotoxins
Jianlin Li1*, Yang Deng1, Yan Liu1, Zhi Ding1, Yichen Li1, Yanhao Jin1, Xuerui Zhu1, Tingting Cai1, Tiesong Zheng1* and Wei Li2
1Department of Food Science and Engineering, Nanjing Normal University, Nanjing 210024, China
2Department of Electronic and Electrical Engineering, The University of Sheffield, Sheffield, S3 7HQ, United Kingdom
- *Corresponding Authors:
- Jianlin Li
Department of Food Science and Engineering
Nanjing Normal University, Nanjing 210024, China
Tel: +86 25 83598286
E-mail: jianlinli82003@aliyun.com
- Tiesong Zheng
Department of Food Science and Engineering
Nanjing Normal University, Nanjing 210024, China
E-mail: tieszheng@sina.com
Received Date: July 19, 2017; Accepted Date: August 17, 2017; Published Date: August 23, 2017
Citation: Li J, Deng Y, Liu Y, Ding Z, Li Y, et al. (2017) High Throughput Detection Methods for Multiplex Mycotoxins. Toxicol Open Access 3:131. doi: 10.4172/2476-2067.1000131
Copyright: © 2017 Li J, et al. This is an open-access article distributed under the terms of the creative commons attribution license, which permits unrestricted use,distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
Mycotoxins are toxic low-molecular-weight compounds which produced by the metabolism of certain fungi species. Due to their toxicity, chemistry stability, diversity and co-occurrence in agriculture products, it is urgently needed to develop the rapid, simple and effective detection technique methods to monitor and prevent mycotoxin contamination in whole food chain. This article gives a review about high throughput detection methods for multiplex mycotoxins which mainly includes chromatographic instrument techniques, microarray chip, suspension array, lateral flow biosensors, surface plasmon resonance (SPR) and nanoparticle-based biosensors. The advantages and disadvantages and the key steps of them have been discussed. The insight and evaluation of the technique progress were given, which would be helpful to further develop this filed.
Keywords
Mycotoxins; High-throughput detection; Research advance
Abbreviations
AFB1-2: Aflatoxin B1-2; AFG1-2: Aflatoxin G1-2; AFM1: AflatoxinM1; DON: Desoxynivalenol; FB1: Fumonisin B1; OTA: Ochratoxin A; ZON: Zearalenone; HT-2: HT-2 Toxin; CIT: Citrinin; ZEN: Zearalanone; NIV: Nivalenol; NEO: Neosolaniol; DAS: Diacetoxyscirpe-nol; FUS-X: Fusarenon-X; 3-ADON: 3- Acetyldeoxynivalenol; 15-ADON: 15-Acetyldeoxynivalenol; TMB: 3,3′, 5,5′-tetramethylbenzidine; QDs: Quantum Dots; HRP: Horseradish Peroxidase; SPR: Surface Plasmon Resonance; Ispr: Imaging SPR; HPLC: High Performance Liquid Chromatography; GC-MS: Gas Chromatography and Mass Spectrometry; LC/MS/MS: Liquid Chromatography and Mass Spectrometry; UPLC-MS/MS: Ultra- Performance Liquid Chromatography-Tandem Mass Spectrometry; IAC: Immunoaffinity Columns; LOD: Limits of Detection; LOQ: Limit of Quantitation; ELISA: Enzyme-Linked Immunosorbent Assay; ESI: Electrospray Ionisation; APCI: Atmospheric Pressure Chemical Ionization; FAO: The Food and Agriculture Organization; FLD: Fluorescence Detector; UV: Ultraviolet Detector; Xmap: Multi Analyte Profiling; QuEChERS: Quick, Easy, Cheap, Effective, Rugged and Safe; SPE: Solid-Phase Extraction; LE: Liquid-Liquid Extraction; SPDE: Solid Phase Dispersion Extraction; PLE: Pressurized Liquid Extraction; SPCMs: Silica Photonic Crystal Microspheres.
Introduction
Mycotoxins are toxic low-molecular-weight compounds which produced by the metabolism of certain fungi species. Nowadays, hundreds of mycotoxins have already been isolated and identified and the most common toxin-producing genera are Aspergillus , Penicillium , Alternaria and Fusarium fungi which correspondingly produced aflatoxins (AFs), ochratoxins (OTA), patulin (PAT), and fusarium toxins [1]. Most of the mycotoxins have been identified as carcinogenic, mutagenic, and teratogenic agents. Many countries and regions have set maximum limits tolerable for the mycotoxins in food and feed to protect human and animal health [2].
Mycotoxins often appear in cereal crops, stored cereals and feedstuff, which can easily enter the food chain when the agricultural commodities are harvested, stored, transported and processed in the absence of proper conditions. The Food and Agriculture Organization (FAO) estimated that about 25% of the world’s food crops are contaminated by mycotoxins, resulting in annual losses of about 1 billion metric tons of food and food products equivalent to about 5 billion dollars per year in Unite States and Canada [3-5]. The mycotoxins display the properties of chemical and thermal stability, which result in that they are difficult to remove once they contaminate the food chain. Therefore, the first priority is to develop the rapid, simple and effective detection technique methods to prevent the mycotoxin contamination from food chain, especial for the developing countries.
Generally, co-occurrence of multiplex mycotoxins is often found in the same sample because a single species of fungi can produce several toxic metabolites, or several species can be present simultaneously, producing different toxins [5]. In addition, various types of mycotoxins also often are present in mixtures of different raw material samples, such as breakfast cereals, flours and bread [5]. These co-occurrence multiplex mycotoxins show additional or synergistic toxic effects [5-10]. It is very necessary to rapidly high throughput screen or detect the multiplex mycotoxins in the samples for accurate risk assessment of food safety. Here, high throughput detection or screening method refers to that it can simultaneously and rapidly detect or screen multiplex target molecules.
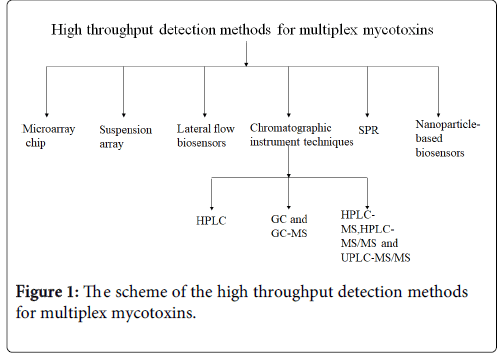
At present, the multiplex mycotoxin detection or screening methods mainly included chromatographic instrument techniques, microarray chip, suspension array, lateral flow biosensors, SPR and nanoparticlebased biosensors (Figure 1). The article will give a short review about these detection methods for multiplex mycotoxins.
Chromatographic Instrument Techniques
Chromatographic instrument techniques mainly include high performance liquid chromatography (HPLC) [11], gas chromatography and mass spectrometry (GC-MS [12], GC- MS/MS [13]), liquid chromatography and mass spectrometry (LC/MS/MS [14]) and other instrumental analysis methods. Generally, chromatographic detection methods are often used for qualitative and quantitative analysis for mycotoxin confirmatory analyses. These techniques, especial for HPLC-MS/MS, are becoming mainstream methods for rapid screening of multiplex mycotoxins because of their high sensitivity, high accuracy, good reproducibility and short detection time.
HPLC
HPLC is one of the most used techniques for qualitative and quantitative of mycotoxin detection, which used different extraction, purification and sensitive detection system equipped with ultraviolet detector (UV), fluorescence detector (FLD), differential detector, photodiode array and ammeter. For example, Chan et al. have established an automated HPLC method with immune affinity column purification and FLD for the simultaneous detection of aflatoxins (AF) and ochratoxin A (OTA) [15]. Limits of detection (LOD) were estimated as 0.2 μg/kg for OTA and AFB1, AFB2, AFG1 and AFG2 and the recovery is 72%~101% [15]. Ainiza et al. developed a reverse-phase HPLC and fluorescence detector (FLD) with a photochemical derivatisation system for simultaneous determination of AFs and OTA using amulti-mycotoxin immunoaffinity column purification. The recovery is 72%~100% for AFs and 73%~113% for OTA. The LOD was 0.1 μg/kg for AFB1/AFG1, 0.05 μg/kg for AFB2/AFG2 and 0.1 μg/kg for OTA [16]. Kong et al. developed a HPLC method with ultrasoundassisted solid-liquid extraction and immunoaffinity column clean-up coupled with on-line post-column photochemical derivatizationfluorescence detection for simultaneous multi-mycotoxin determination of AFs and OTA. The recovery and LOD showed the satisfied results in 13 edible and medicinal nutmeg samples [17]. Recently, Xu et al. set up an ultra HPLC coupled with photo-diode array detection method for the simultaneous determination of deoxynivalenol and its acetylated derivatives with quick, easy, cheap, effective, rugged and safe (QuEChERS) solid-phase extraction (SPE) purification, which has the LOD (21.7-57.4 μg/kg) and quantitation (72.3-191.4 μg/kg) for deoxynivalenolsin in wheat flour and rice [18].HPLC could provide a reliable, high sensitivity, high resolution and good reproducibility results for multiplex mycotoxins. However, the main problem of multiplex mycotoxin analysis is not detection but samples pretreatment for obtaining a selective and fast extraction of mycotoxins from the matrix [19]. Therefore, most of them required complex pretreatment of samples such as multi-mycotoxin immunoaffinity column purification, C18 column purification or supercritical fluid extraction, and post-column derivatization which limited their application in practical. QuEChERS extraction and new SPE purification system for HPLC methods for detection of multiplex mycotoxin should be widely developed and practiced. The detailed sample pretreatment for mycotoxins from matrix can be seen in reference [5].
GC and GC-MS
Though GC and GC-MS are cheaper than HPLC and HPLC-MS, GC and GC-MS have been less widely used for multiplex mycotoxin analysis comparing with HPLC and HPLC-MS. Only a few of the references about GC and GC-MS for multiplex mycotoxin analysis were reported, which may be ascribed to the complicated sampling procedures such as derivatization although methods without derivatization have been reported.
Ryu et al. used GC-MS to simultaneously quantitatively detect seven trichothecene mycotoxins after trimethyl silyl derivatization [20]. Nielsen et al. developed a fast method for simultaneously screening of trichothecences in species of Fusarium , Stachybotrys , Trichoderma and Memnoniella by micro scale extraction and derivatized with pentafluoropropionic anhydride or heptafluorobuturyl imidazole [21]. Rodríguez-Carrasco et al. established GC-MS/MS method to determine 15 mycotoxins and metabolites in human urine with salting-out assisted acetonitrile-based extraction, which displayed a range of 72-109% recoveries and limit of quantitation ranged from 0.25 to 8 μg/kg [22]. Cegielska-Radziejewska et al. established the simultaneous determination of seven trichothecences (T-2, HT-2, DAS, DON, 3-ADON, 15-ADON and NIV) by GC-MS in feed mixtures for broiler chickens [23]. Ibáñez-Vea et al. developed a validated method for the simultaneous determination of eight type-A and type-B trichothecenes in barley by simultaneous extraction sample with acetonitrile-water (84:16), cleaning up with Multisep columns, derivatization with pentafluoropropionic anhydride and imidazole and GC-MS analysis [24]. Escrivá et al. recently reported a method for the simultaneous determination of seven trichothecenes, neosolaniol (NEO), diacetoxyscirpe-nol (DAS), deoxynivalenol (DON), nivalenol (NIV), fusarenon-X (FUS-X), 3-acetyldeoxynivalenol (3-ADON) and 15-acetyldeoxynivalenol (15-ADON) in feed samples with N,Obis( trimethylsilyl) acetamide+trimethylchlorosilane+N-trimethylsilyimidazole (3:2:3) as the derivatization reagent under extracting and purifying samples with acidified mixture of acetonitrile/water [25]. LOQs were between 1 and 10 μg/kg for all studied trichothecenes and recoveries ranged from 62% to 97% [25].
These GC and GC-MS methods for multiplex trichothecene mycotoxins provided the lower LODs, greater selectivity, higher precision and inexpensive analytical performance. However, some mycotoxin specific problems such as non-linearity of calibration curves, poor repeatability, matrix induced over-estimation, and memory effects from previous sample injections, which make them still limited in narrower analytical scope [26].
HPLC-MS, HPLC-MS/MS and Ultra HPLC-MS/MS (UPLCMS/ MS)
Over the last years, a significant number of papers for analysis of multiplex mycotoxins have focused increasingly on HPLC-MS, HPLCMS/ MS and UPLC-MS/MS. Ionization is an important procedure for Liquid chromatographic/mass spectrometric (LC-MS) methods, especially for different food matrix. After atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) have been realized since the mid to late 90s, LC-MS methods have been rapidly developed [27]. LC-MS/LC-MS/MS/UPLC-MS/MS methods for multiplex mycotoxin analysis were listed in Table 1, which showed that ESI interface has been used in majority of LC-MS/MS/UPLCMS/ MS. ESI interface is mainly well suited for the analysis of polar compounds, whereas other atmospheric pressure ionization (API) interfaces like APCI and atmospheric pressure photo ionization (APPI) are highly effective for the analysis of medium and low-polar substances [5]. These multiplex mycotoxin analysis techniques could provide high sensitive, selective, rapid and reliable quantification and confirmation at the low concentration. These methods rely mainly on multiple mycotoxin parallel or sequential sample preparation strategies of one sample followed by separate analysis of each isolated class of mycotoxins in a single run [41].
| LC-MS | Mycotoxins | Matrix | Sample preparation | Column | Ionisation/ion selection | LOD/LOQ (μg/kg) |
Recovery (%) | References |
|---|---|---|---|---|---|---|---|---|
| UPLC-MS/MS | 6 mycotoxins | Wheat flour and rice | QuEChERS-SPE filter | BEH C18 | ESI | 21.7-57.4 | 80-104.4 | [18] |
| UPLC-MS/MS | 36 mycotoxins | wines | QuEChERS | BEH C18 | ESI | 0.1 | 70-120 | [28] |
| LC-MS | FB1,HFB1 | Corn products | LEP and C18 cleanup | 60-RP B | ESI | 5 | 54.6-97.4 | [29] |
| LC-MS/MS | A,B-Tichothecenecs,zearalenone | maize | LEP-MycoSep®#226, 227 cleanup | RP-18 | ESI | 0.3 | 30-99 | [30] |
| UPLC-MS | 17 mycotoxins | Corn feed, peanuts | LEP-MycoSep®#226, cleanup | BEH C18 | ESI | 0.01-0.7 | 70.7-119 | [31] |
| LC-MS/MS | 24 mycotoxins | Sweet pepper | LEP, NH2-SPE column, C18-SPE column | C18 | ESI | 0.32-42.48 | 76-110 | [32] |
| UPLC-MS/MS | 11 mycotoxins | Maize,whea, rice, oat, barley, rye, sorghum, millet | LEP-nylon filter | BEH C18 | ESI | 0.1 | 70-110 | [33] |
| LC-MS/MS | 31 mycotoxins | Wheat, barley, oats | the accelerated solvent extraction | Inertsil ODS-EP | ESI | 1-1250 | 51-122 | [34] |
| UPLC-MS/MS | 12 mycotoxins | beer | SPE with C18 | BEH C18 | ESI | 0.02-0.14 | 70-106 | [35] |
| LC-MS/MS | 99 mycotoxins | Home dust | LE | Gemini® C 18 | ESI | 0.005-2200 | <50for half of samples | [36] |
| LC-MS/MS | 87 mycotoxins | bread | LE | Gemini® C 18 | ESI | 0.02-225 | 55-287 | [37] |
| LC-MS/MS | DON,ZON and their metabolites | Cereal-based food, beer | LE-C18-SPEcleanup, LE-Immunoaffinity, LE-MycoSep226 | RP-C 18 | ESI | 0.5-50 | 89-119 | [38] |
| UPLC-MS/MS | 5 mycotoxins | traditional Chinese medicines | LE-cleanup cartridge; 0.22μm filter | HSS T3 | ESI | 0.29-0.99 | 88.5-119.5 | [39] |
| LC-MS/MS | DON,ZEN,T-2,HT-2 | Wheat and biscuit | LE-Immunoaffinity | C18 | ESI | 0.03-0.33 | 78-109 | [40] |
| LC-MS/MS | Six mycotoxins | Wheat and maize | LE-Immunoaffinity, Ultrasonic LE-syringe filter | RP-C 18 | pressure chemical ionization (APCI) | 1.5-4.7 | 35-175 | [41] |
| LC-MS/MS | Six mycotoxins | urine | LE-Immunoaffinity | C18 | ESI | 0.01-2.2 | 62-96 | [42] |
| LC-MS/MS | Eleven mycotoxins | Cereal samples | LE-0.22μm filter | C18 | ESI APCI | 0.01-20 | 76.8-108.4 | [43] |
| UPLC-MS/MS | 11 mycotoxins | Wheat, rice, oat, maize, barley | LE-0.22μm filter | C18 | ESI APCI | 0.01-25 | 83.5-107.3 | [44] |
| LC-MS/MS | AFB1, AFB2, AFG1, AFG2 | Lotus seeds | LE- Immunoaffinity | Zorbax SBC18 | ESI | 0.003-0.007 | 66.3-110 | [45] |
| LC-MS/MS | Monacolins and citrinin | Red fermented rice | LE- 045μm filter | Zorbax SBC18 | ESI | 0.0005 | 98.3-99.4 | [46] |
| UPLC-MS/MS | 12mycotoxins | pig urine | salting-out assisted LLE | C18 | ESI | 0.07-3.3 | 70-108 | [47] |
| UPLC-MS/MS | 4 Trichothecenes | potato | LE-PriboFast 270 column-0.22μm filter | BEHC18 | ESI | 0.002-0.005 | 77.97-113.28 | [48] |
| UPLC-MS/MS | 6mycotoxins | Feed | LE-carbon nanorubes SPE-0.22μm filter | BEHC18 | ESI | 0.2-0.29 | 95.3-107.2 | [49] |
| UPLC-MS/MS | AFB1,OTA, ZON,ZOL | Milk | LE-Mycosep226 filter | BEHC18 | ESI | 0.003-0.015 | 87-109 | [50] |
| LC-MS/MS | Trichothecenes deoxynivalenol | chicken | LE-HLB cartridge 0.22μm filter | C18 | ESI | 0.16-2.07 | 73.7-106.4 | [51] |
| LC-MS/MS | 16mycotoxins | Dried fruit | QuEXhERS-C18- 0.22μm filter | C18 | ESI | 0.08-15 | 60-135 | [52] |
| LC-MS/MS | 19mycotoxins | Biscuits | SPE- 0.2μm filter | C18 | ESI | 0.04-80.2 | 63-107 | [53] |
| LC-MS/MS | 12mycotoxins | Maize, wheat, corn | LE- Immunoaffinity | C18 | ESI | 0.2-0.5 | 63-88 | [54] |
| LC-MS/MS | 18mycotoxins | wheat | solid phase dispersion extraction (MSPD) - 0.2μm filter | C18 | ESI | 1.0-250 | 68-89 | [55] |
| UPLC-MS/MS | 7 mycotoxins | Dried fruit | Pressurized liquid extraction(PLE)-C18 | PFP column | heated (H-ESI) | 2-110 | 83-103 | [56] |
| LC-MS/MS | 21mycotoxins | coffee beverages | LE- 0.22μm filter | C18 | ESI | 0.02-39.64 | 72-97 | [57] |
| LC-MS/MS | 15mycotoxins | eggs | QuEChERS | C18 | ESI | 0.2-5 | 67.5-105.4 | [58] |
| LC-MS/MS | 8mycotoxins | Apple, orange, cherry and tomato | SPE-0.22μm membrane filter | C18 | ESI | 01-May | 74.2-102.4 | [59] |
| LC-MS/MS | 26mycotoxins | durum wheat grain | LE- 0.22μm filter | C18 | ESI | Feb-70 | 59-110 | [60] |
| UPLC-MS/MS | 12 mycotoxins | Areca catechu | LE- 0.22μm filter | C18 | ESI | 0.1-20 | 85-115 | [61] |
| LC-MS/MS | 5mycotoxins | layer feed | LE- MycoSep 227 | C18 | ESI APCI | 0.9-7.5 | 50-63 | [62] |
| UPLC-MS/MS | 8 mycotoxins | Feed | LE- Immunoaffinity | BEH C18 | ESI | 0.006-0.12 | 91.2-104.1 | [63] |
| LC-MS/MS | 7mycotoxins | vegetable oil | LE-MSPD-0.2μm filter | C18 | ESI APCI | 0.04-2000 | 87.9-106.6 | [64] |
| LC-MS/MS | 7mycotoxins | Wheat, corn, peanut | LE-Immunoaffinity -0.2μm membrane filter | C18 | ESI APCI | 0.04-0.4 | 95.3-103.3 | [65] |
Table 1: Overview on LC-MS/LC-MS/MS/UPLC-MS/MS for mutiplex mycotoxin analysis.
For LC-MS/MS multiplex mycotoxin analysis, the most important and critical steps still is sample pretreatment and sample cleanup. Liquid-liquid extraction (LE), solid phase extraction (SPE), solid phase dispersion extraction (SPDE), pressurized liquid extraction (PLE) and supercritical fluid extraction (SFE) [66] have been used for sample extraction. Currently, the most common extraction solvents employed are methanol/water and acetonitrile/water, although acidified acetone and ethyl acetate/acetonitrile/water have been reported to a lesser extent [67]. Water could increase the solvent enter into the samples and help the release of mycotoxins and increase the extraction efficiency [5,67]. As the improvement of environmental protection and healthful consciousness, organic solutions, especial for hazardous and poisonous solutions, have been avoided as far as possible to use during sample pretreatment. QuEChERS sample preparation approach has been applied in simultaneous extraction of multiplex mycotoxins. However, QuEChERS protocols are different formula as the different chemicals and often are inherently inefficient and reduce the sensitivity of the analytical method [1].
Sample cleanup is often needed to remove any impurities/ interfering material in addition to concentrating the extract prior to analysis and quantification although injection of crude extracts without purification step has been proposed, especial for confirmatory analytical methods. SPE columns, immunoaffinity columns and Mycosep®/Multisep® purification have been usually applied in multiplex mycotoxin analysis. SPE columns are one of the most commonly applied methods of cleanup, such as Florisil, C8, C18 reversed phase and aluminum oxide [67]. The targets were often retained on the surfaces of SPE columns and remove the impurities, then eluted by solvent solution. In some SPE purification protocols, impurities were retained on the surfaces of SPE columns and the eluent was the targets. SPE purification could be affected by pH, type of solvent and ionic strength of sample [5]. Immunoaffinity columns (IAC) could provide the high specificity enrichment for mycotoxins by their antibodies bound to the surfaces of columns. However, the antibodies may lose their activities when the organic solvent is used as extraction solution and eluent. In addition, they are expensive and few commercial companies have produced IAC for the multiplex mycotoxin analysis. Most of the IAC used in references were prepared by authors in their lab. Mycosep®/Multisep® purification are another well-established method for multiplex mycotoxins. Mycosep®#226 and #227 were often used for purification for multiplex mycotoxin analysis [30,32,38,50,62]. These columns comprise different adsorbents (e.g., charcoal and ionexchange resins), which can adsorb proteins, fats and pigments of samples no requirement for activation, washing and elution.
The matrix effects caused by co-elution of matrix components should be carefully considered because they can result in suppression or, more rarely, enhancement of the ionization efficiency of the analyte affecting the accuracy and precision of the results, especial for injection of crude extracts [33,68]. Matrix-matched standards calibration has accurately quantified multiplex mycotoxins in different food matrix without need to use isotope-labelled internal standards [33].
UPLC-MS/MS is being used increasingly in multiplex mycotoxin analysis because its short analysis time, superior resolution and more sensitivity compared with HPLC-MS/MS [5]. However, the cost of analysis is the highest among the MS methods.
Array-based Biosensors for Multiplex Mycotoxin Analysis
Array-based biosensors mainly include DNA microarray, protein microarray and suspension array, which are powerful tools for multiplex target analysis in parallel. The remarkable properties of these techniques lie in its high throughput, automation and integration for analysis equipment. DNA and protein microarray techniques mainly composed of spotting robot, hybridization chamber and chip scanner. DNA microarray usually is applied in high throughput detection for multiplex mycotoxin biosynthesis genes [69]. Protein microarray based on specificity reaction of antibodies and antigens has been used to detect multiplex mycotoxins. Wang et al. established simultaneous determination method for six mycotoxins using indirect competitive immunoassay on a protein microarray by immobilization of complete antigens of six mycotoxins on agarose-modified glass slides [6]. It is reported that the LOD in drinking water were 0.01, 0.24, 15.45, 15.39, 0.05, and 0.01 ng/mL for AFB1, AFM1, DON, OTA, T-2 and ZEN, respectively. The recovery ratios in drinking water ranged from 80%-120%. Though microarray techniques could provide a high throughput and sensitive determination multiplex targets in sample in parallel, their application in practice is limited because of the expensive equipment.
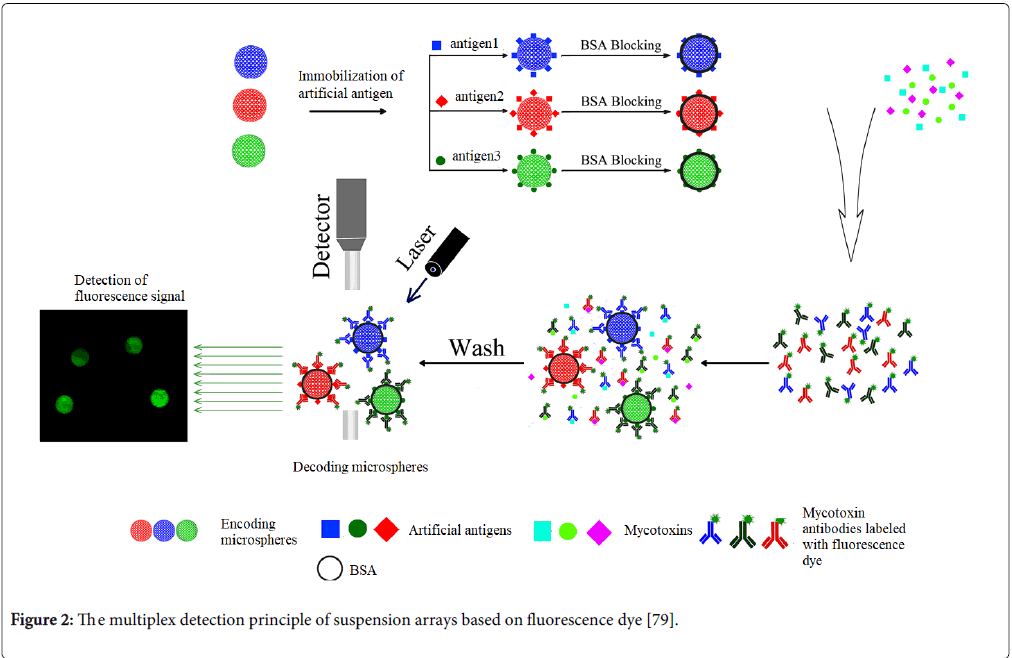
Suspension array techniques have been developed for more than ten years and shown a powerful platform for multiplex mycotoxin analysis because of its high flexibility, fast reaction and good repeatability for detection [70-75]. Unlike planar microarray using the coordinate of positions for encoding different probe molecules, most of suspension array techniques employed the encoding polymer microspheres with spectra as support carriers. The most of multiplex detection principle of suspension arrays based on fluorescence dye immunoassay is seen in Figure 2. The key techniques of suspension array are the encoding and decoding techniques. The most prominent suspension array system comes from Luminex Corporation (Austin, Texas, USA), which has been applied in high throughput screening for multiplex mycotoxins as the mainstream equipment Table 2. The Luminex suspension array Multi Analyte Profiling (xMAP) systems mainly depend on the fluorescent dye encoding techniques for encoding different microspheres and flow cytometer technique for high throughput decoding and reading the signals of targets on the different microspheres. Luminx xAMP system can allow simultaneous measurements of up to 100 different biomolecular interactions in a single well. Compared with the planar microarray, the kinetics of molecular reaction on the surfaces of microspheres has been great improved because of the rotational motion of microspheres in reaction solution. In addition, the suspension array system could easily integrate with sample pretreatment (such as magnetic microspheres for enrichment, purification and detection). More importantly, its cost is not more than one-tenth of planar microarray. Therefore, the suspension array system has great potential in application for multiplex mycotoxin detection.
Figure 2: The multiplex detection principle of suspension arrays based on fluorescence dye [79].
However, suspension array systems encoding with fluorescent dyes are encountering some problems. For example, the fluorescence dyes tend to be quenched or bleached and the optic system are complicated [79,81]. Our group established the easier, simpler and more flexible suspension array system which is based on silica photonic crystal microsphere (SPCM) encoding with their structure colors for multiplex mycotoxin analysis [79-82]. SPCMs for multiplex mycotoxin analysis showed a wide detection linear range and pg/mL LOD [79,81]. The three-dimensional porous microsphere has a large internal surface,bright structure colors and easily been fabricated and manipulated in common lab which make them much more advantages over common solid microspheres. Main suspension arrays for multiplex mycotoxin analysis were summarized in Table 2.
| Suspension array | Microspheres | Mycotoxins | Matrix | Detection principle | LOD/LOQ (μg/kg) | Recovery (%) | References |
|---|---|---|---|---|---|---|---|
| Luminex 200 system | Luminex microspheres | ZEN, FB1, DON, AFB1 | Corn, wheat, feedstuff | direct competitive fluorescent immunoassay | 0.51 | 92.3- 115.5 | [9] |
| BD FACSArray™ Bioanalyzer | BD FACSArray™ microspheres | AFB1, OTA, FB1, DON, ZEA, T-2 | Wheat, pea | direct competitive fluorescent immunoassay | 0.01-75.73 | 80-110 | [70] |
| Luminex100 system | paramagnetic microspheres | AFB1, OTA, FB1, DON, ZEA, T-2 | Feeds | indirect competitive fluorescent immunoassay | 0.29-6.7 | - | [73] |
| Luminex100 | Luminex microspheres | OTA,FB1 | Corn, oat | indirect competitive fluorescent immunoassay | pg | <50 | [74] |
| Luminex100 system | Luminex microspheres | AFB1, T-2, DON, ZON | corn and peanut | indirect competitive fluorescent immunoassay | pg/mL | 80.16-117.65 | [76] |
| Imaging planar bead array analyser | MagPlex, Beads, Invitrogen Dynal | AFB1,OTA, ZON,DON,T-2,FB1 | direct competitive fluorescent immunoassay | 2.5-1000 | - | [77] | |
| Luminex100 FLEXMAP 3D | paramagnetic microspheres Luminex | FB1,OTA,ZEN | Wheat, maize | direct competitive fluorescent immunoassay | 0.7-1270 | - | [78] |
| microarray scanner | Silica photonic crystal microsphere | AFB1, FB1, Citrinin(CIT) | Wheat, peanut, corn | direct competitive fluorescent immunoassay | 0.5-1pg/mL | 74.7-127.9 | [79] |
| microarray scanner | Silica photonic crystal microsphere | FB1, OTA | Wheat, rice corn | direct competitive fluorescent aptamer | 0.16-0.25pg/mL | 76.58-116.38 | [80] |
| multifunctional microplate reader | Silica photonic crystal microsphere | AFB1,FB1,OTA | Wheat, rice corn | indirect competitive chemiluminescent immunoassay | 0.73-1.19pg/mL | 63.5-121.6 | [81] |
| multifunctional microplate reader | Silica-hydrogelphotonic crystal microsphere | AFB1,FB1,OTA | Wheat, rice corn | indirect competitive chemiluminescent immunoassay | 0.4-2.1 pg/mL | 74.96-104.87 | [82] |
| microarray scanner | Silica photonic crystal microsphere | AFB1, FB1, OTA | Wheat, rice corn | Direct fluorescent aptamer | 3.96fg/mL-11.04 pg/mL | 71.20-113.19 | [83] |
Table 2: Overview on suspension arrays for multiplex mycotoxin analysis.
These xAMP systems have given 2-6 mycotoxins analysis results and most of them depended on the immunoassay principles combining fluorescence and chemiluminescence techniques. Theoretically, the microsphere-based arrays could simultaneously detect hundreds of mycotoxins. However, these systems may be limited by the antibody preparation and antibody quantities of mycotoxins. Fortunately, the antibodies of the common mycotoxins can easily be obtained from commercial company. Therefore, it is possible that these systems replace the traditional enzyme-linked immunosorbent assay (ELISA) methods because of their obvious advantages.
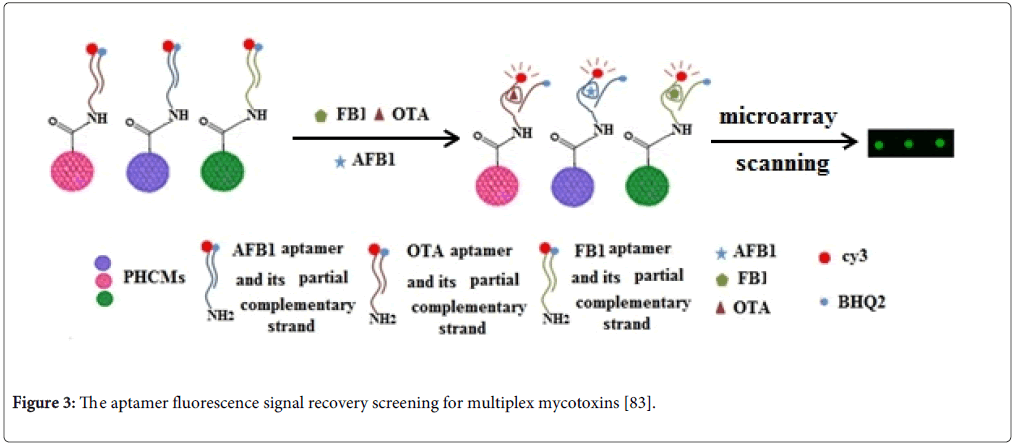
Recently, aptamer techniques on the microsphere array carriers have been established to detect the multiplex mycotoxins [80,83]. The principles of aptamer techniques on microsphere array carriers were based on the changes of fluorescence signal before and after mycotoxins binding to their aptamers (Figure 3). The methods are designed as one-step detection for multiplex mycotoxins. They have a great potential for replacing the immunoassay methods for multiplex mycotoxin analysis because they are simple, easily operated, high sensitive and cost-effective.
Figure 3: The aptamer fluorescence signal recovery screening for multiplex mycotoxins [83].
Multiplex lateral flow biosensors analysis for mycotoxins
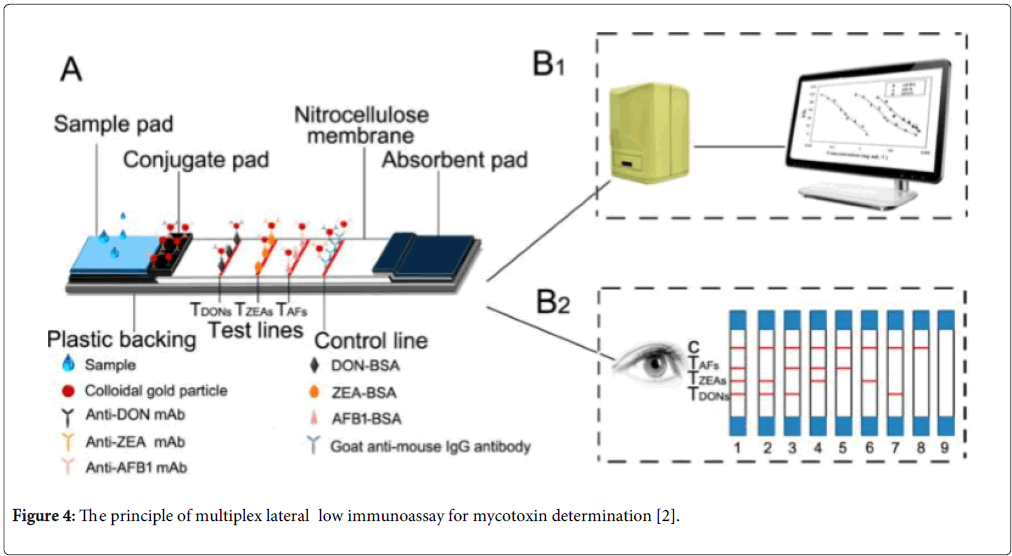
Lateral flow biosensors are self-operating devices that perform rapid assays on a membrane or gel in a chromatographic manner from a single sample addition [84]. The one-step lateral-flow methods often use colloidal gold-labelled antibodies as the indicator visible to the naked eye and specific reagents to bind its antigen immobilized on the membrane surface. Multiplex lateral flow techniques are based on the traditional single-plex lateral flow biosensor for the multiplex analyte detection. Generally, artificial antigens of multi-mycotoxins were immobilized on the surface of membrane or gel and the competitive immunoassay protocol was performed on the surface of membrane among the targets and artificial antigens to binding to labelled mycotoxin antibodies (Figure 4). Compared with HPLC-MS/MS methods, though the number of mycotoxins for multiplex analysis is limited, lateral flow methods are simple, rapid and low cost and could provide qualitative, semiquantitative or quantitative visual on-site testing. These techniques have been rapidly developed recently because they allow the operation and interpretation for the non-skilled personnel. For example, Kolosova et al. developed a qualitative onestep test for simultaneous assay for DON and ZEA using colloidal gold-labelled monoclonal antibodies [85]. It can provide the cut-off levels of 1500 and 100 μg/kg for DON and ZEA, respectively. Burmistrova et al. used alkaline phosphatase labelled antigens to develop colors for non-instrumental multiplex semiquantitative and qualitative detection of OTA, ZEN and FB1 in wheat, and maize samples [86]. Kong et al. established a gold nanoparticle-based semiquantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins [87]. Other multiplex lateral flow analysis for mycotoxins were summarized in the Table 3.
Figure 4: The principle of multiplex lateral low immunoassay for mycotoxin determination [2].
| Mycotoxins | Matrix | Membrane material | Color development | Sample clean-up | LOD or Cut-off level (μg/kg) | References |
|---|---|---|---|---|---|---|
| AFB1,ZEA,DON | Wheat, maize | nitrocellulose membrane | Colloidal gold nanoparticles | without | 1,50 and 60 | [2] |
| DON, ZEA | wheat | Hi-Flow Plus 75 | Colloidal gold | without | 1500,100 for DON and ZEA | [85] |
| OTA, FB1,ZEN, | Wheat, maize, silage | Immunodyne ABC | 5-bromo-4-chlor-3-indolyl-phosphate/nitro-blue-terazolium | glass microfiber filter | 2.5,50,1000 for OTA, ZEN, FB1 | [86] |
| ZEAs, DONs,T-2s, AFs, FBs | Cereal smaples | Nitrocellulose (NC) high flow-plus | Colloidal gold nanoparticles | without | 0.25-1,5-500,1-10,0.5-2.5 and 5-25 for ZEAs, DONs,T-2s,AFs, FBs | [87] |
| AFB1, OTA, DON, ZON,FB1 | corn, wheat, peanut | polyvinylidene fluoride | 3,3’,5,5’-tetramethylbenzidine (TMB) | without | 20,60,1000,20 and 250 for AFB1, ZON,DON, OTA, FB1 | [88] |
| AFB1,OTA,ZEA | Maize, rice, peanut | nitrocellulose membrane | Colloidal gold nanoparticles | without | LOD 0.25, 0.5, and 1for AFB1, OTA, ZEA | [89] |
| ZEA, DON, T-2/HT-2, FB1 /FB2 | Wheat, maize | nitrocellulose membrane | Colloidal gold nanoparticles | without | Qualitative | [90] |
| ZEN,FB1 | Wheat, corn | nitrocellulose membrane | Colloidal gold nanoparticles | without | 0.35and5.23ng/mL For ZEN and FB1 | [91] |
| T-2,HT-2 | wheat | polyethylene filters or sepharose gel | TMB | without | 0.55 and 1.7ng/g | [92] |
| AFB1, OTA, ZEN, DON, | maize, peanuts, and cassava flour | sepharose 4B,Immunodyne ABC membranes | TMB | C18 clean-up sorbent | 3,1,250,1000,200 for sepharose 4B 3,5,700,175 for membrane For OTA,AFB1,DON and ZEN | [93] |
| FB1,DON,T2,ZEA | Wheat, oats, maize | nitrocellulose membrane | Colloidal gold nanoparticles | without | 280,400,1400 ,3200 and for 80,400,1400 ZEA,T2,DON And FB1 in maize and wheat/oats | [94] |
| AFB1,ZEA, OTA | Corn, rice, peanut | nitrocellulose membrane | Colloidal gold nanoparticles | without | 0.10-0.13,0.42-0.46,0.19-0.24for AFB1, ZEA OTA | [95] |
Table 3: Overview on lateral flow biosensors for multiplex mycotoxin analysis.
The color development mainly depended on the colloidal gold nanoparticles and enzyme substrate chromogenic reagents, which could provide the qualitative response and the quantitative results by means of a colorimetric reader. For example, Song et al. developed qualitative and semiquantitative triplex lateral flow immunoassay with LOD of 0.05,1 and 3 μg/kg for AFB1, ZEA and DON, respectively. The strip reader gave the relative optical density between the signal of a positive sample and the blank, which allow establish the calibration curves for the three mycotoxins [2]. 3, 3’, 5, 5’-tetramethylbenzidine (TMB) was often used as color development of horseradish peroxidase (HRP) which catalyzes TMB to produce blue color. Although the colloidal gold nanoparticles and TMB have shown good sensitivity and low limits of detection for color development, different signal enhancement strategies have been developed to significantly improve LOD, sensitivity and stability of the system, which is strongly related with the development of nanomaterial techniques. Graphene, quantum dots, nano-silver and magnetic nanoparticles have been introduced in the system and showed the stable, enhanced signal and wide detection linear ranges [96]. In addition, except nitrocellulose membrane as the support substrate, the three-dimensional porous smart nanomaterial may bring the field to a new level.
Nevertheless, the lateral flow devices still have some problems when they have been applied in the real samples. As the increase of analysis kinds of mycotoxins in the single device, the large of reagents will be consumed in the system [84]. Most of the systems just give qualitative or semiquantitative response by naked-eye and the false-positive or false-negative results are often reported. In addition, unspecific adsorptions coming from different matrix compounds often occur in the membrane or gel, which could result in invalid or failed detection.
Other biosensor techniques for multiplex mycotoxin analysis
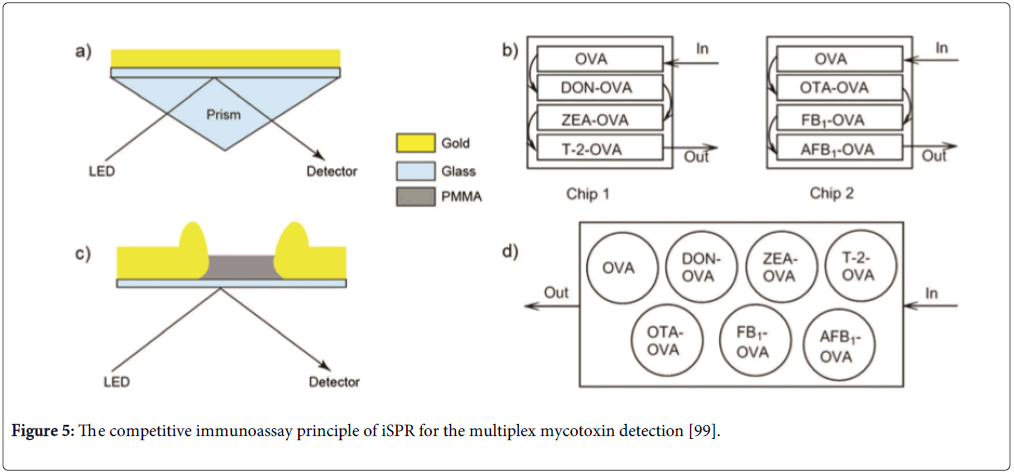
Other biosensor analysis systems for multiplex mycotoxins mainly include label-free techniques (such as surface plasmon resonance (SPR)) and nanoparticle-based biosensor techniques. SPR techniques are the typical optical label-free in real time biosensors for multiplex mycotoxins. SPR continuously monitors changes of refractive index of the biorecognition layer on the sensor surface. Nielen group developed a competitive inhibition immunoassay using the imaging SPR (iSPR) technique for the simultaneous detection of DON and ZEN, which showed 84 and 68 μg/kg for DON, 64 and 40 μg/kg for ZEN in maize and wheat samples, respectively [97]. Recently, they used the technique to detect DON and DON, ZEA, T-2, OTA, OTA, FB1and AFB1 on nanostructure chip surfaces for beer and barley samples [98,99]. The competitive immunoassay principle of iSPR for multiplex mycotoxin detection is seen in Figure 5. They reported the nanostructure chip could be regenerated for 450 cycles [98] and 60 cycles [99] after each cycle. The system can be used for in-field or at-line detection of DON in beer and barley without preconcentration, while OTA in beer requires an additional enrichment step [98]. The LODs in beer were 17 ng/mL for DON and 7 ng/mL for OTA [97]. The LOD in barley (in μg/kg) were determined to be 26 for DON, 6 for ZEA, 0.6 for T-2, 3 for OTA, 2 for FB1 and 0.6 for AFB1 [99]. The detection platform could provide a rapid and semi-quantitative screening for multiplex mycotoxins prior to LC-MS/MS [99]. Hu et al. developed a sensitive method for detection of AFB1, OTA and ZEN using iSPR with gold nanoparticles as signal amplification tags in gold chip surface [100]. The competitive immunoassay format was performed on the gold chip surface and then the secondary antibody-conjugated gold nanoparticles were used to bind with monoclonal antibodies for further amplification of the iSPR signal. They showed that the LODs were 8, 30 and 15 pg/mL for AFB1, OTA and ZEN and dynamic ranges covering three orders of magnitude. For the detection for multiplex mycotoxins, much more volume reagents are needed in the system. Furthermore, non-purification samples may result in false response because of other compounds in matrix. Therefore, the focus of the platform should be on miniaturization of the chip and integration of pretreatment of samples with detection.
Figure 5: The competitive immunoassay principle of iSPR for the multiplex mycotoxin detection [99].
Nanoparticle-based biosensor techniques for multiplex mycotoxin analysis have been rapidly developed due to the booming progress in nanomaterial field. Graphene, quantum dots, nano-silver, upconversion and magnetic nanoparticles have been designed as the labeled probe or color development for multiplex mycotoxin analysis. Immunoassay and aptamer principles have been developed to detect multiple mycotoxins. Wu et al. established multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of OTA and FB1 with the LOD of 0.02 ng/mL for OTA and 0.1 ng/mL for FB1 [101]. Chen et al. applied antigen-modified magnetic nanoparticles as biosensor probes and antibody-functionalized improved upconversion nanoparticles as signal probes to develop an ultrasensitive fluorescence biosensor, which showed the LOD of 0.001 ng/mL and detection linear range of 0.001-0.1 ng/mL for AFB1 and DON in peanut oils [102]. Saeger group used quantum dot (QD) nanolabels to develop the multiplex fluorescent immunosorbent simultaneous analysis method which has LODs of 3.2, 0.6, 0.2, 10 and 0.4 μg/kg for DON, ZEN, AFB1, T-2 and FB1 in maize [103]. Then they used QD@SiO2 and silica-coated liposomes loaded with QDs as labels to establish the multiplex fluorescent immunosorbent assay with the LODs of 6.1 and 5.3, 5.4, and 4.1, 2.6, and 1.9 μg/kg for DON, ZEN and AFB1 [104], 16.2 and 18, 2.2 and 2.6 μg/kg for ZEN and AFB1 in maize and wheat. Zhang et al. designed a fluorescent aptasensor by DNA-scaffolded silver nanoclusters coupling with Zn-ion signal-enhancement for simultaneous detection of OTA and AFB1, which showed the LOD of 0.2 and 0.3 pg/mL for OTA and AFB1 in rice, corn, and wheat. This field has a great potential for developing the simple,rapid and sensitive sample pretreatment and detection kit for multiplex mycotoxins.
Conclusion
Co-occurrence of multi-mycotoxins in the plant source products and their synergistic toxic effects has been demonstrated in previous reports. Multiplex mycotoxin analysis has a vital significance in preventing human and livestock food chain to avoid their contaminations. LC-MS/MS equipped with ESI has been mainstream technique for multiplex mycotoxin analysis in confirmation analysis. UPLC-MS/MS method would be next generation technique for multiplex mycotoxin analysis because of their obvious advantages over LC-MS/MS in short analysis time, superior resolution and more sensitivity. Sample pretreatment and sample cleanup would still be focus for the chromatographic instrument techniques in multiplex mycotoxin analysis.
For the large quantities of the screening samples and on-site rapid detection, suspension array and lateral flow biosensor techniques for multiplex mycotoxins analysis will be simple, rapid, cost-efficient and practical. These techniques are strongly depended on the development of nanotechnology and specific probe molecules. The stability, sensitivity and specificity of them would be the key regions to break through. In addition, the integration between sample pretreatment and detection techniques would be paid more attention in these systems. As the development of aptamer techniques, the aptamer-suspension array or aptamer-lateral flow biosensor will insult in the simpler, cheaper, faster and more convenient methods for multiplex mycotoxin analysis. However, for analysis of the “masked” or conjugated multiplex mycotoxins, HPLC-MS/MS or UPLC-MS/MS is irreplaceable techniques and urgently needed to further research.
Acknowledgments
We gratefully acknowledge the financial supports from National Natural Science Foundation of China no.31471642 and no.31071542, Natural Science Foundation of Jiangsu Province no. BK20131398 and 100 Talents Program of Nanjing Normal University.
The authors have declared no conflict interest.
References
- Anfossi L, Giovannoli C, Baggiani C (2016) Mycotoxin Detection. Curr Opin Biotech 37: 120-126.
- Song S, Liu N, Zhao Z, Njumbe Ediage E, Wu S, et al. (2014) Multiplex lateral flow immunoassay for mycotoxin determination. Anal Chem 86: 4995-5001.
- Turner NW, Bramhmbhatt H, Szabo-Vezse M, Poma A, Coker R, et al. (2015) Analytical methods for determination of mycotoxins: An update (2009-2014). Anal Chim Acta 901: 12-33.
- Al-Taher F, Banaszewski K, Jackson L, Zweigenbaum J, Ryu D, et al. (2013) Rapid Method for the Determination of Multiple Mycotoxins in Wines and Beers by LC-MS/MS Using a Stable Isotope Dilution Assay. J Agric Food Chem 61: 2378-2384.
- Pereira VL, Fernandes JO, Cunha SC (2014) Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci Tech 36: 96-136.
- Wang YK, Yan YX, Li SQ, Wang HA, Ji WH, et al. (2013) Simultaneous Quantitative Determination of Multiple Mycotoxins in Cereal and Feedstuff Samples by a Suspension Array Immunoassay. J Agric Food Chem 61: 10948-10953.
- Streit E, Schatzmayr G, Tassis P, Tzika E, Marin D, et al. (2012) Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 4: 788-809.
- Pamel EV, Verbeken A, Vlaemynck G, Boever JD, Daeseleire E (2011) Ultrahigh-Performance Liquid Chromatographic–Tandem Mass Spectrometric Multimycotoxin Method for Quantitating 26 Mycotoxins in Maize Silage. J Agric Food Chem 59: 9747-9755.
- Soleimany F, Jinap S, Abas F (2012) Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry Food Chem 130: 1055-1060.
- Brera C, Debegnach F, De Santis B, Pannunzi E, Berdini C, et al. (2011) Simultaneous determination of aflatoxins and ochratoxin A in baby foods and paprika by HPLC with fluorescence detection: a single-laboratory validation study. Talanta 83: 1442-1446.
- Ferreira I, Fernandes JO, Cunha SC (2012) Mycotoxins in Plants and Plant Products: Cereals and Cereal Products. Food Control 27: 188-193.
- Olsson J, Börjesson T, Lundstedt T, Schnürer J (2002) Detection and quantification of ochratoxin A and deoxynivalenol in barley grains by GC-MS and electronic nose. Int J Food Microbiol 72: 203-214.
- Gong XM, Ren YP, Dong J, Sun J, Li J, et al. (2011) Determination of Mycotoxin Biomarkers in Eggs by Liquid Chromatography-Tandem Mass Spectrometry Coupled with Matrix Solid Phase Dispersion. Instrum Anal 30: 6-12.
- Chan D, MacDonald SJ, Boughtflower V, Brereton P (2004) Simultaneous determination of aflatoxins and ochratoxin A in food using a fully automated immunoaffinity column clean-up and liquid chromatography–fluorescence detection. J Chromatogr A 1059: 13-16.
- Ainiza WWM, Jinap S, Sanny M (2015) Simultaneous determination of aflatoxins and ochratoxin A in single and mixed spices. Food Control 50: 913-918.
- Kong WJ, Liu SY, Qiu F, Xiao XH, Yang MH (2013) Simultaneous multi-mycotoxin determination in nutmeg by ultrasound-assisted solid-liquid extraction and immunoaffinity column clean-up coupled with liquid chromatography and on-line post-column photochemical derivatization-fluorescence detection. Analyst 138: 2729-2739.
- Xu JJ, Zhou J, Huang BF, Cai ZX, Xu XM, et al. (2016) Simultaneous and rapid determination of deoxynivalenol and its acetylate derivatives in wheat flour and rice by ultra high performance liquid chromatography with photo diode array detection J Sep Sci 39: 2028-2035.
- Josephs RD, Krska R, Grasserbauer M, Broekaert JA (1998) Determination of trichothecene mycotoxins in wheat by use of supercritical fluid extraction and high-performance liquid chromatography with diode array detection or gas chromatography with electron capture detection. J Chromatogr A 795: 297-304.
- Ryu JC, Yang JS, Song YS, Kwon OS, Park J, Chang IM (1996) Survey of natural occurrence of trichothecene mycotoxins and zearalenone in Korean cereals harvested in 1992 using gas chromatography/mass spectrometry. Food Addit Contam 13: 333-341.
- Nielsen KF, Thrane U (2001) Fast methods for screening of trichothecenes in fungal cultures using gas chromatography–tandem mass spectrometry. J Chromatogra A 929: 75-87.
- Rodríguez-Carrasco Y, Moltó JC, Mañes J, Berada H (2014) Development of a GC–MS/MS strategy to determine 15 mycotoxins and metabolites in human urine. Talanta 128: 125-131.
- Cegielska-Radziejewska R, Stuper-Szablewska K, Szablewski T (2013) Microflora and mycotoxin contamination in poultry feed mixtures from western Poland. Ann Agric Environ Med 20: 30-35.
- Ibáñez-Vea M, Lizarraga E, González-Peñas E (2011) Simultaneous determination of type-A and type-B trichothecenes in barley samples by GC–MS. Food Control 22: 1428-1434.
- Escrivá L, Manyes L, Font G, Berrada H (2016) Analysis of trichothecenes in laboratory rat feed by gas chromatography-tandem mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 33: 329-338.
- Köppen R, Koch M, Siegel D, Merkel S, Maul R, et al. (2010) Determination of mycotoxins in foods: current state of analytical methods and limitations. Appl Microbiol Biotechnol 86: 1595-1612.
- Zöllner P, Mayer-Helm B (2006) Trace mycotoxin analysis in complex biological and food matrices by liquid chromatography–atmospheric pressure ionisation mass spectrometry. J Chromatogra A 1136: 123-169.
- Pizzutti IR, Kok A, Scholten J, Righi LW, Cardoso CD, et al. (2014) Development, optimization and validation of a multimethod for the determination of 36 mycotoxins in wines by liquid chromatography–tandem mass spectrometry. Talanta 129: 352-363.
- Hartl M, Humpf HU (1999) Simultaneous Determination of Fumonisin B1 and Hydrolyzed Fumonisin B1 in Corn Products by Liquid Chromatography/Electrospray Ionization Mass Spectrometry. J Agric Food Chem 47: 5078-5083.
- Berthiller F, Schuhmacher R, Buttinger G, Krsk R (2005) Rapid simultaneous determination of major type A- and B-trichothecenes as well as zearalenone in maize by high performance liquid chromatography–tandem mass spectrometry. J Chromatogra A 1062: 209-216.
- Ren YP, Zhang Y, Shao SL, Cai ZX, Feng L, et al. (2007) Simultaneous determination of multi-component mycotoxin contaminants in foods and feeds by ultra-performance liquid chromatography tandem mass spectrometry. J Chromatogra A 1143: 48-64.
- Monbaliu S, Van Poucke C, Van Peteghem C, Van Poucke K, Heungens K, et al. (2009) Development of a multi-mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Commun Mass Spectrom 23: 3-11.
- Beltrán E, Ibáñez M, Sancho JV, Hernández F (2009) Determination of mycotoxins in different food commodities by ultra-high-pressure liquid chromatography coupled to triple quadrupole mass spectrometry. Rapid Commun Mass Spectrom 23: 1801-1809.
- Kokkonen MK, Jestoi MN (2009) A Multi-compound LC-MS/MS Method for the Screening of Mycotoxins in Grains. Food Anal Methods 2: 128-140.
- Romero-González R, Vidal JLM, Aguilera-Luiz MM, Frenich AG (2009) Application of Conventional Solid-Phase Extraction for Multimycotoxin Analysis in Beers by Ultrahigh-Performance Liquid Chromatography−Tandem Mass Spectrometry J Agric Food Chem 57: 9385-9392.
- Vishwanath V, Sulyok M, Labuda R, Bicker W, Krska R (2009) Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 395: 1355-1372.
- Sulyok M, Krska R, Schuhmacher R (2007) A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal Bioanal Chem 389: 1505-1523.
- Vendl O, Berthiller F, Crews C, Krska R (2009) Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC–MS–MS. Anal Bioanal Chem 395: 1347-1354.
- Han Z, Liu XS, Ren YP, Luan LJ, Wu YJ (2010) A rapid method with ultra-high-performance liquid chromatography-tandem mass spectrometry for simultaneous determination of five type B trichothecenes in traditional Chinese medicines. J Sep Sci 33: 1923-1932.
- Tanaka H, Takino M, Sugita-Konishi Y, Tanaka T, Leeman D, et al. (2010) Determination of Fusarium mycotoxins by liquid chromatography/tandem mass spectrometry coupled with immunoaffinity extraction Rapid Commun Mass Sp 24: 2445-2452.
- Capriotti AL, Foglia P, Gubbiotti R, Roccia C, Samperi R, et al. (2010) Development and validation of a liquid chromatography/atmospheric pressure photoionization-tandem mass spectrometric method for the analysis of mycotoxins subjected to commission regulation (EC) No. 1881/2006 In cereals. J Chromatogr A 1217: 6044-6051.
- Solfrizzo M, Gambacorta L, Lattanzio VMT, Powers S, Visconti A (2011) Simultaneous LC-MS/MS Determination of Aflatoxin M1, Ochratoxin A, Deoxynivalenol, De-Epoxydeoxynivalenol, α and β-Zearalenols and Fumonisin B1 in Urine as a Multi-Biomarker Method to Assess Exposure to Mycotoxins Anal Bioanal Chem 401: 2831-2841.
- Soleimany F, Jinap S, Faridah A, Khatib A (2012) A UPLC–MS/MS for simultaneous determination of aflatoxins, ochratoxin A, zearalenone, DON, fumonisins, T-2 toxin and HT-2 toxin, in cereals. Food Control 25: 647-653.
- Liu SY, Qiu F, Kong WJ, Wei JH, Xiao XH, et al. (2013) Development and validation of an accurate and rapid LC-ESI-MS/MS method for the simultaneous quantification of aflatoxin B1, B2, G1 and G2 in lotus seeds. Food Control 29: 156-161.
- Mornar A, Sertić M, Nigovic B (2013) Development of a Rapid LC/DAD/FLD/MSn Method for the Simultaneous Determination of Monacolins and Citrinin in Red Fermented Rice Products J Agric Food Chem 61: 1072-1080.
- Song SQ, Ediage EN, Wu AB, Saeger SD (2013) Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry J Chromatogr A 1292: 111-120.
- Xue HL, Bi Y, Wei JM, Tang YM, Zhao Y, et al. (2013) New Method for the Simultaneous Analysis of Types A and B Trichothecenes by Ultrahigh-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry in Potato Tubers Inoculated with Fusarium sulphureum. J Agric Food Chem 61: 9333-9338.
- Ying YF, Wu YL, Wen Y, Yang T, Xu XQ, et al. (2013) Simultaneous determination of six resorcylic acid lactones in feed using liquid chromatography–tandem mass spectrometry and multi-walled carbon nanotubes as a dispersive solid phase extraction sorbent. J Chromatogr A 1307: 41-48.
- Huang LC, Zheng N, Zheng BQ, Wen F, Cheng JB, et al. (2014) Simultaneous determination of aflatoxin M1, ochratoxin A, zearalenone and α-zearalenol in milk by UHPLC-MS/MS. JQ Food Chem 146: 242-249.
- Xu LX, Zhang GJ, Guo CN, Zhang YP, Zhang Y, et al. (2014) Simultaneous determination of major type-B trichothecenes and the de-epoxy metabolite of deoxynivalenol in chicken tissues by HPLC–MS/MS. J Sep Sci 37: 642-649.
- Azaiez I, Giusti F, Sagratini G, Mañes J, Fernández-Franzón M (2014) Multi-mycotoxins Analysis in Dried Fruit by LC/MS/MS and a Modified QuEChERS Procedure. Food Anal Methods 7: 935-945.
- Capriotti AL, Cavaliere C, Foglia P, Samperi R, Stampachiacchiere S, et al. (2014) Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography–tandem mass spectrometry. Comparison of different extraction procedures. J Chromatogr A 1343: 69-78.
- Lattanzio VM, Ciasca B, Powers S, Visconti A (2014) Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid chromatography–tandem mass spectrometry after multi-toxin immunoaffinity clean up. J Chromatogr A 1354: 139-143.
- Blesa J, Moltó JC, Akhdari SE, Mañes J, Zinedine A (2014) Simultaneous determination of Fusarium mycotoxins in wheat grain from Morocco by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Control 46: 1-5.
- Campone L, Piccinelli AL, Celano R, Russo M, Valdés A, et al. (2015) A fully automated method for simultaneous determination of aflatoxins and ochratoxin A in dried fruits by pressurized liquid extraction and online solid-phase extraction cleanup coupled to ultra-high-pressure liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 407: 2899-2911.
- García-Moraleja A, Font G, Mañes J, Ferrer E (2015) Development of a new method for the simultaneous determination of 21 mycotoxins in coffee beverages by liquid chromatography tandem mass spectrometry. Food Res Int 72: 247-255.
- Zhu RY, Zhao ZY, Wang JH, Bai B, Wu AB, et al. (2015) A simple sample pretreatment method for multi-mycotoxin determination in eggs by liquid chromatography tandem mass spectrometry. J Chromatogr A 1417: 1-7.
- Wang M, Jiang N, Xian H, Wei DZ, Shi L, et al. (2016) A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1429: 22-29.
- Juan C, Covarelli L, Beccari G, Colasante V, Mañes J (2016) Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control 62: 322-329.
- Liu HM, Luo JY, Kong WJ, Liu QT, Hu YC, et al. (2016) UFLC-ESI-MS/MS analysis of multiple mycotoxins in medicinal and edible Areca catechu. Chemosphere 150: 176-183.
- Bernhardt K, Valenta H, Kersten S, Humpf HU, Dänicke S (2016) Determination of T-2 toxin, HT-2 toxin, and three other type A trichothecenes in layer feed by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS)--comparison of two sample preparation methods. Mycotoxin Res 32: 89-97.
- Hu XF, Hu R, Zhang ZW, Li PW, Zhang Q, et al. (2016) Development of a multiple immunoaffinity column for simultaneous determination of multiple mycotoxins in feeds using UPLC–MS/MS M. Anal Bioanal Chem 408: 6027-6036.
- Sharmili K, Jinap S, Sukor R (2016) Development, optimization and validation of QuEChERS based liquid chromatography tandem mass spectrometry method for determination of multimycotoxin in vegetable oil. Food Cont 70: 152-160.
- Zhang ZW, Hu XF, Zhang Q, Li PW (2016) Determination for multiple mycotoxins in agricultural products using HPLC–MS/MS via a multiple antibody immunoaffinity column. J Chromatogr B 1021: 145-152.
- Zougagh M, Ríos A (2008) Supercritical fluid extraction of macrocyclic lactone mycotoxins in maize flour samples for rapid amperometric screening and alternative liquid chromatographic method for confirmation. J Chromatogra A 1177: 50-57.
- Meneely JP, Ricci F, Egmond HPV, Elliott CT (2011) Current methods of analysis for the determination of trichothecene mycotoxins in food. Trend Anal Chem 30: 192-203.
- Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom 20: 2649-2659.
- Schmidt-Heydt M, Geisen R (2007) A microarray for monitoring the production of mycotoxins in food. Int J Food Microbiol 117: 131-140.
- Czeh A, Mandy F, Feher-Toth S, Torok L, Mike Z, et al. (2012) A flow cytometry based competitive fluorescent microsphere immunoassay (CFIA) system for detecting up to six mycotoxins. J Immunol. Methods 384: 71-80.
- Leng Y, Sun K, Chen X, Li W (2015) Suspension arrays based on nanoparticle-encoded microspheres for high-throughput multiplexed detection Chem Soc Rev 44: 5552-5595.
- Ediage EN, Mavungu JDD, Goryacheva IY, Peteghem CV, Saeger SD (2012) Multiplex flow-through immunoassay formats for screening of mycotoxins in a variety of food matrices Anal Bioanal Chem 403: 265-278.
- Peters J, Bienenmann-Ploum M, Rijk TD, Haasnoot W (2011) Development of a multiplex flow cytometric microsphere immunoassay for mycotoxins and evaluation of its application in feed. Mycotoxin Res 27: 63-72.
- Anderson GP, Kowtha VA, Taitt CR (2010) Detection of Fumonisin B1 and Ochratoxin A in Grain Products Using Microsphere-Based Fluid Array Immunoassays. Toxins 2: 297-309.
- Leng YK, Wu WJ, Li L, Lin K, Sun K, et al. (2016) Magnetic/Fluorescent Barcodes Based on Cadmium-Free Near-Infrared-Emitting Quantum Dots for Multiplexed Detection. Adv Funct Mater 26: 7581-7589.
- Wang Y, Ning BN, Peng Y, Bai JL, Liu M, et al. (2013) Application of suspension array for simultaneous detection of four different mycotoxins in corn and peanut. Biosens Bioelectron 41: 391-396.
- Peters J, Cardall A, Haasnoot W, Nielen MW (2014) 6-Plex microsphere immunoassay with imaging planar array detection for mycotoxins in barley. Analyst 139: 3968-3976.
- Peters J, Thomas D, Boers E, Rijk TD, Berthiller F, et al. (2013) Colour-encoded paramagnetic microbead-based direct inhibition triplex flow cytometric immunoassay for ochratoxin A, fumonisins and zearalenone in cereals and cereal-based feed. Anal Bioanal Chem 405: 7783-7794.
- Deng GZ, Xu K, Sun Y, Chen Y, Zheng TS, et al. (2013) High Sensitive Immunoassay for Multiplex Mycotoxin Detection with Photonic Crystal Microsphere Suspension Array. Anal Chem 85: 2833-2840.
- Sun Y, Xu J, Li W, Cao B, Wang DD, et al. (2014) Simultaneous Detection of Ochratoxin A and Fumonisin B1 in Cereal Samples Using an Aptamer–Photonic Crystal Encoded Suspension Array. Anal Chem 86: 11797-11802.
- Xu K, Sun Y, Li W, Xu J, Cao B, et al. (2014) Multiplex chemiluminescent immunoassay for screening of mycotoxins using photonic crystal microsphere suspension array. Analyst 139: 771-777.
- Xu J, Li W, Liu R, Yang Y, Lin QX, et al. (2016) Ultrasensitive low-background multiplex mycotoxin chemiluminescence immunoassay by silica-hydrogel photonic crystal microsphere suspension arrays in cereal samples. Sensor Actuat B-Chem 232: 577-584.
- Yang Y, Li W, Shen P, Liu R, Li YW, et al. (2017) Aptamer fluorescence signal recovery screening for multiplex mycotoxins in cereal samples based on photonic crystal microsphere suspension array Sensor. Actuat B-Chem 248: 351-358.
- Li J, Macdonald J (2016) Multiplexed lateral flow biosensors: technological advances for radically improving point-of-care diagnoses. Biosens Bioelectron 83: 177-192.
- Kolosova AY, De Saeger S, Sibanda L, Verheijen R, Van Peteghem C (2007) Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of zearalenone and deoxynivalenol. Anal Bioanal Chem 389: 2103-2107.
- Burmistrova NA, Rusanova TY, Yurasov NA, Goryacheva IY, Saeger SD (2014) Multi-detection of mycotoxins by membranebased flow-through immunoassay.Multi-detection of mycotoxins by membrane based flow-through immunoassay. Food Cont 46: 462-469.
- Kong DZ, Liu LQ, Song SS, Suryoprabowo S, Li AK, et al. (2016) A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 8: 5245-5253.
- He QH, Xu Y, Wang D, Kang M, Huang ZB, et al. (2012) Simultaneous multiresidue determination of mycotoxins in cereal samples by polyvinylidene fluoride membrane based dot immunoassay. Food Chem 134: 507-512.
- Li X, Li P, Zhang Q, Li R, Zhang W, et al. (2013) Multi-Component Immunochromatographic Assay for Simultaneous Detection of Aflatoxin B1, Ochratoxin A, and Zearalenone in Agro-Food. Biosens Bioelectron 49: 426-432.
- Lattanzio VMT, Holst CV, Visconti A (2013) Experimental design for in-house validation of a screening immunoassay kit. The case of a multiplex dipstick for Fusarium mycotoxins in cereals. Anal Bioanal Chem 405: 7773-7782.
- Wang YK, Yan YX, Ji WH, Wang HA, Li SQ, et al. (2013) Rapid Simultaneous Quantification of Zearalenone and Fumonisin B1 in Corn and Wheat by Lateral Flow Dual Immunoassay. J Agric Food Chem 61: 5031-5036.
- Goftman VV, Beloglazova NV, Ediage EN, Saeger SD, Dietrich R, et al. (2012) Rapid immunochemical tests for qualitative and quantitative determination of T-2 and HT-2 toxins. Anal Methods 4: 4244-4249.
- Ediage EN, Di Mavungu JD, Monbaliu S, Van Peteghem C, De Saeger S (2012) A validated multianalyte LC-MS/MS method for the quantification of 25 mycotoxins in cassava flour, peanut cake and maize samples. Bioanal Chem 403: 265-278.
- Lattanzio VM, Nivarlet N, Lippolis V, Della Gatta S, Huet AC, et al. (2012) Multiplex dipstick immunoassay for semi-quantitative determination of Fusarium mycotoxins in cereals. Anal Chim Acta 718: 99-108.
- Chen YQ, Chen Q, Han MM, Zhou JY, Gong L, et al. (2016) Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem 213: 478-484.
- Quesada-González D, Merkoçi A (2015) Nanoparticle-based lateral flow biosensors. Biosens Bioelectron 73: 47-63.
- Dorokhin D, Haasnoot W, Franssen MCR, Zuilhof H, Nielen MWF (2011) Imaging surface plasmon resonance for multiplex microassay sensing of mycotoxins. Anal Bioanal Chem 400: 3005-3011.
- Joshi S, Annida RM, Zuilhof H, Beek TAV, Nielen MWF (2016) Analysis of Mycotoxins in Beer Using a Portable Nanostructured Imaging Surface Plasmon Resonance Biosensor. J Agric Food Chem 64: 8263-8271.
- Joshi S, Segarra-Fas A, Peters J, Zuilhof H, van Beek TA, et al. (2016) Multiplex surface plasmon resonance biosensing and its transferability towards imaging nanoplasmonics for detection of mycotoxins in barley. Analyst 141: 1307-1318.
- Hu WH, Chen HM, Zhang HH, He GL, Li X, et al. (2014) Sensitive detection of multiple mycotoxins by SPRi with gold nanoparticles as signal amplification tags. J Colloid Interf Sci 431: 71-76.
- Wu S, Duan N, Ma X, Xia Y, Wang H, et al. (2012) Multiplexed Fluorescence Resonance Energy Transfer Aptasensor between Upconversion Nanoparticles and Graphene Oxide for the Simultaneous Determination of Mycotoxins. Anal Chem 84: 6263-6270.
- Chen Q, Hu W, Sun C, Li H, Ouyang Q (2016) Synthesis of improved upconversion nanoparticles as ultrasensitive fluorescence probe for mycotoxins Anal Chim Acta 938: 137-145.
- Beloglazova NV, Speranskaya ES, Wu A, Wang Z, Sanders M, et al. (2014) Novel multiplex fluorescent immunoassays based on quantum dot nanolabels for mycotoxins determination. Biosens Bioelectron 62: 59-65.
- Beloglazova NV, Foubert A, Gordienko A, Tessier MD, Aubert T, et al. (2016) Sensitive QD@ SiO2-based immunoassay for triplex determination of cereal-borne mycotoxins. Talanta 160: 66-71.
- Beloglazova NV, Goryacheva OA, Speranskaya ES, Aubert T, Shmelin PS, et al. (2015) Silica-coated liposomes loaded with quantum dots as labels for multiplex fluorescent immunoassay. Talanta 134: 120-125.
- Zhang J, Xia YK, Chen M, Wu DZ, Cai SX, et al. (2016) A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with Zn(II)-ion signal-enhancement for simultaneous detection of OTA and AFB1 Sensor. Actuat B-Chem 235: 79-85.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 6095
- [From(publication date):
September-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 5016
- PDF downloads : 1079