High Serum Level of Secreted Frizzled-Related Protein 5 (sfrp5) is Associated with Future Cardiovascular Events
Received: 13-Feb-2017 / Accepted Date: 17-Mar-2017 / Published Date: 25-Mar-2017
Abstract
Objectives: Novel biomarkers to predict future risk of coronary artery disease, especially in the early stage, are warranted. The goal of this study was to determine whether SFRP5, a novel anti-inflammatory adipokine, is associated with future cardiovascular events.
Methods: This is a 4-year follow-up study that included 81 subjects with CAG (+) and 87 subjects with CAG (-). SFRP5 levels was analyzed by ELISA. 2-year and 4-year follow-up were conducted for the occurrence of major adverse cardiac events (MACE), including unstable angina pectoris, ischemic stroke, nonfatal myocardial infarction, revascularization, and cardiovascular death.
Results: In multivariate Cox regression analysis, high SFRP5 significantly predicted patients’ MACE with an HR of 2.174 (95% CI 1.169, 4.041; p=0.014) at 2-year follow-up, and with an HR of 1.974 (95% CI 1.138, 3.426; p=0.016) at 4-year follow-up respectively. The incidence of MACE was significantly increased in the high SFRP5 group for CAG(-) patients ((HR (95%CI) 3.326(1.275, 8.680), p=0.014 for 2-year; HR (95%CI) 2.590(1.122, 5.978), p=0.026) for 4-year), as well as for patients over 65 years (HR(95%CI) 4.931(1.989, 12.222), p<0.001 for 2-year; HR(95%CI) 3.194(1.541, 6.621), p=0.002 for 4-year, respectively).
Conclusion: A high serum concentration of SFRP5 is associated with the occurrence of future CV events,especially in the elderly.
Keywords: SFRP5; Coronaryarterydisease; Cardiovascularevent; Angiography; Hypertension; Inflammation
78758Introduction
Coronary artery disease (CAD), as a metabolic and inflammatory disease, carried a high incidence of hospital readmission and a high mortality risk, making it one of the deadliest diseases in the world [1]. Novel indicators as a prediction of these outcomes of CAD patients in an early stage is essential [2].
Adipose tissue secrets multiple adipokines, modulating the systemic inflammatory responses that contribute to inflammatory disorders such as metabolic syndrome, atherosclerosis and coronary artery disease [3]. Secreted frizzled-related protein 5 (SFRP5), as an emerging adipokine, played an important role in the glucose metabolism and diabetes mellitus [4-8]. Recently, SFRP5 has been identified as a novel adipokine with the anti-inflammatory function [9]. In the cardiovascular setting, studies about SFRP5 is indeed limited. Previous studies have shown that the SFRP5/Wnt5a regulatory system influenced the angiotensin II (AngII)-induced cardiomyocyte hypertrophy through the AT1 receptor/Rho/ROCK1/JNK signaling pathway, downregulating
BNP and TNF-a at the same time [10]. It could also diminish the cardiac inflammation to play a protective role [11]. Notably, a crosssectional study demonstrated for the first time that in the subjects with CAD (stenosis over 50%), the serum SFRP5 levels were significantly lower than those in the non-CAD (stenosis<50%) subjects, low SFRP5 levels may contribute to CAD [12]. However, in recent studies, the role of SFRP5 in the prediction of future cardiovascular events were not under the notice, making it uncertain.
The aim of this 4-year follow-up study was to investigate whether SFRP5 is associated with future cardiovascular events in both CAG(+) and CAG(-) subgroups, in terms of a composite primary endpoint of combined occurrence of major adverse cardiovascular events (MACE).
Methods
Participants
All patients were prospectively recruited in a cardiac catheterization laboratory at the cardiology department of Shanghai Tenth People’s Hospital (China). The study population was Shanghai residents undergoing coronary angiography to evaluate suspected or established CAD for clinical indications (most frequently chest pain, dyspnea on exertion), by physician’s decision. Participating CAG(+)patients had a coronary stenosis >50% at catheterization while CAG(-) group had a coronary stenosis <50%.
In accordance with non-inclusion criteria, subjects with heart failure (left ventricular ejection fraction <30%) or cardiomyopathies were excluded. In addition, we excluded patients with neoplasm and hematologic disorders, severe liver or renal disease (CKD>4), acute infection, acute state of a chronic infectious or inflammatory disease and history of cardiac procedures (percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)).
The diagnoses of T2DM were based on the diagnostic criteria made by World Health Organization (WHO) [13].
This protocol was approved by the Shanghai Tenth People’s Hospital Ethical Review Board, conducted in accordance with the Declaration of Helsinki. Written informed consents were obtained from all subjects.
Biochemical measurements
Serum was isolated from blood samples collected after overnight fasting, and stored at -80â��. Serum liver and renal function as well as the metabolic parameters like lipid profile were measured with standard laboratory techniques on colorimetric enzymatic assay systems (Roche MODULAR P-800, Switzerland). Levels of serum SFRP5 was measured with enzyme-linked immune-sorbent assays (ELISAs) (Human ELISA kit, Wuhan USCN Science Co, Ltd, China). ELISA’s detection range was 1.56-100 ng/mL. Intra-assay and inter-assay CVs were 10% and 12% for SFRP5.
Angiography
Patients were conducted with a coronary angiography through a radial artery with standard judkins technique. We defined CAG(+) as the presence of luminal diameter stenosis ≥ 50% in the left anterior descending artery (LAD), right coronary artery (RCA), left circumflex coronary artery (LCX) and their main branches. Over 50% luminal narrowing of the left main trunk stenosis was considered as two-vessel disease. We categorized the severity of coronary atherosclerosis by the number of coronary vessels with significant stenosis as 0, 1, 2, or ≥ 3 vessels, respectively. The imaging procedures were conducted by 2 professional interventional cardiologists, masked to the cohort data.
Clinical follow-up for endpoints
Follow-up was carried out in 2013.12.1-2013.12.31 and 2015.12.1- 2015.12.31, respectively. For the entire cohort of 168 subjects, the primary outcome was a composite of major adverse cardiovascular events (MACE), including unstable angina pectoris (UAP), ischemic stroke, nonfatal myocardial infarction (MI), revascularization (PCI or CABG) and cardiovascular death. UAP was defined by a chest pain with the troponin T value between 0.004-0.1 ng/mL. Nonfatal MI was defined by a rise of either troponin I >1.0 ng/mL or troponin T >0.1 ng/mL with canonical chest pain symptoms and/or characteristic electrocardiographic changes. Coronary revascularization with either PCI or CABG was defined with a history of either PCI or CABG which were confirmed by a review of the medical records. Ischemic stroke was defined as the new onset of a neurological deficiency symptoms lasting for at least 24 h with evidence from either magnetic resonance imaging or computed tomography. A death diagnosis was made on the basis of a death certificate given by our hospital or others’. The endpoint was a combination of telephone contact and review of the computerized medical records of clinic visits and hospitalizations, conducted by a blinded end-point evaluating committee.
Statistical Analysis
Baseline demographic and clinical characteristics are presented as mean ± SD, median (interquartile range), or frequency counts, and the differences between the two groups were evaluated with t-test or the Mann-Whitney test, as appropriate. Kolmogorov-Smirnov test were preferred to determine whether the data were normally distributed. The assessment of relations of SFRP5 with other biomedical indicators were evaluated using the multivariate regression. Games Howell test was performed to compare the SFRP5 levels in subgroups of different number of lesion vessels. Adjusted efficacy (hazard ratio) was estimated using the Cox regression model controlling for the confounders including age, gender, DM, hypertension history, CAD, smoking habits, and usage of statin. Kaplan-Meier curves were performed to show the MACE-free survival. In our analysis, we took the median value of the serum SFRP5 to distinguish high/low levels, and participants were divided into elder (over 65 years old) and younger (≤65 years old) subgroups separately. Statistical analysis was performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL). A two-sided significant level of 5% was considered as statistical significance.
Results
Characteristics of study participants at baseline
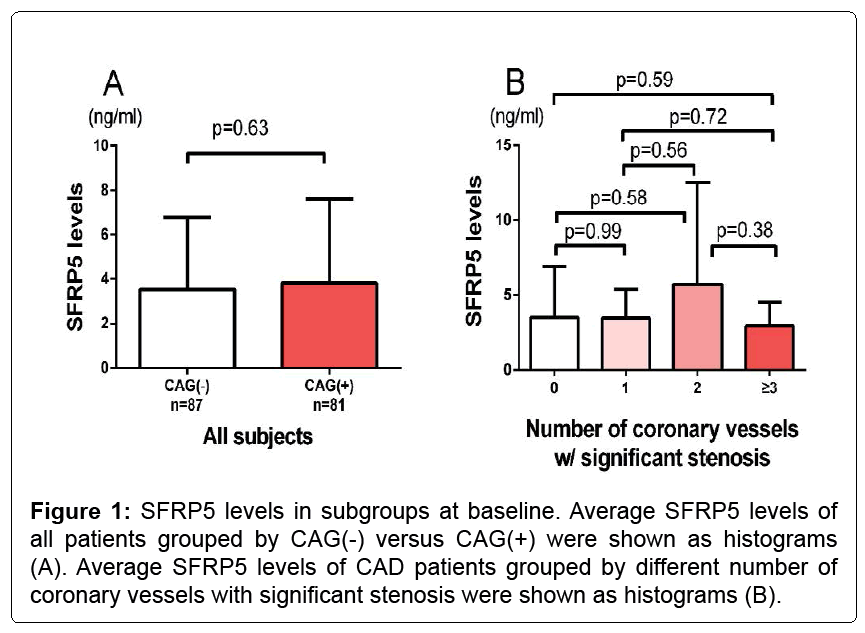
The characteristics of a total of 168 subjects including CAG(+) and CAG(-) participants were shown in Table 1. No significant differences were found between CAG(+) and CAG(-) subjects with respect to age, BMI, systolic blood pressure, blood urine nitrogen, triglyceride (TG), hypertension or diabetes and antihypertensive medications including ACEI/ARB, CCB. Serum hs-CRP levels in CAG(-) subjects were significantly lower than in CAG(+) subjects (5.15 ± 3.26 and 11.56 ± 5.89 ng/mL, respectively; p<0.001). And creatinine (76.6 ± 23.1 vs. 84.8 ± 26.1 umol/L, p=0.02) and uric acid levels (328.9 ± 112.4 vs. 366.0 ± 97.1 umol/L, p=0.04) were also greater in the CAG(+) group. For this entire cohort, the CAG(+) participants had a better lipid profile including a lower total cholesterol (4.8 ± 1.0 vs. 4.3 ± 1.1 mmol/L, p=0.009) and a lower LDL-c (2.8 ± 0.8 vs. 2.4 ± 0.9 mmol/L, p=0.02) than the CAG(-) group (Table 1), presumably as a result of the statin usage (4(5) vs. 19(23), p<0.001). Of note, as shown in Figure 1A, there was no significant difference of SFRP5 between CAG(+) and CAG(-) group (p=0.63, Figure 1A).
| Characteristic | All (n=168) | CAG (-) (n=87) | CAG (+) (n=81) | p |
|---|---|---|---|---|
| Age, years | 65 ± 11 | 65 ± 12 | 64 ± 10 | 0.38 |
| Male, n (%) | 93(55) | 41(47) | 52(64) | 0.03 |
| BMI, kg/m2 | 24.5 ± 3.7 | 24.5 ± 3.6 | 24.7 ± 3.8 | 0.8 |
| Currently smoking, n (%) | 33(20) | 13(15) | 20(25) | 0.12 |
| SBP (mmHg) | 144 ± 23 | 145 ± 24 | 142 ± 22 | 0.28 |
| SFRP5 (ng/mL) | 2.86(2.10) | 2.80(1.93) | 2.92(2.15) | 0.63 |
| hsCRP (ng/mL) | 8.55 ± 5.78 | 5.15 ± 3.26 | 11.56 ± 5.89 | <0.001 |
| cTNT (ng/mL) | 0.01(0.01) | 0.003(0.01) | 0.01(0.02) | 0.01 |
| BUN (mmol/L) | 5.85 ± 1.85 | 5.83 ± 1.87 | 5.86 ± 1.85 | 0.91 |
| Cr (umol/L) | 80.8 ± 24.9 | 76.6 ± 23.1 | 84.8 ± 26.1 | 0.02 |
| UA (umol/L) | 347.4 ± 106.3 | 328.9 ± 112.4 | 366.0 ± 97.1 | 0.04 |
| TC (mmol/L) | 4.5 ± 1.1 | 4.8 ± 1.0 | 4.3 ± 1.1 | <0.01 |
| TG (mmol/L) | 1.46(0.91) | 1.36(0.62) | 1.56(0.97) | 0.17 |
| HDL-c (mmol/L) | 1.1(0.4) | 1.2(0.5) | 1.0(0.2) | 0.03 |
| LDL-c (mmol/L) | 2.6 ± 0.9 | 2.8 ± 0.8 | 2.4 ± 0.9 | 0.02 |
| Diabetes mellitus, n (%) | 40(24) | 19(22) | 20(25) | 0.53 |
| Hypertension, n (%) | 108(64) | 56(64) | 52(64) | 0.9 |
| Statin, n (%) | 23(14) | 4(5) | 19(23) | <0.001 |
| ACEI/ARB, n (%) | 25(15) | 14(16) | 11(14) | 0.92 |
| CCB, n (%) | 31(18) | 15(17) | 16(20) | 0.35 |
| 2-year MACE, n (%) | 47(28) | 22(25) | 25(31) | 0.26 |
| 4-year MACE, n (%) | 58(35) | 27(31) | 31(38) | 0.21 |
Note: Data are shown as the mean ± SD, median (interquartile range), or frequency counts, as appropriate. CAG (+): at least 1 coronary stenosis >50% at catheterization. BMI: Body mass index; CAD: Coronary artery disease; SFRP5: Secreted frizzledrelated protein 5; hs-CRP: High-sensitivity Creactive protein; ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker; CCB: Calcium channel blocker; UA: Uric acid; Cr: Creatine; BUN: Blood urine nitrogen; TC: Total cholesterol; TG: Triglycerides; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low density lipoprotein-cholesterol
Table 1: Characteristics of the subjects.
Relationships between serum SFRP5 levels and conventional risk factors and disease severity
Table 2 showed that, a significantly higher rate of DM was found in the low SFRP5 group (31% vs. 17%, low vs. high SFRP5, p=0.045). For all patients, there is no significant differences between high and low SFRP5 groups with respect to the other prescribed conventional risk factors including age, gender, smoking state, hypertension, TG, total cholesterol (TC), LDL, blood pressure, proBNP, hsCRP, CAG(+). As for the number of coronary vessels with significant stenosis, which may identify the CAD severity, there is no significant differences of SFRP5 between groups of different lesion vessel numbers (Figure 1B).
| Characteristic | Low SFRP5 group | High SFRP5 group | |
|---|---|---|---|
| All subjects(n=168) | n=84 | n=84 | p value |
| CAG (+), n (%) | 39(46) | 42(50) | 0.76 |
| DM, n (%) | 26(31) | 14(17) | 0.045 |
| Smoking, n (%) | 17(20) | 16(19) | 0.99 |
| Hypertension, n (%) | 52(62) | 56(67) | 0.63 |
| Age, years | 65.5 ± 10.6 | 64.4 ± 11.7 | 0.5 |
| SBP (mmHg) | 142 ± 22 | 146 ± 23 | 0.2 |
| proBNP (pg/ml) | 124 (210.3) | 128.7 (412.2) | 0.42 |
| cTNT (ng/mL) | 0.003(0.01) | 0.01(0.02) | 0.36 |
| LDL-c (mmol/L) | 2.62 ± 0.88 | 2.60 ± 0.87 | 0.89 |
| hsCRP (ng/ml) | 8.8 ± 6.2 | 8.4 ± 5.7 | 0.8 |
| 2-year MACE, n (%) | 17(20) | 30(36) | 0.016 |
| 4-year MACE, n (%) | 23(27) | 35(42) | 0.023 |
Note: Data are shown as the mean ± SD, median (interquartile range), or frequency counts, as appropriate. CAG (+): at least 1 coronary stenosis >50% at catheterization
Table 2: Characteristics of the subjects grouped by SFRP5 levels.
Results of the 2-year and 4-year follow-up
The median follow-up of this study was 2.15 years (2-year) and 4.16 years (4-year) respectively. During the inclusion period, 4 patients (2.4%) at 2-year follow-up and 6 patients (3.6%) at 4-year follow-up dropped out of our study. These dropouts in our study was as a result of losing contact including both phone contact and medical records.
The occurrence of MACE for the participants
73 MACE occurred in 47 patients (28.7% of 164 patients) at 2-year follow-up including 37 UAP, 14 nonfatal MIs, 9 ischemic strokes, 11 revascularizations and 3 cardiovascular deaths. 118 MACE occurred in 58 patients (35.8% of 162 patients) at 4-year follow-up including 49 UAP, 25 nonfatal MIs, 16 ischemic strokes, 20 revascularizations and 8 cardiovascular deaths (Tables 3 and 4).
| 2-year follow-up | 4-year follow-up | |||
|---|---|---|---|---|
| Participants | HR (95%CI) | P | HR (95%CI) | P |
| All | 2.174 (1.169, 4.041) | 0.014 | 1.974 (1.138, 3.426) | 0.016 |
| Non-CAD group | 3.326 (1.275, 8.680) | 0.014 | 2.590 (1.122, 5.978) | 0.026 |
| Cad group | 1.884 (0.791, 4.489) | 0.153 | 1.845 (0.852, 3.992) | 0.12 |
| Young group | 0.702 (0.255, 1.933) | 0.494 | 0.894 (0.350, 2.284) | 0.815 |
| Old group | 4.931 (1.989, 12.222) | <0.001 | 3.194 (1.541, 6.621) | 0.002 |
Note: Age, gender, low/high SFRP5, DM, hypertension history, CAD, Smoking, and usage of statin are forced into the model, and CAD was excluded when it’s applied to the CAD/Non-CAD group.
Table 3: Cox regression results of surviving from MACE with low/high SFRP5 levels.
| Events | 2-year MACE (n=47) | 4-year MACE (n=58) |
|---|---|---|
| Unstable angina pectoris, n (%) | 37(79) | 49(84) |
| Nonfatal MI, n (%) | 14(30) | 25(43) |
| Ischemic stroke, n (%) | 9(19) | 16(28) |
| Revascularization, n (%) | 11(23) | 20(34) |
| Cardiovascular death, n (%) | 3(6) | 8(14) |
Note: 1 patient was carried home in an agonal stage because of the social custom that Shanghai people should die in their own home (2- year follow-up). And this case was confirmed by telephone later. Other cardiovascular death was diagnosed on the basis of a death certificate given by our hospital or others’. No non-cardiovascular death occurred during follow up.
Table 4: Number of events in 2-year follow up and 4-year follow up.
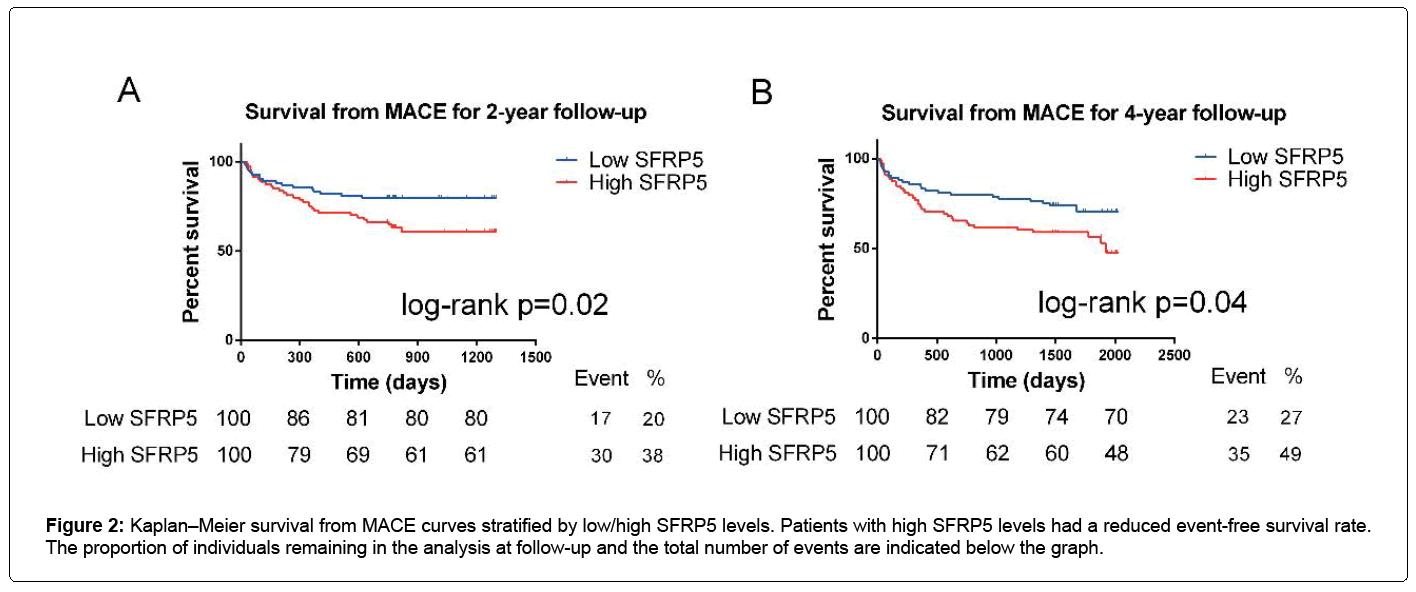
For all the participants, Figure 2 shows that a high SFRP5 level was significantly associated with a high MACE rate at 2-year follow-up (logrank p=0.024), as well as at 4 years (log-rank p=0.036) (Figures 2A and 2B).
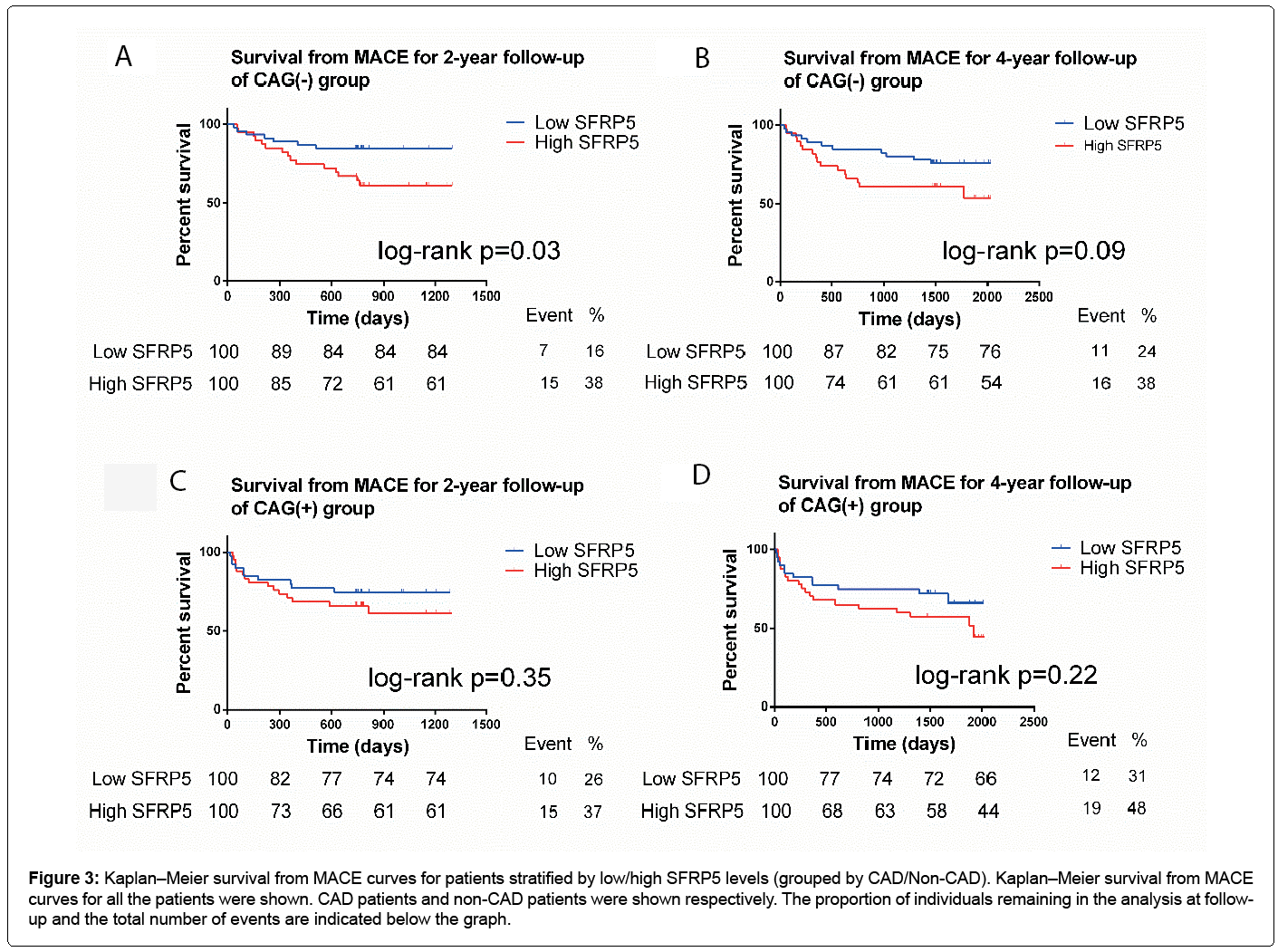
Figure 3 was designed based on the CAG(+) and CAG(-) groups. For CAG(-) participants, a high SFRP5 level was significantly associated with a high MACE rate at 2-year follow-up (log-rank p=0.025) (Figure 3). In the 4-year follow-up, this trend was not significant (log-rank p=0.096) (Figure 3). And there was also a consistent trend for CAG(+) patients with CAD but it did not reach statistical significance (Figure 3).
Figure 3: Kaplan–Meier survival from MACE curves for patients stratified by low/high SFRP5 levels (grouped by CAD/Non-CAD). Kaplan–Meier survival from MACE curves for all the patients were shown. CAD patients and non-CAD patients were shown respectively. The proportion of individuals remaining in the analysis at followup and the total number of events are indicated below the graph.
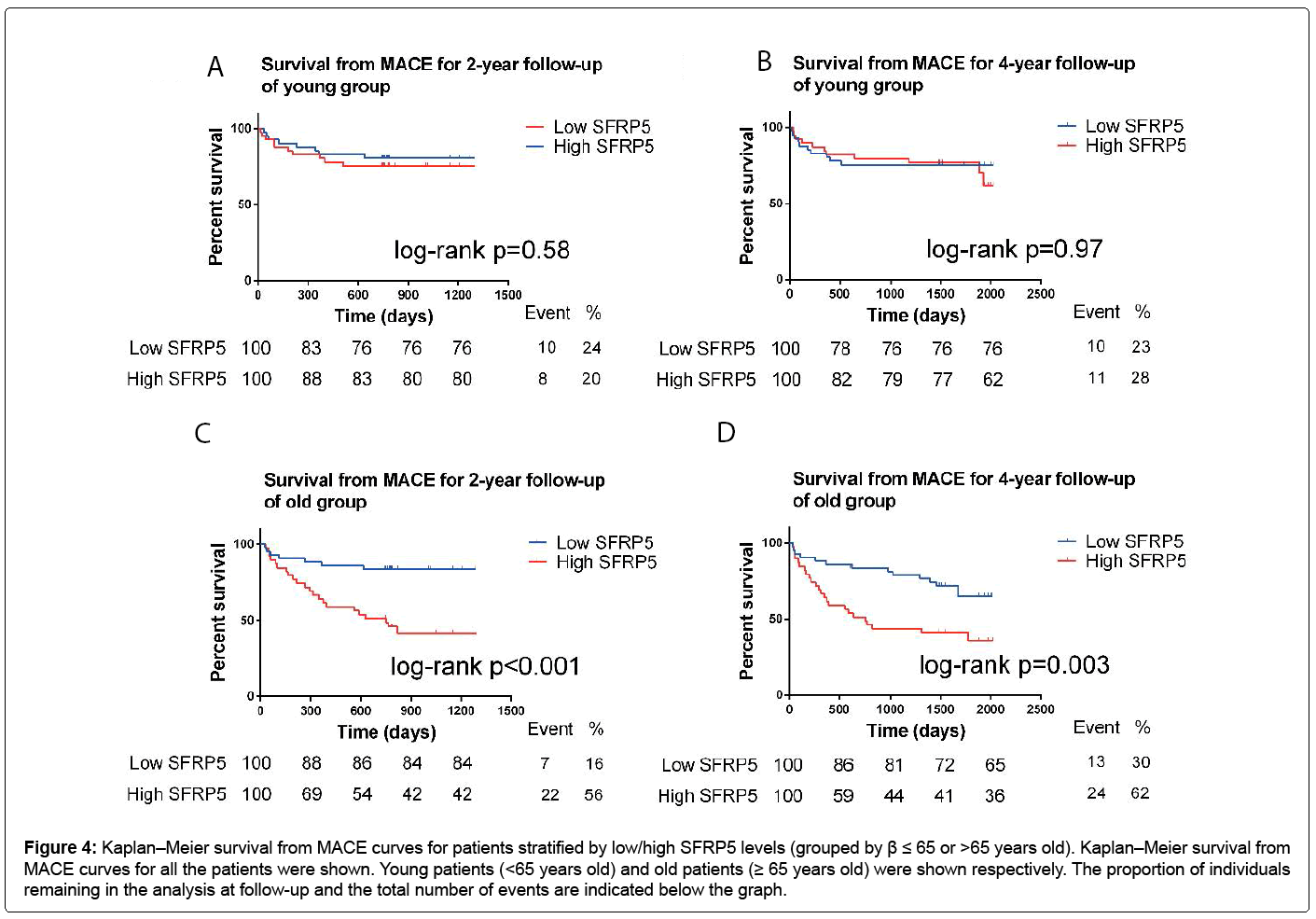
For the participants over 65 years old, a high SFRP5 level was significantly associated with a high MACE rate at 2-year follow-up (log-rank p<0.001) and 4-year follow-up (log-rank p=0.003) (Figure 4). But for people who were ≤ 65 years old, there was no obvious relationships between the SFRP5 levels and the MACE rate (Figure 4).
Figure 4: Kaplan–Meier survival from MACE curves for patients stratified by low/high SFRP5 levels (grouped by β ≤ 65 or >65 years old). Kaplan–Meier survival from MACE curves for all the patients were shown. Young patients (<65 years old) and old patients (≥ 65 years old) were shown respectively. The proportion of individuals remaining in the analysis at follow-up and the total number of events are indicated below the graph.
Hazard ratio of MACE associated with a high SFRP5 level
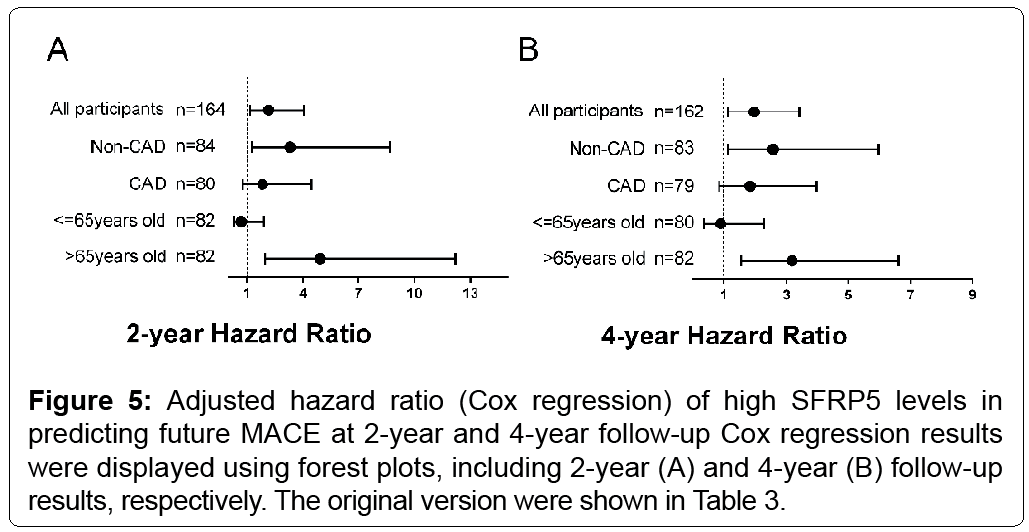
Considering the adjusted Cox model with a satisfactory proportional hazard assumption to all the adjusted covariates including age, gender, DM, hypertension history, CAG(+), smoking and usage of statin, MACE rate was significantly higher in the high SFRP5 group. All participants had an HR of 2.174 (95% CI 1.169, 4.041; p=0.014) at 2-year follow-up, and HR of 1.974 (95% CI 1.138, 3.426; p=0.016) at 4-year follow-up, respectively. CAG(-) Patients had an HR of 3.326 (95% CI 1.275, 8.680; p=0.014) at 2-year follow-up and an HR of 2.590 (95% CI 1.122, 5.978; p=0.026) at 4-year follow-up (Table 3 and Figure 5). No significant relationships were found in CAG(+) patients (Table 3 and Figure 5). Patients aged over 65 years had an HR of 4.931 (95% CI 1.989, 12.222; p<0.001) at 2-year and an HR of 3.194 (95%a CI 1.541, 6.621; p=0.002) at 4-year follow-up. As for patients aged 65 years or younger, no significant relationships were found (Table 3 and Figure 5).
Discussion
In the literature, there were limited data available on the prognostic significance of SFRP5 in the field of cardiovascular disease, but SFRP5 was always considered as a potential CV biomarker [10-12]. Our study is the first one evaluating the association between circulating SFRP5 levels and future MACE. We demonstrated that increased SFRP5 levels was significantly associated with the occurrence of MACE, especially in patients over 65 years old.
The mean age of the 168 patients (93 males, 55%) was 65 ± 11 years. 108 patients (64%) had a history of hypertension, 40 (24%) had diabetes mellitus and 33 (20%) were current smokers. We found no significant differences in the distribution of gender and age between high/low SFRP5 groups, which is consistent with the previous studies [6,12]. Of note, pro-BNP, LDL-c, cTNT and hs- CRP were unaffected by low or high SFRP5 levels. Whether SFRP5 is associated with DM remains controversial [7,14]. In our study, we found that DM rate in patients with low SFRP5 was significantly higher than those with high SFRP5.
In our study, there is no significant difference of SFRP5 levels between CAG(+) and CAG(-) groups. There is even no significant difference of SFRP5 levels between the groups of different lesion vessel numbers. However, a previous cross-sectional study revealed that, for a group of 185 participants who were from Kagawa Prefectural Central Hospital, serum SFRP5 levels were significantly associated with CAD (stenosis over 50%). And for the younger group (<65 years old), SFRP5 serves as a strong CAD indicator [12]. There were several reasons to explain this difference. First of all, for both of these two studies, the limited sample size has impact on the results at baseline. On the other hand, it has been widely accepted that there are always differences between ethnics in many aspects such as pharmacokinetics [15]. And for the biological marker, the existence of such differences between the ethnics can also be accepted [16]. Finally, though SFRP5 has been considered as a metabolism-associated adipokine in many other aspects such as inflammation, oxidative stress and role of biomarker [6,17,18], the role of SFRP5 in human cardiovascular physiology remains unknown.
We illustrated that increased baseline serum SFRP5 was significantly associated with future MACE, especially for participants over 65 years old. Such association has some underlying reasons. Previous studies revealed that WNT signaling pathway can be antagonized by SFRPs by primarily binding to the WNT proteins [19]. Moreover, SFRP5 can block the production of inflammatory mediators from the macrophage by targeting the downstream substances in the non-canonical WNT5a signaling [9,20]. These findings revealed the anti-inflammatory effect of SFRP5. Coronary heart disease, for example, is also an inflammatory disease. CAD is characterized by a progressive inflammation and activation of macrophage. And WNT is also involved in these aspects, including endothelial dysfunction and macrophage activation [21]. Therefore, the elevated SFRP5 levels may indicate a protective compensatory response to inflammation. And SFRP5 may serve a vital protective role in the occurrence of CAD. Given these observations, high serum SFRP5 concentrations may indicate the occurrence and development of CAD as well as other cardiovascular disease.
We observed that, for all the patients, a high SFRP5 level was significantly associated with 2-year and 4-year MACE. And for CAG(-) patients, the high SFRP5 group had a significantly higher MACE rate at 2-year follow-up. However, there was only a trend at 4-year followup, it did not reach significant. As previously reported, for a same cohort of patients, survival rate from MACE at 4-year follow-up may be confounded [22,23]. It would not be as clear as what it was in the first 2 years. And after adjusting confounders in Cox’s regression model, a significantly high incidence of MACE in high SFRP5 group at 4-year follow-up became significant, which further proved the influence of confounders. Moreover, we also found that, for CAD patients, there was only a trend, it did not reach statistical significant. There are many contributors of CAD. As the development of CAD, the impact of SFRP5 concentrations may be also confounded. Therefore, for those who suffered from stenosis of over 50% already, it may has no clear significance of distinction.
We did find a significant association between high SFRP5 levels and high MACE rate in elderly persons. Previous studies revealed that the risk of cardiovascular event increased with aging [22,24]. Thus, considering high SFRP5 level as a risk factor, its impact would also increase with aging. It’s understandable that high SFRP5 levels in the elderly (over 65 years old) have a significant association with MACE.
Our study had several strengths. This is the first study which revealed SFRP5 as an emerging cardiovascular biomarker. SFRP5 can serve as a tool for future risk assessment of cardiovascular disease. Second, our study used a prospective cohort design, and only 4 patients (2.4%) at 2-year follow-up and 6 patients (3.6%) at 4-year follow-up dropped out of our study. Several limitations should also be considered in this study. First, the control group was not general population, they were patients who also suffered from some mild CVDs like hypertension. Second, this study had a limited number of the participants (n=168). These results required a larger sample size to validate. Third, the superiority of SFRP5 over current prognostic biochemical markers deserves further investigation, though we found that SFRP5 is relatively independent from other prognostic parameters.
Conclusion
We demonstrated for the first time that high serum concentration of SFRP5 is associated with the future cardiovascular events, independent from other conventional risk factors. Furthermore, the impact of high SFRP5 seems to be greater in elderly. Measurement of circulating SFRP5 levels may be used to evaluate the risk of future cardiovascular event.
Acknowledgments
This study was supported by grants from National Natural Science Foundation of China (No.81300239), and Shanghai Sailing Program (NO.15YF1409400).
References
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) Heart disease and stroke statistics-2015 update: A report from the American Heart Association. Circulation 131: e29-322.
- White HD, Chew DP (2008) Acute myocardial infarction. The Lancet 372: 570-584.
- Nakamura K, Fuster JJ, Walsh K (2014) Adipokines: A link between obesity and cardiovascular disease. J Cardiol 63: 250-259.
- Â Mori H, Prestwich TC, Reid MA, Longo KA, Gerin I, et al. (2012) Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest 122: 2405-2416.
- Â Jura M, Jaroslawska J, Chu DT, Kozak LP (2016) Mest and Sfrp5 are biomarkers for healthy adipose tissue. Biochimie 124: 124-133.
- Carstensen M, Herder C, Kempf K, Erlund I, Martin S, et al. (2013) Sfrp5 correlates with insulin resistance and oxidative stress. Eur J Clin Invest 43: 350-357.
- Â Hu Z, Deng H, Qu H (2013) Plasma SFRP5 levels are decreased in Chinese subjects with obesity and type 2 diabetes and negatively correlated with parameters of insulin resistance. Diabetes Res Clin Pract 99: 391-395.
- Mori H, Prestwich TC, Reid MA, Longo KA, Gerin I, et al. (2012) Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest 122: 2405-2416.
- Â Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, et al. (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329: 454-457.
- Â Jin X, Guo B, Yan J, Yang R, Chang L, et al. (2015) Angiotensin II increases secreted frizzled-related protein 5 (sFRP5) expression through AT1 receptor/Rho/ROCK1/JNK signalling in cardiomyocytes. Mol Cell Biochem 408: 215-222.
- Jura M, Jaroslawska J, Chu DT, Kozak LP (2016) Mest and Sfrp5 are biomarkers for healthy adipose tissue. Biochimie 124: 124-133.
- Carstensen M, Herder C, Kempf K, Erlund I, Martin S, et al. (2013) Sfrp5 correlates with insulin resistance and oxidative stress. Eur J Clin Invest 43: 350-357.
- Hu Z, Deng H, Qu H (2013) Plasma SFRP5 levels are decreased in Chinese subjects with obesity and type 2 diabetes and negatively correlated with parameters of insulin resistance. Diabetes Res Clin Pract 99: 391-395.
- Â Cheng L, Zhang D, Chen B (2015) Declined plasma sfrp5 concentration in patients with type 2 diabetes and latent autoimmune diabetes in adults. Pak J Med Sci 31: 602-605.
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, et al. (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329: 454-457.
- Haines AP, Levin AG, Fritsche HA (1979) Ethnic-group differences in serum levels of carcinoembryonic antigen. Lancet 2: 969.
- Flehmig G, Scholz M, Kloting N, Fasshauer M, Tonjes A, et al. (2014) Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PloS one 9: e99785.
- Jin X, Guo B, Yan J, Yang R, Chang L, et al. (2015) Angiotensin II increases secreted frizzled-related protein 5 (sFRP5) expression through AT1 receptor/Rho/ROCK1/JNK signalling in cardiomyocytes. Mol Cell Biochem 408: 215-222.
- Nakamura K, Sano S, Fuster JJ, Kikuchi R, Shimizu I, et al. (2016) Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J Biol Chem 291: 2566-2575.
- Miyoshi T, Doi M, Usui S, Iwamoto M, Kajiya M, et al. (2014) Low serum level of secreted frizzled-related protein 5, an anti-inflammatory adipokine, is associated with coronary artery disease. Atherosclerosis 233: 454-459.
- Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539-553.
- Canivell S, Rebuffat S, Ruano EG, Kostov B, Siso-Almirall A, et al. (2015) Circulating SFRP5 levels are elevated in drug-naive recently diagnosed type 2 diabetic patients as compared with prediabetic subjects and controls. Diabetes Metab Res Rev 31: 212-219.
- McLeod HL (2002) Pharmacokinetic differences between ethnic groups. Lancet 359: 78.
- Flehmig G, Scholz M, Kloting N, Fasshauer M, Tonjes A, et al. (2014) Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PloS one 9: e99785.
Citation: Ji H, Li H, Zhuang J, Su Y, Wen J, et al. (2017) High Serum Level of Secreted Frizzled-Related Protein 5 (sfrp5) is Associated with Future Cardiovascular Events. Cardiovasc Ther 2: 115.
Copyright: © 2017 Ji H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4634
- [From(publication date): 0-2017 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 3719
- PDF downloads: 915