High Risk of Acute Exacerbation of Interstitial Lung Disease In Relapsed Small Cell Lung Cancer Treated With Amrubicin
Received: 01-Aug-2019 / Accepted Date: 14-Aug-2019 / Published Date: 20-Aug-2019
Abstract
Background: The safety and efficacy of chemotherapy for relapsed small cell lung cancer (SCLC) with interstitial lung disease (ILD) is uncertain. ILD is a risk factor for acute exacerbation (AE)-ILD.
Methods: From January 2009 to December 2017, we retrospectively analyzed relapsed SCLC patients with ILD who received chemotherapy at Nagoya Medical Center. ILD was diagnosed from the clinical features and highresolution computed tomography (HRCT). The objectives were to evaluate the incidence of AE-ILD for relapsed SCLC with each agent and overall survival (OS) of the patients from second-line chemotherapy.
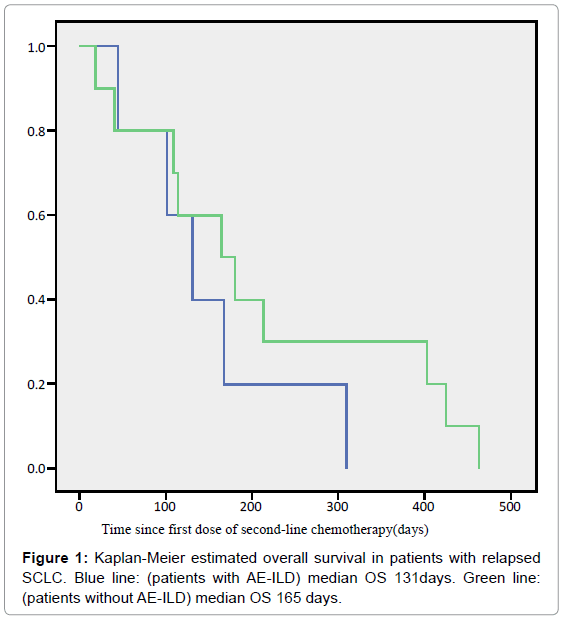
Results: In total, 16 relapsed SCLC patients with ILD were treated. In the process of receiving chemotherapy, six patients (regimens: amrubicin/topotecan 5/1, usual interstitial pneumonia (UIP) pattern/non-UIP pattern 4/2) developed AE-ILD. None of the patients administered paclitaxel (PTX) or nanoparticle albumin bound paclitaxel (nab-PTX) developed AE-ILD. The incidence of AE-ILD treated with amrubicin was 45.5% (5/11). Median overall survival of the patients with and without AE from the start of second-line chemotherapy was 131 days and 165 days, respectively; the difference between the two groups was not statistically significant (p=0.324).
Conclusions: Our results indicated that amrubicin may have a higher risk of AE-ILD. However, PTX regimens could be a safe option for relapsed SCLC with ILD
Keywords: Small cell lung cancer; Interstitial lung disease; Acute exacerbation; Amrubicin
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. Small cell lung cancer (SCLC) accounts for 15% to 20% of lung cancer [1]. SCLC is characterized by a high rate of invasion and rapid cellular proliferation. SCLC also shows high sensitivity to chemotherapy and radiotherapy; however, the response duration is relatively short. The standard chemotherapy regimen for SCLC patients is combined platinum agents and etoposide or platinum agents plus irinotecan, which is the most frequently used combination and yields a median survival period of approximately 9–12 months in clinical trials [2,3].
Interstitial lung disease (ILD) is characterized by damage to the lung parenchyma by inflammation and fibrosis. Preexisting ILD is a risk factor for acute exacerbation (AE) for chemotherapy-related ILD (AE-ILD). AE-ILD may bring on a fatal state. Whether chemotherapy is feasible for patients with SCLC with ILD remains unclear because patients with ILD have been excluded from most prospective clinical trials. In clinical practice, patients with SCLC with ILD have been carefully treated with cytotoxic chemotherapy.
Patients with advanced SCLC with ILD treated with etoposide and platinum agent combination chemotherapy gain benefits, with safety equivalent to what is seen in patients without ILD [4]. For secondline chemotherapy, nogitecan (NGT) or paclitaxel (PTX) is effective for treating patients with relapsed SCLC with ILD [5,6]. However, the safety and efficacy of chemotherapy for patients with relapsed SCLC with ILD has not been elucidated, while a previous study showed that computed tomography (CT) findings were associated with AE-ILD [7].
We conducted a retrospective study to evaluate the safety and efficacy of chemotherapy for treating patients with relapsed SCLC with ILD. Additionally, we retrospectively analyzed pretreatment CT and investigated the clinical course of patients with relapsed SCLC with ILD.
Patients and Methods
The medical records of patients with SCLC with ILD treated with cytotoxic chemotherapy at Nagoya Medical Center between January 2009 and December 2017 were retrospectively reviewed. The primary endpoint of this study was to evaluate the incidence of AE-ILD for relapsed SCLC with ILD with each agent. Secondary endpoint was to evaluate the overall survival (OS) of the patients with SCLC with ILD who received second-line chemotherapy; in short, to find an optimal drug and whether the patients obtained survival benefit.
The clinical characteristics, treatment outcomes and survival of these patients were analyzed using data obtained from their medical records. ILD was diagnosed from the patients’ medical histories, physical examinations and radiological abnormalities that were consistent with the characteristics of bilateral lung fibrosis, such as ground-glass opacity and consolidation, with or without reticular shadow. In patients with ILD, usual interstitial pneumonia (UIP) and other types of pneumonia were diagnosed based on CT features defined by the International Consensus Statement of the European Respiratory Society [8]. We excluded patients with apparent pulmonary infection, pulmonary embolism, or heart failure.
Chemotherapy-sensitive relapse was defined as relapse at an interval of >90 days after completion of first-line chemotherapy and refractory relapse was defined as no response to first-line chemotherapy or relapse within 90 days of completion of the first-line chemotherapy [9].
All responses were defined according to the Response Evaluation Criteria in Solid Tumors version 1.1 criteria. OS was measured from the start of second-line treatment until death. OS was determined using the Kaplan-Meier method. Statistical analyses were performed using a statistical software program (PASW Statistics 18; SPSS Inc., Chicago, IL). Results were considered statistically significant when the p value was less than or equal to 0.05.
The Institutional Ethics Review Board of Nagoya Medical Center approved the study protocol and the research was conducted in accordance with the principles of the World Medical Association Declaration of Helsinki; patients were informed that they could withdraw consent at any time during treatment.
Results
A total of 266 consecutive patients were diagnosed with SCLC at our institution. Twenty-one of these patients had ILD and 16 received second-line chemotherapy.
Patient characteristics
Patient clinical characteristics are summarized in Table 1. All patients were Japanese; 13 were men (81%) and 3 were women (19%) with a median age of 70 years (range= 61–82 years). Of the 16 analyzed patients, eight (50%) had limited disease (LD) and eight (50%) showed extended disease (ED). All patients were current or former smokers. Of these, seven patients (44%) were identified with a UIP pattern, and nine (56%) had a non-UIP pattern. Four patients had sensitive relapses, and 12 refractory relapses. 11 patients received carboplatin (CBDCA) plus etoposide (ETP) and five patients received cisplatin (CDDP) plus ETP.
| Sex | |
| Men/Women | 13/3 |
| Age, years | |
| Median (range) | 70 (61-82) |
| Stage | |
| LD/ED | 8/8 |
| ECOG performance status | |
| 0/1/2 | 5/7/4 |
| Smoking status | |
| Current/Former/Never | 7/9/0 |
| ILD pattern | |
| UIP/non-UIP | 7/9 |
| Relapse type | |
| Sensitive/Refractory | 4/12 |
| 1st line chemotherapy | |
| CBDCA+ETP/CDDP+ETP | 11/5 |
Table 1: Patients’ Characteristics (n=16). The study included 16 patients. ECOG: Eastern Cooperative Oncology Group; LD: Limited Disease, ED: Extended Disease, ILD: Interstitial Lung Disease, UIP: Usual Interstitial Pneumonia, CBDCA: Carboplatin, CDDP: Cisplatin, ETP: Etoposide.
Incidence of AE-ILD and treatment outcome
Of the 16 patients with SCLC with ILD, six (38%) developed AEILD. Table 2 summarizes the treatment outcomes of chemotherapy for relapsed SCLC with ILD. Eleven patients received amrubicin (AMR), six received paclitaxel (PTX), two received nanoparticle albumin-bound PTX (nab-PTX), three received Topotecan (TOP), and two received platinum agents plus ETP (re-challenge). AE-ILD was observed in 46% (5/11) and 33% (1/3) of patients treated with AMR and TOP, respectively. The patients with AE-ILD are summarized in Table 2. Two of six patients had UIP patterns (33%). Treatment for AE-ILD was administered to one patient. The other patients improved with corticosteroid therapy. The median OS from the day secondline chemotherapy started was 131 days [95% confidence interval (CI): 67–195 days] and 165 days [95% CI: 63–267 days] for patients with and without AE-ILD, respectively (Figure 1). No statistically significant difference in OS was observed between the AE-ILD and non-AE-ILD groups (p=0.324). Moreover, one patient was excluded from the Kaplan-Meier curve because AE-ILD developed after thirdline chemotherapy (Table 3, patient 3). The disease control rates of each chemotherapeutic regimen were 27%, 17%, 50%, 33% and 100%, respectively, as described in Table 4.
| Regimen | N | AE-ILD(%) | DCR (%) |
|---|---|---|---|
| AMR | 11 | 5/11 (46) | 27 |
| PTX | 6 | 0 | 17 |
| Nab-PTX | 2 | 0 | 50 |
| TPO | 3 | 1/3 (33) | 33 |
| Platinam+ETP | 2 | 0 | 100 |
Table 2: Treatment outcome of chemotherapy in patients with relapsed SCLC. AE-ILD: Acute Exacerbation-Interstitial Lung Disease; DCR: Disease Control Rate; CR: Complete Responce; PR; Partial Response; SD: Stable Disease; AMR: Amrubicin; PTX: Paclitaxel; nab-PTX: Nanoparticle Albumin-Bound PTX; TOP: Topotecan; ETP: Etoposide.
| Patient | Age/Sex | PS | Regimen | ILD pattern | Stage | Relapse type | Line | Grade |
|---|---|---|---|---|---|---|---|---|
| 1 | 76/F | 1 | AMR | UIP | LD | Sensitive | 2 | 5 |
| 2 | 63/M | 0 | AMR | Non UIP | ED | Refractory | 2 | 2 |
| 3 | 74/F | 1 | AMR | Non UIP | LD | Refractory | 2 | 2 |
| 4 | 78/M | 2 | TPO | UIP | ED | Refractory | 3 | 3 |
| 5 | 78/M | 0 | AMR | Non UIP | LD | Refractory | 2 | 2 |
| 6 | 62/M | 0 | AMR | Non UIP | ED | Refractory | 2 | 3 |
Table 3: Summary of patients who developed AE-ILD.
| Event | Gr1 | Gr2 | Gr3 | Gr4 | Gr5 | All Grades |
|---|---|---|---|---|---|---|
| Leukocytopenia | 1 | 1 | 2 | 4 | 0 | 8 |
| Neutropenia | 0 | 1 | 2 | 4 | 0 | 7 |
| Thrombocytopenia | 0 | 2 | 2 | 0 | 0 | 4 |
| Anemia | 1 | 2 | 1 | 0 | 0 | 4 |
| Febrile neutropenia | 0 | 0 | 1 | 1 | ||
| Neurotoxicity | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 0 | 3 | 1 | 0 | 1 | 5 |
The study included 11 patients.
Table 4: Treatment-related adverse events of AMR.
Toxicity without AE-ILD
Of the 11 patients treated with AMR after second-line chemotherapy, grade 4 leukocytopenia and grade 4 neutropenia occurred in 36% and 36%, respectively (Table 4). Toxicity of patients with PTX or nab-PTX is shown in Table 5. No patients had AE-ILD. Additionally, no grade 4 adverse events including hematotoxicity showed among the patients who received PTX or nab-PTX.
| Event | Gr1 | Gr2 | Gr3 | Gr4 | Gr5 | All Grades |
|---|---|---|---|---|---|---|
| Leukocytopenia | 1 | 1 | 1 | 0 | 0 | 3 |
| Neutropenia | 0 | 1 | 1 | 0 | 0 | 2 |
| Thrombocytopenia | 0 | 1 | 0 | 0 | 0 | 1 |
| Anemia | 2 | 0 | 0 | 0 | 0 | 2 |
| Febrile neutropenia | 1 | 0 | 0 | 1 | ||
| Neurotoxicity | 1 | 0 | 1 | 0 | 0 | 2 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 |
The study included 8 patients.
Table 5: Treatment-related adverse events of PTX and nab-PTX.
Discussion
Drug-induced AE-ILD, a potentially fatal adverse effect, may occur in relapsed SCLC patients with ILD who undergo chemotherapy. The optimal chemotherapy for such patients remains controversial [5,6,10].
In the present study, the total incidence of AE-ILD was 38% (6/16). We found a relatively high incidence of ILD in SCLC patients with AMR, a promising agent for treating SCLC. Most patients with relapsed SCLC were treated with standard chemotherapy [11]. Previous studies already reported that AMR had a higher rate (33%) of AE-ILD in SCLC patients with ILD [12]. However, CT image evaluation, whether with a UIP pattern or non-UIP pattern was not described in this report. Kenmotsu et al. reported that AE-ILD was significantly more common in patients with lung cancer with a UIP pattern [7]. A recent study demonstrated the safety of nivolumab, the drug considered to have a high incidence of AE-ILD, for patients with lung cancer with a non- UIP pattern ILD [12,13].
In this study, as previously reported, AE-ILD was found in 46% (5/11) of relapsed SCLC patients with ILD, while interstitial pneumonitis due to chemotherapy occurred in 4.5% (3/67) of relapsed SCLC patients without ILD. The difference between the two groups was statistically significant (p<0.001). Our study showed that patients with a non-UIP pattern on CT who were administered AMR developed AEILD more often than those with the UIP pattern (Table 3). Our finding could indicate that the risk of AE-ILD with AMR did not depend on the CT image findings.
To the best of our knowledge, the present study is the first to report the incidence of AE-ILD in patients with relapsed SCLC who received AMR with HRCT pattern.
This may indicate that SCLC patients with ILD treated with AMR have a higher rate of AE-ILD even if they have a non-UIP pattern. Additionally, severe adverse events (grade 4/5), such as bone marrow suppression, were observed in the AMR group (Table 4).
The phase III trial demonstrated that the median survival with best supportive care was 13.9 weeks [14]. In this retrospective study, OS with AE-ILD was 131 days (18.7 weeks), and without AE-ILD was 165 days (23.6 weeks; Figure 1). These results indicate that patients with relapsed SCLC with ILD could obtain survival benefits. We need to evaluate this with a larger study size because the present study had a small sample size drawn from a single institution.
Although the difference between the two groups was not statistically significant in the present study, grade 5 AE-ILD occurred in one patient. AE-ILD may bring on a fatal state. Which agents are safe and effective for treating patients with relapsed SCLC with ILD is unclear. However, a previous study demonstrated that PTX or nab- PTX plus CBDCA was effective and feasible for treating non-small cell lung cancer (NSCLC) [15,16]. Additionally, the efficacy of PTX for previously treated SCLC patients was assessed in a phase II study [17]. In the present study, the efficacy of PTX or nab-PTX monotherapy for relapsed SCLC was demonstrated by disease control rates (DCR) of 17% and 50%, respectively [15,16]. Even though the efficacy of PTX or nab-PTX monotherapy was poor compared with previous studies, AEILD was not observed in this study (Table 2). Additionally, the toxicity included AE-ILD of PTX or nab-PTX, showed as summarized in Table 5. Grade 4/5 adverse events did not occur in this group. PTX or nab- PTX monotherapy was relatively safe in our study.
Use of PTX either as a monotherapy or in combination with CBDCA has been demonstrated for SCLC patients without ILD [17-19]. The studies that specifically focused on SCLC patients with ILD are listed in Table 6. In these studies, chemotherapy containing PTX was reported to be a relatively safe and effective option for SCLC patients with ILD [5,10]. This shows the important role that PTX may play, not only in NSCLC with ILD, but also in SCLC with ILD.
| Regimen | N | AE-ILD (%) | Grade5 (%) | DCR (%) |
|---|---|---|---|---|
| CBDCA+PTX (10) | 17 | 0 | 0 | 58.8 |
| AMR (12) | 12 | 4(33.3) | 1(25) | N.D |
| PTX (5) | 10 | 2(20) | N.D | 40.0 |
| NGT (17) | 23 | 5 (21.7) | 3 (60) | N.D |
| AMR (our study) | 11 | 5 (45.4) | 1 (20) | 27.2 |
| PTX (our study) | 8 | 0 | 0 | 25.0 |
N.D: Not Described; CBDCA: Carboplatin.
Table 6: Comparison with previous studies.
Nab-PTX is an albumin-bound, 130-nm particle form of PTX that exhibits a higher activity and lower toxicity than solvent-based PTX (sb-PTX). A phase III study found that administering nab-PTX in combination with CBDCA achieved a higher response rate compared with CBDCA plus PTX (33% vs. 25%, respectively) (20). Moreover, nab-PTX may replace the conventional treatment with PTX as it is more effective for treating patients with NSCLC. Additionally, a recent multicenter prospective study demonstrated that nab-PTX is safe for patients with NSCLC with ILD [20,21]. Therefore, we often administer nab-PTX to SCLC patients with ILD.
A recent study demonstrated that re-challenge chemotherapy could be effective for relapsed SCLC patients with ILD [22]. Two patients were administered re-challenge chemotherapy, CBDCA plus ETP as a second-line chemotherapy (Table 2). Although evaluation is difficult because of the low patient number, re-challenge chemotherapy for relapsed SCLC with ILD may be feasible for patients with sensitive relapse.
SCLC is the most aggressive type of lung cancer and survival following relapse from first-line treatment is generally poor [1]. Topotecan monotherapy was effective for relapsed SCLC without ILD [13]. Enomoto et al. reported its efficacy and safety for relapsed SCLC with ILD [23]. However, because 33% (1/3) of patients with topotecan monotherapy developed AE-ILD, we could not demonstrate the safety of this drug.
Our findings have several limitations. First, the present study is limited by the small number of patients involved and by the retrospective nature of the study. As described above, chemotherapy for SCLC patients with ILD is associated with a higher risk of AEILD. Thus, several of the previous studies we investigated on relapsed SCLC patients with ILD were also small. The safety and efficacy of chemotherapy must be confirmed in a prospective randomized large-scale study. Second, none of the patients had undergone lung biopsies and no autopsies were performed. AE-ILD resulting from chemotherapy could not be confirmed histogenetically.
Conclusion
Relapsed SCLC patients with ILD with AMR had a higher risk of developing AE-ILD even for patients with a non-UIP pattern. Chemotherapy containing PTX or nab-PTX for relapsed SCLC patients with ILD may be safe for relapsed SCLC with ILD.
References
- Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, et al. (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346: 85-91.
- Okamoto H, Watanabe K, Kunikane H, Yokoyama A, Kudoh S, et al. (2007) Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 97: 162-169.
- Minegishi Y, Kuribayashi H, Kitamura K, Mizutani H, Kosaihira S, et al. (2011) The feasibility study of Carboplatin plus Etoposide for advanced small cell lung cancer with idiopathic interstitial pneumonias. J Thorac Oncol 6: 801-807.
- Fujimoto D, Shimizu R, Kato R, Sato Y, Kogo M, et al. (2015) Second-line Chemotherapy for Patients with Small Cell Lung Cancer and Interstitial Lung Disease. Anticancer Res 35: 6261-6266.
- Enomoto Y, Inui N, Imokawa S, Karayama M, Hasegawa H, et al. (2015) Safety of topotecan monotherapy for relapsed small cell lung cancer patients with pre-existing interstitial lung disease. Cancer Chemother Pharmacol 76: 499-505.
- Kenmotsu H, Naito T, Kimura M, Ono A, Shukuya T, et al. (2011) The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol 6: 1242-1246.
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, et al. (2015) An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care 192: 3-19.
- Inoue A, Sugawara S, Yamazaki K, Maemondo M, Suzuki T, et al. (2008) Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol 26: 5401-5406.
- Mouri A, Yamaguchi O, Miyauchi S, Shiono A, Utsugi H, et al. (2019) Combination therapy with carboplatin and paclitaxel for small cell lung cancer. Respir Investig 57: 34-39.
- Horita N, Yamamoto M, Sato T, Tsukahara T, Nagakura H, et al. (2016) Amrubicin for relapsed small-cell lung cancer: a systematic review and meta-analysis of 803 patients.
- Yoh K, Kenmotsu H, Yamaguchi Y, Kubota K, Ohmatsu H, et al. (2010) Severe interstitial lung disease associated with amrubicin treatment. J Thorac Oncol 5: 1435-1438.
- Fujimoto D, Yomota M, Sekine A, Morita M, Morimoto T, et al. (2019) Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: A multicenter, open-label single-arm phase II trial. Lung Cancer 134: 274-278.
- O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cuceviá B, et al. (2006) Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 24: 5441-5447.
- Minegishi Y, Sudoh J, Kuribayasi H (2011) The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer 71: 70-74.
- Igawa S, Nishinarita N, Takakura A, Ozawa T, Harada S, et al. (2018) Real-world evaluation of carboplatin plus a weekly dose of nab-paclitaxel for patients with advanced non-small-cell lung cancer with interstitial lung disease. Cancer Manag Res 10: 7013-7019.
- Yamamoto N, Tsurutani J, Yoshimura N, Asai G, Moriyama A, et al. (2006) Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res 26: 777-781.
- Sugiyama K, Kogure Y, Torii A, Shiraishi K, Yamada A, et al. (2019) Solvent-based paclitaxel or nab-paclitaxel for heavily treated relapsed/refractory small cell lung cancer: Retrospective single-institution observational study.
- Mori K, Kamiyama Y, Kondo T, Kano Y, Kodama T (2006) Pilot phase II study of weekly chemotherapy with paclitaxel and carboplatin for refractory or relapsed small-cell lung cancer. Cancer Chemother Pharmacol 58: 86-90.
- Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, et al. (2012) Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 30: 2055-2062.
- Usui Y, Usui Y, Kenmotsu H, Mori K (2018) A multicenter single-arm phase II study of nab-paclitaxel/carboplatin for non-small cell lung cancer patients with interstitial lung disease. Annals of Oncology 29: 493-547.
- Nasu S, Suzuki H, Moriizumi K, Hara Y, Tanaka S, et al. (2019) Re-challenge chemotherapy in patients with sensitive relapse small-cell lung cancer and interstitial lung disease. J Thorac Dis 11: 514-520.
- Enomoto Y, Inui N, Imokawa S, Karayama M, Hasegawa H, et al. (2015) Safety of topotecan monotherapy for relapsed small cell lung cancer patients with pre-existing interstitial lung disease. Cancer Chemother Pharmacol 76: 499-505.
Citation: Niwa H, Torii A, Yamada A, Kogure Y, Kitagawa C, et al. (2019) High Risk of Acute Exacerbation of Interstitial Lung Disease In Relapsed Small Cell Lung Cancer Treated With Amrubicin. J Oncol Res Treat 4: 141.
Copyright: © 2019 Niwa H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 2126
- [From(publication date): 0-2019 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1399
- PDF downloads: 727