Research Article Open Access
High Frequency Embryogenic Callus Induction and Whole Plant Regeneration in Japonica Rice Cv. Kitaake
| Saroj Kumar Sah*, Ajinder Kaur and Jagdeep Singh Sandhu | ||
| School of Agricultural Biotechnology, Punjab Agricultural University, India | ||
| Corresponding Author : | Saroj Kumar Sah School of Agricultural Biotechnology Punjab Agricultural University, India Tel: +91-9779569724 E-mail: saroj-biotec@pau.edu, saroj1021@gmail.com |
|
| Received April 22, 2014; Accepted May 28, 2014; Published June 09, 2014 | ||
| Citation: Sah SK, Kaur A, Sandhu JS (2014) High Frequency Embryogenic Callus Induction and Whole Plant Regeneration in Japonica Rice Cv. Kitaake. J Rice Res 2:125. doi: 10.4172/jrr.1000125 | ||
| Copyright: © 2014 Sah SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Rice Research: Open Access
Abstract
A new and rapid protocol for production of embryogenic callus and plant regeneration has been reported in Oryza sativa cv. Kitaake. Callus cultures were established from seeds of Kitaake on MS medium supplemented with 2,4-D (3.0 mg/l), BAP (0.25 mg/l) and proline (0.6 g/l) gelled with phytagel (3.0 g/l), after 9 days of seed culturing. The embryogenic calli were regenerated on regeneration medium, i.e. MS medium supplemented with BAP (3.0 mg/l) and NAA (0.2 mg/l) and gelled with agar (8.0 g/l) in combination with phytagel (2.0 g/l) and regeneration occurred in 18 days. The regeneration percentage achieved was very high i.e 82.66% on the above medium. Rooting was achieved on half strength MS medium. The plantlets were hardened and transferred to soil in earthen pots. The regeneration protocol so developed through callus formation was highly reproducible. The plants showed normal growth and flowering under glass house conditions as well as in field conditions.
| Keywords | |
| MS medium; Kitaake; Shoot regeneration | |
| Introduction | |
| World population is increasing day by day and by the year 2050, it is expected to reach 9.1 billion. In order to feed this population, food production needs to be increased by 70% of the present production and global agricultural production must be increased by 60-110% [1-3]. Current rate of grain production are not sufficient to meet the increasing food demand. Nearly 2.4% increase in grain yield per year is needed to double the crop production by 2050. But at current rates, approximately 60%, 42%, 38% and 55% increase in maize, rice, wheat and soybean production, respectively is possible by that time [4]. Increase in rice yield by 1.0% per year, may not result in any change in the per capita rice harvest by 2050. Rice, wheat, maize and soybean provide nearly 43% of global dietary energy and 40% of daily protein supply [4]. More than two billion people world-wide totally depend upon rice and in Asia rice is the staple food, where it provides 40-70% of the total food calories consumed [5]. In Afghanistan, India, Bangladesh, Laos, Vietnam and Cambodia, rice production is doubling, but significant reduction of yield is found in some local parts of India like Uttar Pradesh, Maharashtra and Tamilnadu and there is no significant change in per capita rice harvest in Pakistan, Nepal, Malaysia and South Korea. Rice provides approximately 30% and 27% of the dietary energy in India and China, respectively [4]. Due to abiotic stresses like drought, submergence tolerance and salinity, production of rice is decreased, therefore, the production of abiotic stress-tolerant rice cultivars is the main priority [6]. Since conventional breeders have been trying to improve the quality and quantity of rice for many years, but they have not been able to solve these issues. With the help of biotechnological techniques the quality and quantity of rice can be improved. Many techniques of biotechnology are directed towards the improvement of conventional plant breeding processes, such as introduction of novel genes by genetic transformation, protoplast fusion for the production of male-sterile lines, production of haploids for attaining rapid homozygosity and somaclonal variation for introducing trait variability, plastid engineering [7-9]. For successful carrying out genetic transformation in rice, establishment of efficient plant regeneration in vitro is a pre-requisite [10-12]. Till now, different protocols have been developed to initiate callus from explants, such as mature embryos [13-18], immature embryos [19,20-22], mature seeds [22-25], root segments [15,26], coleoptile [27,28] and leaf bases [9]. Genetic engineering is strongly dependent on genotype and availability of an efficient in vitro plant regeneration method. In general, because of poor regeneration abilities, cultivars are recalcitrant to various biotechnological advances [29]. Identification and screening of useful cultivars for embryogenic callus formation and subsequent plant regeneration in vitro are the key steps in rice genetic improvement program by using biotechnological application [30]. Genotype and nutrient media are two of the most important factors which affect callus induction and subsequent plant regeneration. Keeping in view the above facts, an attempt was made to develop an easy, rapid and highly efficient regeneration protocol through high frequency embryogenic callus induction using mature seeds as explants and found its successful applicability to japonica rice (cv. Kitaake). The cultivar Kitaake was selected for standardization of various factors critical for enhanced transformation and regeneration as it has a very short life cycle (9 weeks) and is widely used in genetic transformation [31]. Kitaake can be efficiently transformed by A. tumefaciens-mediated T-DNA approach. Jung et al. [32] have generated several thousand T-DNA insertional mutants and overexpressed or silenced several hundred genes in Kitaake. | |
| In the present study, different components of the regeneration medium viz., growth hormones and gelling agents were standardized. The modified regeneration medium triggered production of a large number of shoots from a small number of calli and also promoted their fast growth, and hence has an edge over the existing protocols where the regeneration step requires maximum time. Using this protocol, significantly high regeneration frequency (up to 82%) was achieved in the tested cultivar Kitaake. | |
| This speedy, yet less labour intensive protocol overcomes major limitations associated with genetic manipulation in rice. Moreover, our protocol uses mature seeds as explants, which can easily be obtained in large quantity throughout the year and kept viable for a long time. Such an easy, efficient and generalized large protocol has the potential to be used for crop improvement and gene function studies on the model monocot plant rice. | |
| Materials and Methods | |
| Plant material | |
| The experiment was conducted on japonica rice cv. Kitaake, the seeds of which were procured from the Department of Biotechnology, National Institute of Agrobiological Resources, Japan. The research work was carried out at the Plant Tissue Culture and Genetic Transformation Laboratories, G.S. Khush Labs, School of Agricultural Biotechnology, Punjab Agricultural Univ ersity, Ludhiana, India during 2010-2013. | |
| Methodology | |
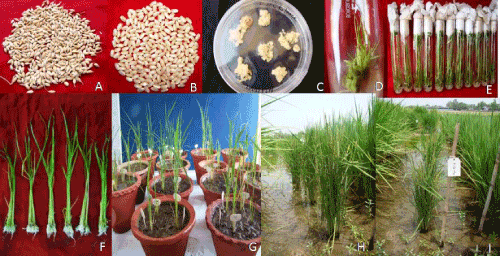
| Mature dry seeds of Kitaake (Figure 1A) were used and in vitro regeneration protocol was standardized following somatic embryogenesis mode. For this purpose, healthy dehusked seeds of Kitaake (Figure 1B) were surface sterilized with 70% ethanol (v/v) for 1 min, followed by 10-15 mins sterilization in mercuric chloride (0.1%). Seeds were then washed 5-6 times with sterile distilled water and dried on autoclaved Whatman paper (3mm) for 3-5 min. For callus induction, eight to ten seeds were inoculated per petriplate on callus induction medium (CIM) and incubated at 25 + 1°C in dark. | |
| Tissue culture media | |
| Different media components viz., carbohydrate source, agar concentration and combination of phytagel, agar and hormones were optimized for rice callus induction and regeneration. Sucrose or maltose at a concentration of 3%, 4% or 5% was used as carbohydrate source in the callus induction and regeneration media. The media were solidified with 0.8%, 1% or 1.2% (w/v) agar. The callus induction and regeneration media used were supplemented with proline (600 mg/l). The callus induction media was also supplemented with 2,4-D ( 3.0 mg/l) and BAP(0.5 mg/l). The regeneration medium comprised of NAA (0.2 mg/l) and BAP (3.0 mg/l). After evaluating each of the agents mentioned above singly, the combined effect of these ingredients was also evaluated in a series of experiments and data were taken on days to callus formation, callus induction frequency (%), efficiency of embryogenic calli formation efficiency (%), days to green bud formation and regeneration frequency (%). | |
| Non embryogenic calli were discarded and only embryogenic calli were selected. The embryogenic calli were cut into approximately 3 equal pieces and subcultured again onto fresh CIM and kept in dark. Calls induction frequency (%) was calculated as follows: | |
 |
|
| The frequency of developing embryogenic calli was calculated as follows: | |
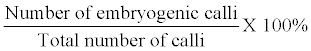 |
|
| After a week of subculture, pieces of callus (2-4 mm in diameter) were placed in a test tube containing regeneration medium. Regeneration frequency (%) was calculated as follows: | |
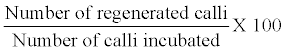 |
|
| For the development of roots, the regenerated shoots were shifted to jam jars containing rooting medium (comprising half strength of MS salts (½ MS), 20 g/l sucrose and 5 g/l agar, pH 5.8) and maintained at 25 + 10°C in light for 10 days. | |
| Statistical analysis | |
| Statistical analysis was performed by Completely Randomized Design (CRD) with the help of CPCS1 (a computer program package for the analysis of commonly used experimental design) [33]. All the data were subjected to analysis of variance (ANOVA). CD values at 5% level of significance were calculated to compare mean values and interpretations were done accordingly. | |
| Results and Discussion | |
| For conducting genetic transformation of any crop, a rapid and robust tissue culture system is a prerequisite. More than 5 month old calli start losing their regeneration capacity, so calli age is an important factor that influence callus regeneration. Thus, selecting the most suitable medium to improve the quality of calli is an important step towards callus regeneration. Regeneration frequency also depends on genotype and its interaction with culture conditions [34]. Influence of genotype on plant regeneration has been observed in several rice cultivars belonging to japonica, indica and japonica-indica hybrids [15]. Some of these showed high capacity for plant regeneration while others did not. Abe and Futsuhara [16] reported that callus formation from mature seeds, and subsequent regeneration in 60 different japonica, indica and javanica cultivars indicated both intra and inter-varietal differences. Genetic markers have been used to find genes involved in the induction of embryogenic calli [35-37], but these genes have not yet been isolated [34]. In addition to genotype, various factors including physiological and developmental status of the explant, composition and concentration of the basal salts, organic components and plant growth regulators in the culture medium are known to influence callus induction and plant regeneration ability in rice [38]. The first success in regeneration of plants from callus, derived from mature seeds of rice, was obtained by Nishi et al [39]. Numerous explants have since been used by different workers for callus induction and plant regeneration like leaf sheath [40], anthers or pollen [41,42], immature embryos [43], immature panicles [44,45], roots [46], young inflorescences [47], leaf bases [48] and mature embryos [34]. Among these explants, immature embryos and mature seeds are most commonly used for callus induction and subsequent studies. | |
| The frequency of plant regeneration depends upon the type of callus and various reports have described the nature and properties of highly regenerable calli, also termed as embryogenic calli. Some of the factors that affect plant regeneration efficiency from callus are the concentrations of gelling agents, osmoticum and different combinations of plant growth regulators [49]. Kavi-Kishor [50] reported that osmolarity of both growth and regeneration media was important for obtaining highly regenerable rice callus and retaining its regeneration potential. It has been observed that low water content of callus cultured on a medium containing mannitol and a high concentration of agar were key factors for efficient regeneration of plants from callus [51]. | |
| Therefore, an effort was made to obtain high frequency of regeneration using mature seed derived embryogenic callus in case of japonica rice cv Kitaake. The results reported here suggested that callus induction and regeneration in Kitaake were influenced by the type and concentration of carbon source. | |
| Effect of different concentrations of sucrose and maltose was studied on different events like days to callus formation, callus induction, embryogenic calli formation, days to green bud formation and regeneration frequency in cv. Kitaake, and significant differences among different events were found. (Table 1) Supplementation of culture medium with 4% maltose showed best results for days to callus formation (~9 days), per cent callus induction (90.33%), embryogenic callus formation (57.66%) and shoot regeneration (82.66 %). For days to green bud formation, 5% maltose showed the best result however, the regeneration percentage was lesser than 4% maltose. Upon comparing 4% maltose with the other sugar concentrations it was found to produce more significant results in respect to all the events mentioned in the Table 1 except days to green bud formation. The highest amount of callus induction (90.33 %) and regeneration (82.66%) was achieved with 4% maltose. Media containing 3% maltose yielded 61.66 % callus induction and 31.66% regeneration. The presence of maltose (4%) instead of sucrose in the media also lowered the number of days to callus formation in Kitaake. | |
| Previous reports have also found maltose to be a better carbon source for regeneration [52-54]. According to Lentini et al. [55], maltose has been described as an agent that may regulate osmotic potential of cellular environment of callus. Since sucrose promotes in vitro production of ethylene in excised tissues causing the browning of callus, the substitution of maltose for sucrose may help to protect the calli by reducing the production of ethylene. Similar results that maltose is better than sucrose as the carbohydrate source in both the subculture and differentiation media were obtained by Lin et al. [56]. It is likely that the two sugars exhibit different bioavailability to rice under culture condition. | |
| Another factor enhancing the callus induction and regeneration in Kitaake is the gelling agent. Agar concentration in the callus induction and regeneration media was also important in enhancing the frequencies of callus induction, embryogenic calli and regeneration. Of different levels of agar (0.8%, 1% and 1.2%) evaluated, the medium containing 1% agar was the most favourable and 0.8% proved to be least favourable as shown in Table 2. While increasing the concentration of agar it increased the callus induction and also affected the regeneration as well. Use of 1% agar led to formation of 68.66% callus induction and subsequent regeneration of 46.77%. The use of 1.2% agar led to 57.18% callus induction and 38.04% regeneration. | |
| Use of phytagel in combination with agar leads to increase in embryogenic calli frequency and regeneration frequency (71.83%). Effect of different concentrations of agar (0.8, 1.0 and 1.2%), phytagel (0.3%) and combination of agar and phytagel (0.8% agar + 0.2% phytagel) on percent callus was studied. Among these phytagel (0.3%) showed statistically higher callus induction frequency (72.73%) as compared to other gelling agents. The callus induction frequency also increased upon increasing the concentration of agar from 0.8 to 1.2 %, but to a lesser extent as compared to phytagel (0.3%; Table 3). For regeneration, 1.0% agar showed statistically better shoot induction (45.56%) as compared to other agar concentrations. Upon increasing the concentration of agar from 0.8 to 1.0 %, the regeneration percentage increased, but decreased at 1.2%. In combination agar and phytagel (0.8% agar + 0.2% phytagel) showed significantly better regeneration (71.83 %) in Kitaake amongst all treatments (Table 3). Similar results that agar concentration influences callus induction and regeneration has been reported by Zaidi et al. [52]. According to Lee et al. [57], agar influences shoot regeneration by regulating the humidity of in vitro culture conditions. Agar contains agropectins and some other organic impurities that might have inhibitory effect on explant growth and callus proliferation. Phytagel has been reported to be free from such impurities [58]. Gelrite was found to enhance callus induction and in vitro regeneration in Swarna (89.9%) and Mahsuri (93.4%) rice varieties [52]. Although use of agar as gelling agent to promote in vitro regeneration of rice has been reported [59-61,57], but Saharan et al. [62] and Mensens et al. [63] have reported higher regeneration frequency in indica rice varieties on media solidified with gelrite. Lin and Zhang [56] also have reported that 0.3% phytagel give higher callus induction. The addition of cytokinin (BAP) and auxin (NAA) to regeneration medium enhanced the frequency of shoot regeneration from callus cultures [24,25]. Use of casein hydrolysate was found to be beneficial for generation of embryogenic calli in japonica rice [62,64,65]. Use of proline in the medium has also been reported to be effective for the initiation and maintenance of embryogenic calli [61,66]. | |
| The combined action of all the supplements led to higher efficiency of callus induction, formation of embryogenic calli and regeneration. For the callus induction, it is proposed that the best media among all the tested combinations is MS media supplemented with 40 g/l maltose, 0.3 g/l casein hydrolysate, 0.6 g/l proline, 3.0 mg/l 2,4-D, 0.25 mg/l BAP gelled with 3.0 g/l phytagel (Figure 1C). MS media supplemented with 30 g/l sucrose, 3 mg/l BAP, 0.2 mg/l NAA gelled with 8 g agar/l and 2 g/l phytagel (Figure 1D) is proposed and for rooting, half strength MS supplemented with 20 g/l sucrose and 5g/l agar was found to be highly responsive (Figures 1E and F). The plants were transferred to the pots in the green house (Figure 1G) after hardening and were transplanted in the field after 10 days (Figure 1H and I). | |
| Thus in the present study, it was observed that the callus induction and regeneration was greatly affected by the carbon source and gelling agent. By using this protocol, a total of 49 transgenic lines of Kitaake using biolistic approach was developed. Thus, protocol reported here might help in crop improvement strategies. | |
| Acknowledgements | |
| The authors are thankful to Dr. Kuldeep Singh, Director, School of Agricultural Biotechnology, Punjab Agricultural University (PAU) for providing the necessary research facilities, and Dr. Darshan Singh Brar, Honorary Adjunct Professor, School of Agricultural Biotechnology, PAU for valuable suggestions during the entire work. We are also thankful to Department of Biotechnology, National Institute of Agrobiological Resources, Japan for providing rice seeds of Kitaake. Saroj Kumar Sah acknowledges Indian Council for Cultural Relations and Ministry of Foreign Affairs, New Delhi, India for providing fellowship during M.Sc. programme. | |
References |
|
|
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Basmati Rice
- Drought Tolerence
- Golden Rice
- Leaf Diseases
- Long Grain Rice
- Par Boiled Rice
- Raw Rice
- Rice
- Rice and Aquaculture
- Rice and Nutrition
- Rice Blast
- Rice Bran
- Rice Diseases
- Rice Economics
- Rice Genome
- Rice husk
- Rice production
- Rice research
- Rice Yield
- Sticky Rice
- Stress Resistant Rice
- Unpolished Rice
- White Rice
Recommended Journals
Article Tools
Article Usage
- Total views: 17813
- [From(publication date):
August-2014 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 12775
- PDF downloads : 5038
