Review Article Open Access
Heterogeneity of Parkinsonism in Familial Frontotemporal Dementia: Insights from Genetic Clues on the Pathogenesis
Chin-Hsien Lin*
Department of Neurology, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- *Corresponding Author:
- Chin-Hsien Lin
Department of Neurology
National Taiwan University Hospital
Taipei 100, Taiwan
Tel: +886-2-23123456
Fax: +886- 2-23418395
E-mail: chlin@ntu.edu.tw
Received date August 09, 2016; Accepted date August 13, 2016; Published date August 20, 2016
Citation: Lin CH (2016) Heterogeneity of Parkinsonism in Familial Frontotemporal Dementia: Insights from Genetic Clues on the Pathogenesis. J Alzheimers Dis Parkinsonism 6:257. doi:10.4172/2161-0460.1000257
Copyright: © 2016 Lin CH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Frontotemporal dementia (FTD) is a clinically, genetically and pathologically heterogeneous disorder characterized by personality changes, language impairment, and deficits of executive functions, often overlapped with Parkinsonism syndrome and motor neuron disorder. Owing to the advancement in the field of molecular genetics in the familiar forms of FTD, several recent breakthroughs have provided notable insights into the pathogenesis of this neurodegenerative disorder. Nevertheless, Parkinsonism in FTD has received relatively less attention. Given that Parkinsonism feature is found in approximately 15-20% of patients with FTD, there is a need to understand Parkinsonism in FTD in order to obtain a better landscape of this disease spectrum. Recent advances have revealed mechanistic links between Parkinsonism and frontotemporal dementia, as well as between other neurodegenerative diseases, such as amyotrophic lateral sclerosis. Here, we summarize recent genetic advances and molecular findings in familial FTD with Parkinsonism and delineate the mechanisms underlying this overlapped neurodegenerative disorder.
Keywords
Frontotemporal dementia; Parkinsonism; Gene; Pathogenesis
Introduction
Neurodegenerative disorders are a major health problem with a prevalence of 135 million people affected worldwide, that is estimated to double by 2050, based on World Health Organization predictions [1]. Specific protein inclusions and depositions causing neuronal dysfunction define most neurodegenerative diseases at the pathological level, and the clinical picture is generally defined by the affected brain regions. In the past 30 years, the genetic causes of inherited forms of neurodegenerative disorders have been identified and the molecular components of the inclusions have been discovered. In most cases, pathogenic mutations cause disease through an overproduction of the amyloidogenic protein or an increase in the protein’s propensity to aggregate, with resulting neurodegeneration. A major class of neurodegenerative diseases, collectively known as tauopathies, is characterized by intracellular inclusions consisting of an abnormally modified microtubule-binding tau protein. Primary tauopathies are a major class of frontotemporal lobar degeneration (FTLD) neuropathology and can present clinically with several forms of frontotemporal dementia [2] and parkinsonian syndromes (i.e., Progressive Supranuclear Palsy Syndrome, PSP; Corticobasal Syndrome, CBS) [3]. Uncovering these neurodegenerative disease-causing genes and understanding the molecular functions of encoded proteins will shed light on the mechanisms that underlie the sporadic forms of disease.
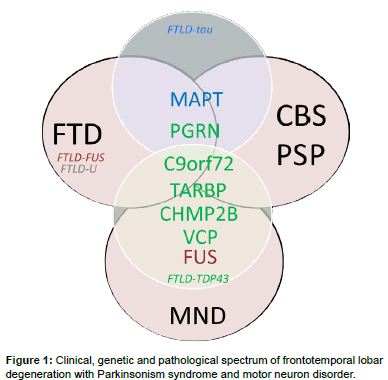
Frontotemporal dementia denotes a clinical disorder involving neurodegeneration of the frontal and temporal cortices, leading to behavioral changes and language disturbances in the presence of relatively intact memory and visuospatial functions. FTD accounts for 5-15% of all cases of dementia and is the second most common cause of dementia in the age group of less than 65 [4]. FTD is clinically divided into a behavioral variant FTD (bvFTD) and primary progressive aphasia [5]; the latter can be further subdivided into semantic dementia [6], progressive non-fluent aphasia and logopenic progressive aphasia [5]. Compared with the most common Alzheimer’s dementia, FTD has an increased association with parkinsonism and, in some cases, with motor neuron disease, especially amyotrophic lateral sclerosis (ALS) [7]. Parkinsonism is usually present in patients with bv FTD, but is rarely seen in those with primary progressive aphasia [8]. Parkinsonism features may present before, during, or after the development of behavioral or language disturbances in patients with FTD. The extrapyramidal system symptoms present as bradykinesia, rigidity, and abnormal posture but rarely as tremor. This overlapped clinical spectrum of FTD with Parkinsonism, ranging from typical Parkinson’s disease to parkinsonism-plus syndromes, such as PSP and CBS, has increased the clinical variability of this neurodegenerative disorder (Figure 1) [9,10].
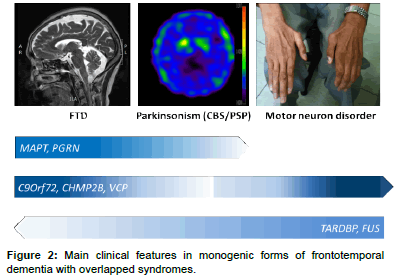
The heterogeneity of FTD is also reflected in post-mortem pathology findings. The subtypes of underlying pathological changes in patients with FTD are classified based on the pattern and constituents of major protein depositions in neurons and referred to as FTLDs [11]. The main category is FTLD with tau pathology (FTLD-tau), in which neurons and glial cells contain inclusions of hyperphosphorylated tau protein [12]. More than half of FTLD patients present with tau-negative but ubiquitin-positive inclusions, referred to as FTLD-ubiquitin (FTLD-U). In 80–95% of this group, inclusions consist of transactive response DNA-binding protein 43 kDa (TDP-43) [7], referred to as FTLD-TDP [13]. The remaining TDP-43–negative FTLD-U cases have inclusions of fused-in-sarcoma protein (FUS), referred to as FTLDFUS (Figure 2) [14]. However, the inclusion protein of a small number of FTLD-U patients remains unclear. Of note, the pathology findings for most patients with the logopenic progressive aphasia variant are Alzheimer’s disease pathology with β-amyloid accumulations [15]. One recent study even reported α-synuclein neuronal inclusions in patients with FTD [16].
Parkinsonism is found in approximately 15-20% of patients with FTD [6,17], but few studies have focused on the clinical and genetic aspects of parkinsonism in FTD. The clinical, genetic, and pathological overlap between FTD and parkinsonism syndrome often leads to extensive phenotypic variability, even among different members of the same family who carry an identical disease mutation. For this reason, we aim to highlight recent genetic advances in familial FTD with parkinsonism and delineate the mechanisms underlying the extreme phenotypic heterogeneity that characterizes this disease.
Genetics of FTD with parkinsonism
About 40% of FTD patients have at least one relative with dementia, and in 10-15%, family history is consistent with an autosomal dominant inheritance [18]. These families are characterized as having at least three affected family members in two generations specifically with FTD, motor neuron disease, or one of the parkinsonism syndromes, i.e., PSP or CBS. Among the different subtypes of FTD, bv FTD is the most prominent with family history (~ 30-50%) and combined with features of parkinsonism, while semantic dementia appears to be the least hereditary subtype of FTD (<20%) [19]. Currently, the best understood forms of autosomal dominant FTD associated with parkinsonism are those linked to chromosome 17, with mutations in the microtubuleassociated protein tau (MAPT) and progranulin (PGRN) genes, and to chromosome 9, with mutations in the chromosome 9 open reading frame 72 (C9ORF72) gene [20]. Since 1998, when the MAPT gene was first linked to familial FTD with parkinsonism [21], several other genes have been identified, including chromatin-modifying protein 2B (CHMP2B), valosin-containing protein (VCP), transactive DNAbinding protein (TARDBP), and FUS (Table 1) [7,20,22-24].
More than 50 mutations in the MAPT gene have been identified in families with FTD and parkinsonism linked to chromosome 17q (FTDP-17), with accumulation of hyperphosphorylated tau protein in neurons and/or glial cells [25]. The frequency of MAPT gene mutations is highly variable, ranging from 0-3% in sporadic FTD to 9-21% in familial cases (Table 1). The mode of inheritance is autosomal dominant. For FTDP-17 patients with parkinsonism as the initial presentation, most missense mutations are in exon 10 of the MAPT gene [10,26,27]. The penetrance of MAPT mutation carriers is more than 95% and the clinical presentation is variable and can include an initially good response to levodopa therapy, mimicking Parkinson’s disease [27]. The age of onset varies by specific mutation and within the same family carrying the same mutation, but in general, disease onset is between ages 45 and 60 (Table 1). The parkinsonism features of MAPT mutation carriers include relatively symmetric bradykinesia, axial and limb rigidity, and postural instability but rarely resting tremor. Although patients may initially show some responses to levodopa, it is not sustained [28]. There is no phenotype–genotype correlation in MAPT mutation carriers; however, it has been previously shown that the dementia-dominant group correlates with the H1/H2 genotype of MAPT gene while the parkinsonism-dominant group correlates with the H1/H1 genotype [29].
| Genes | Inheritance | Mutation frequency | Penetrance | Onset age | Associated symptoms |
|---|---|---|---|---|---|
| MAPT | AD | 9-21% in familial FTD,0-3% in sporadic FTD | >95% | 45-60 Y/O | bvFTD, PPA, PSP, CBS, seizures, AD |
| PGRN | AD | 4-23% in familial FTD,2-8% in sporadic FTD | 50% at 60 Y/O,90% at 70 Y/O | 35-83 Y/O | bvFTD, AD, PD, PSP, CBS |
| C9Orf72 | AD | 18-30% in FTD-MND,12-18% in familial FTD,2-4% in sporadic FTD | Incomplete | 33-75 Y/O | Often combined with MND (or ALS) |
| CHMP2B | AD | Rare in FTD | N.A. | N.A. | Often combined with MND (or ALS) |
| VCP | AD | Extremely rare in isolated FTD >50% in IBMPFD |
100% for IBMPFD, 30% for FTD |
40-55Y/O | |
| TARDBP | AD | 2-3% in familial FTD-MND, rare in isolated FTD |
N.A. | N.A. | Often combined with MND (or ALS) |
| FUS | AD | <3% in familial FTD-MND | N.A. | N.A. | Often combined with MND (or ALS) |
Table 1: Summary of genes associated with Parkinsonism with frontotemporal dementia.
Alternative splicing of exons 2, 3 and 10 of the MAPT gene gives rise to six isoforms of tau protein: three isoforms containing three amino acid repeats [16] and three isoforms with four repeats (4R). Tauopathies are classified by the predominance of tau isoforms found in cytoplasmic inclusions: those with inclusions predominantly composed of tau with 3R tau, those with predominantly 4R tau, or those with an equal ratio of 3R:4R tau [30]. The axonal protein tau is involved in the establishment and maintenance of neuronal morphogenesis through the activity of binding microtubules, which are regulated by the levels and sites of phosphorylation [31]. Tauopathies are believed to be caused by aberrant hyperphosphorylation of tau, leading to the assembly of variable neurotoxic tau aggregates and deposition of insoluble tau fibers in both neurons and glia (Figure 3) [32]. MAPT pathogenic mutations are thought to cause disease by either 1) inhibiting the normal microtubulebinding function of tau, 2) promoting tau protein aggregation, or 3) affecting the splicing of exon 10 to result in imbalances between 3R/4R tau isoforms [33]. As a result, specific neuropathological findings (e.g., tau isoform predominance, inclusion morphology/ultrastructure) vary for each specific mutation but universally include neuronal and glial tau inclusions together with neurodegeneration throughout the CNS, with a particular propensity for frontal and temporal neocortex, limbic structures, and basal ganglia [34].
Among parkinsonism syndromes caused by MAPT mutations, PSP and CBS are 4R tauopathies, which commonly combine the cognitive and behavioral features of FTD. PSP is pathologically characterized by intra-neuronal globose tau inclusions in the brainstem, subthalamic nucleus, and dentate nucleus of the cerebellum and non-neuronal inclusions, i.e., glial “tufted astrocytes” and oligodendrocytic “coiled bodies” in the white matter of the neocortex [35]. The most common clinical presentation of PSP is an atypical Parkinsonian syndrome characterized by prominent axial rigidity, early falling, oculomotor palsy and poor response to dopaminergic therapy. Overlapping syndromes of PSP and FTD, especially bvFTD, are largely due to MAPT mutations, especially p.delN296, p.S305S and p.N279K mutations [26,27]. However, no clear association has been identified among the presence, onset, and severity of cognitive decline in PSP patients with MAPT mutations [10]. In addition to familial cases, polymorphisms in the MAPT gene have been linked to increased risk for sporadic PSP. A recent genome-wide association study found a link between increased risk for sporadic PSP and a haplotype of an inverted sequence of polymorphisms in linkage disequilibrium, i.e., the H1 haplotype, and several variants in MAPT [36]. These findings support the role of MAPT in both familial and sporadic cases of PSP overlapped with FTD.
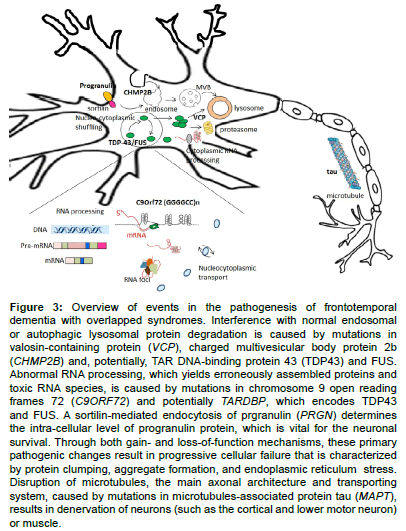
Figure 3 : Overview of events in the pathogenesis of frontotemporal dementia with overlapped syndromes. Interference with normal endosomal or autophagic lysosomal protein degradation is caused by mutations in valosin-containing protein (VCP), charged multivesicular body protein 2b (CHMP2B) and, potentially, TAR DNA-binding protein 43 (TDP43) and FUS. Abnormal RNA processing, which yields erroneously assembled proteins and toxic RNA species, is caused by mutations in chromosome 9 open reading frames 72 (C9ORF72) and potentially TARDBP, which encodes TDP43 and FUS. A sortilin-mediated endocytosis of prgranulin (PRGN) determines the intra-cellular level of progranulin protein, which is vital for the neuronal survival. Through both gain- and loss of function mechanisms, these primary pathogenic changes result in progressive cellular failure that is characterized by protein clumping, aggregate formation, and endoplasmic reticulum stress. Disruption of microtubules, the main axonal architecture and transporting system, caused by mutations in microtubules-associated protein tau (MAPT), results in denervation of neurons (such as the cortical and lower motor neuron) or muscle.
CBS, another 4R tauopathy presenting as a parkinsonism syndrome, has a considerable clinicopathological overlap with PSP. CBS is associated with a large burden of tau-positive diffuse “astrocytic plaques” and “ballooned neurons” in the grey matter of limbic and neocortical structures and with tau-positive “coiled bodies” and astrocytic tau inclusions in white matter [35]. The basal ganglia and brainstem contain hyperphosphorylated tau inclusions as well, and sometimes the morphology is difficult to distinguish from PSP [35]. One recent study evaluating parkinsonism in patients with FTD showed that 22% (70/319) of patients demonstrated parkinsonism features [17]. Specifically, among 70 FTD patients with parkinsonism, 15 were clinically diagnosed as CBS and 7 initially diagnosed as PSP. A recent study also has shown that CBS could be an initial presentation of mutations in exon 10 of the MAPT gene [37]. These findings highlight the phenotypic heterogeneity and clinical overlap of syndromes caused by MAPT mutations.
PGRN
Since the discovery in 2006 of PGRN mutations in FTD patients with ubiquitin-positive, tau-negative inclusions, [38,39] more than 70 pathological mutations have been reported. The mode of inheritance is autosomal dominant. Progranulin protein is a secreted growth factor with unclear function that is highly expressed in neurons of the cerebral cortex and hippocampus and in the cerebellum [40]. The post-mortem findings of patients with PGRN mutations are ubiquitin-positive inclusions that do not contain progranulin [38]. These pathology observations are consistent with the fact that most PGRN mutations are nonsense, frameshift, or splice-site mutations producing mutant mRNAs, which are degraded by nonsense-mediated decay that prevents expression of truncated proteins [38,39]. Therefore, neurodegeneration results from a partial loss of progranulin function rather than from abnormal toxic gain of function and aggregation of the mutant protein.
Mutations in the PGRN account for up to approximately 4-23% of familial and 2-8% of sporadic patients with FTD (Table 1). PGRN mutations show an age-dependent penetrance, with 50% at age 60 and 90% at age 70 [2]. The clinical presentation in patients with PGRN mutations is highly variable. Age at symptom onset varies significantly, ranging from 35 to 83 years, with an average of 60 years. The most common clinical presentation in patients carrying PGRN mutations is bv FTD, followed by progressive non-fluent aphasia and CBS [2,41]. In a study recruiting 24 families including 32 symptomatic PGRN mutation carriers, parkinsonism features were reported in about 40% of patients, and 3.3% were clinically diagnosed as CBS [2]. In addition, 25% of patients with PGRN mutations have hallucinations and delusions as the predominant symptoms, leading to a misdiagnosis of dementia with Lewy bodies [42]. Furthermore, an Alzheimer’s dementia-like phenotype with episodic memory deficit, which occurs in 10-30% of patients with FTD, has also been reported as an initial clinical feature of the c.154delA mutation of PGRN [43].
Because the penetrance is not 100% and all reported mutations cause a progranulin haploinsufficiency, other factors may influence progranulin expression in patients carrying PGRN mutations. Genetic variants in TMEM106B, [44] a gene encoding the still uncharacterized transmembrane protein 106B, are reported to delay the onset of disease in PGRN mutation carriers by increasing levels of progranulin [45,46]. The expression level of progranulin is also regulated by factors that mediate progranulin endocytosis, including microRNAs (e.g. miR-29b and miR-107) and sortilin, a receptor for neurotrophic factors (Figure 3) [47-50]. However, no obvious modulatory effects have been observed of the MAPT haplotype or ApoE genotype on the clinical presentation of PGRN mutation carriers [51,52]. Recently, a complete progranulin deficiency due to a homozygous PGRN mutation was found to cause the rare lysosomal storage disease neuronal ceroid lipofuscinosis, suggesting that lysosomal storage disorders and PGRN-associated FTD may share a common mechanism [53].
C9ORF72
In 2011, two independent groups identified a heterozygous expanded hexanucleotide repeat (GGGGCC, G4C2), located between the non-coding exons 1a and 1b of the C9orf72 gene on chromosome 9, as a cause of familial FTD with or without ALS [7,20]. The inheritance pattern is autosomal dominant, although the penetrance is not complete (Table 1). The number of repeat units of the C9orf72 expansion in healthy controls is 2-24, but the repeat numbers can range from several hundred to thousands of copies [7,20]. So far, there is no clear evidence for genetic anticipation, although some studies have identified earlier disease onset in subsequent generations [54].
C9orf72 repeat expansions are about as common as mutations in the MAPT gene and PGRN gene in patients with familial FTD (Table 1). The clinical phenotype is highly heterogeneous and variable even within families affected by the same mutation. The most common clinical presentations are bv FTD, followed by motor neuron disorder, especially ALS (Figure 2) [55]. The mean age at onset is around 55 years (33-75 years). Previous reports have shown that C9orf72 repeat expansions contribute to 18-30% of cases of familial FTD with motor neuron disorders and to 12-18% of those involving familial FTD (Table 1). Parkinsonism is reported in about 30-48% of patients with C9ORF72 expansions [56,57]. Most patients present with levodopa-unresponsive akinetic–rigidity features, and tremors are usually not associated [58- 60]. During the course of the disease, most patients eventually develop some abnormalities of behavior, language, and cognitive changes. Compared to MAPT and PGRN mutation carriers, cases involving C9Orf72 mutations rarely present as CBS or PSP syndromes [61].
The function of the C9orf72 protein is still largely unknown, and the pathogenesis underlying the C9orf72 mutation remains unclear. Both haplo-insufficiency through loss of gene expression [62,63] and gain-of-function mechanisms with secondary RNA toxicity caused by sequestration of RNA-binding proteins have been reported [64]. The pathological hallmarks of patients carrying abnormal GGGGCC expansions of C9orf72 are TDP-43 immunoreactive inclusions, which have been observed in many cerebral structures, including the cerebral cortex, hippocampus, basal ganglia, substantia nigra and lower motor neurons of the brainstem and spinal cord [65]. Recent studies have shown that these TDP-43–positive inclusions also consist of dipeptide repeat (DPR) proteins, which are the result of the bidirectional translation of the non-coding GGGGCC repeats through a mechanism of non–ATG-initiated translation, called RAN translation (Figure 3) [66,67]. These observations suggest that DPR aggregation due to RAN translation could be the initial pathological event that possibly triggers TDP-43 accumulation in neurons [68]. Despite recent findings implicating both loss-of-function and gain-of-function mechanisms with new insights into the roles of DPR proteins and RAN translation, the relative contribution of these mechanisms and the molecular pathways that lead to neurodegeneration are yet to be elucidated.
CHMP2B
The CHMP2B gene, located at chromosome 3p11.2, was identified in 2005 in a large Danish family with an autosomal FTD [23]. The mutation occurs in a splice acceptor site, resulting in the production of two variants of C-terminally truncated CHMP2B proteins. A subsequent study in a Belgian FTD cohort identified a familial FTD patient with a distinct truncation mutation, CHMP-2BQ165X, that leads to the loss of the final 49 amino acids, providing further evidence that C-terminal truncations of CHMP2B lead to FTD [69]. Since then, several studies have found missense mutations in the CHMP2B gene that contribute to some families with familial FTD with and without ALS (less than 1%) [70,71]. The presence of CHMP2B gene mutations in a small number of families has been reported to be accompanied by parkinsonism features, dystonia, and myoclonus [72]. However, the mutation frequency of the CHMP2B gene in patients with FTD and parkinsonism is low (Figure 1). Further studies enrolling more patients are needed to elucidate the role of this gene in parkinsonism syndrome.
The protein encoded by CHMP2B is charged multivesicular body (MVB) protein 2B, a subunit of the endosomal sorting complex required for transport (ESCRT)-III, which mediates the bending and fusion of cellular membranes [73]. This membrane manipulation by ESCRT complexes is essential for the maturation of endosomes, which acquire numerous intraluminal vesicles to form late endosomes, or MVBs [73]. MVBs ultimately fuse with lysosomes or autophagosomes to allow degradation of the endosomal content. Mutant CHMP2B protein affects the maturation of both endosomes and autophagosomes (Figure 3) [74,75]. Consistent with these observations, one recent study using transgenic mice with the FTD-causative mutant CHMP2B showed that the animals develop a lysosomal storage pathology characterized by large neuronal lysosomal and late-endosomal aggregates, findings also observed on neuropathological assessment in patients carrying CHMP2B mutations [76]. Of note, PGRN, another FTD-causative gene, and TMEM106B, a potential genetic risk factor for FTD, also play a role in regulating endolysosomal and lysosomal functions [45,46,53]. In addition, one recent study showed that the endogenous function of CHMP2B is required for maintaining the stability and complexity of neuronal dendritic spines and trees [77]; the process of dendritic arborization depends on an intact endosomal trafficking system [78]. These findings imply that impaired lysosomal degradation and endosomal trafficking dysfunction are key pathways in FTD associated with the CHMP2B mutation.
VCP
The VCP gene, located on chromosome 9p13.3, encodes valosincontaining protein. A variant has been identified in North American families with an autosomal dominant multisystem syndrome involving inclusion body myopathy, Paget’s disease of the bone, and frontotemporal dementia [79]. The frequency of VCP mutations in FTD is rare, accounting for less than 2% of cases [80]. The disease usually begins at the age of 40 with myopathic symptoms as the initial presentation in the majority of patients, followed by adult-onset Paget disease of bone [79]. FTD, especially bvFTD, ALS, and parkinsonism features, may usually develop later in the disease course. Rarely, VCP mutations have been found in patients with a dementia-only phenotype [80] and typical Parkinson’s disease with resting tremor and levodopa responsiveness [81].
Valosin-containing protein is one of the ATPases associated with various cellular activities and is involved in multiple cellular processes, including protein degradation via both proteasome and autophagy pathways, membrane fusion, transcriptional activation, apoptosis, and molecular chaperoning (Figure 3) [82]. However, the exact molecular mechanism remains elusive, as does the interaction with other FTDcausing disease proteins in the pathogenesis of FTD with other neurodegenerative systems, including muscles, motor neurons, and the extrapyramidal system.
TARDBP
Mutations in the TARDBP gene were initially described in 2008 in patients with ALS, in both familial and sporadic forms [83,84]. The most common phenotype of TARDBP mutations is ALS (Figure 2), which accounts for 2-3% of familial ALS patients (Table 1). Some TARDBP mutation carriers also uncommonly manifest FTD, especially bvFTD, and Parkinson’s disease with some levodopa responsiveness [85,86]. The inheritance mode is autosomal dominant with unclear penetrance.
The TARDBP-encoded protein, TDP-43, is a ubiquitously expressed and highly conserved ribonucleoprotein. TDP-43 is postulated to be involved in transcriptional activity, messenger RNA splicing, exon skipping, and microRNA regulation (Figure 2). Studies have found that in neurodegenerative disorders, TDP-43 can be redistributed from the nucleus to the cytoplasm and sequestered into inclusions that consist mainly of abnormally phosphorylated and C-terminally truncated TDP-43 fragments [87,88] .Because TDP-43 inclusions have also been observed in non–TARDBP-associated genetic forms of FTD, including those involving PGRN, VCP, and C9orf72 mutations, these findings suggest that dysregulation of TDP-43 might be a common downstream pathogenic pathway in these neurodegenerative disorders.
FUS
FUS, located on chromosome 16, was identified in 2009 in families with ALS and expands the genetic heterogeneity of FTD [89]. FUS mutations have also been identified in patients with FTD-motor neuron disease/ALS and are rare (less than 3%) genetic causes of familial cases of FTD and ALS (Table 1 and Figure 2) [90]. Case reports have described a pure FTD phenotype without motor neuron involvement in FUS mutation carriers [24,91]. Rare FUS mutation carriers present with parkinsonism features [24].
The FUS protein is the most characteristic pathological marker of the tau-negative and/or TDP-43–negative FTLD cases (Figure 1). The FUS protein belongs to the family of DNA/RNA-binding proteins, which are ubiquitously expressed, multifunctional binding proteins involved in various cellular processes, including cell proliferation, DNA repair, transcription regulation, and multiple levels of RNA and microRNA processing (Figure 3) [92]. TDP-43 is also an RNA processing protein, and both FUS and TARDBP dysfunction affect global cellular RNA regulation [87,88,92]. In addition, the recent discovery that expression of the mutant C9Orf72 gene may induce RNA foci that could sequester RNA binding proteins such as TDP-43 and FUS highlights a further possibly important mechanism of RNA dysfunction in this neurodegenerative spectrum [20]. Furthermore, TDP-43 and FUS could bind and regulate key aggrephagy-related genes whereas dysfunction of aggrephagy leads to cytoplasmic re-localization and aggregation of TDP-43 [93]. VCP protein, another FTD-causative gene product, is linked to aggrephagy and lysosomal degradation pathways, highlighting the interaction between RNA dysfunction and the autophagosome/lysosomal degradation pathway in the disease process.
Conclusion and Future Directions
Clinical, genetic, and pathological heterogeneity are characteristic features of FTD. The clinical phenotypes can include memory impairment, personality changes, and language impairment and are variably associated with parkinsonism features and motor neuron disorders. The neuropathology is also strikingly variable, encompassing FTLD-tau (mostly found in patients carrying MAPT mutations), FTLDTDP (observed in patients carrying PGRN, C9orf72, TARDBP, or VCP mutations), FTLD-FUS (mostly found in ALS patients carrying FUS mutations), and FTLD-U. In the past decade, considerable progress has been made toward unraveling the genetic causes of FTD with parkinsonism or motor neuron disorders. The most common causative genes for FTD with parkinsonism syndrome are MAPT, PGRN, and C9ORF72, but they are associated with a wide range of phenotypes. Mutations in other genes, including TARDBP, VCP, CHMP2B and FUS, although rare genetic causes of familial FTD with overlapping neurodegenerative features, could explain a small number of the remaining familial cases. Numerous attempts have been made to identify an in vivo biomarker that can accurately predict the underlying neuropathology and causative variant, but a clear phenotype–genotype pathology association has not yet been established. Alterations in the protein degradation system and RNA processing pathway are pivotal in familial forms of FTD with parkinsonism and/or motor neuron disorders, but the mechanism of neuronal specificity in the frontal–temporal cortex, basal ganglia, and spinal motor neurons remains elusive.
As parkinsonism is a common clinical feature associated with FTD, our current review provides updated information and a better understanding of this disease spectrum. Considering the vast clinical, genetic, and pathological heterogeneity associated with this disease, the development of accurate biomarkers is mandatory for guiding genetic diagnosis and for defining distinctive mechanism-based therapeutic approaches in future clinical trials.
References
- Gammon K (2014) Neurodegenerative disease: Brain windfall. Nature 515: 299-300.
- Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, et al. (2008) Phenotype variability in progranulin mutation carriers: A clinical, neuropsychological, imaging and genetic study. Brain 131: 732-746.
- Kovacs GG (2015) Invited review: Neuropathology of tauopathies: Principles and practice. NeuropatholApplNeurobiol 41: 3-23.
- Bird T, Knopman D, VanSwieten J, Rosso S, Feldman H, et al. (2003) Epidemiology and genetics of frontotemporal dementia/Pick's disease. Ann Neurol 54 Suppl 5: S29-31.
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, et al. (2011) Classification of primary progressive aphasia and its variants. Neurology 76: 1006-1014.
- Seelaar H, Kamphorst W, Rosso SM, Azmani A, Masdjedi R, et al. (2008) Distinct genetic forms of frontotemporal dementia. Neurology 71: 1220-1226.
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, et al. (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72: 257-268.
- Fujioka S, Wszolek ZK (2011) Clinical aspects of familial forms of frontotemporal dementia associated with Parkinsonism. J MolNeurosci 45: 359-365.
- Pan XD, Chen XC (2013) Clinic, neuropathology and molecular genetics of frontotemporal dementia: A mini-review. TranslNeurodegener 2: 8.
- Siuda J, Fujioka S, Wszolek ZK (2014) Parkinsonian syndrome in familial frontotemporal dementia. Parkinsonism RelatDisord 20: 957-964.
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, et al. (2001) Clinical and pathological diagnosis of frontotemporal dementia: Report of the work group on frontotemporal dementia and Pick's disease. Arch Neurol 58: 1803-1809.
- Kersaitis C, Halliday GM, Kril JJ (2004) Regional and cellular pathology in frontotemporal dementia: Relationship to stage of disease in cases with and without Pick bodies. ActaNeuropathol 108: 515-523.
- Grossman M, Wood EM, Moore P, Neumann M, Kwong L, et al. (2007) TDP-43 pathologic lesions and clinical phenotype in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Arch Neurol 64: 1449-1454.
- Mackenzie IR, Munoz DG, Kusaka H, Yokota O, Ishihara K, et al. (2011) Distinct pathological subtypes of FTLD-FUS. ActaNeuropathol 121: 207-218.
- Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, et al. (2011) Subtypes of progressive aphasia: Application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain 134: 3030-3043.
- Aoki N, Boyer PJ, Lund C, Lin WL, Koga S, et al. (2015) Atypical multiple system atrophy is a new subtype of frontotemporal lobar degeneration: Frontotemporallobar degeneration associated with alpha-synuclein. ActaNeuropathol 130: 93-105.
- Kertesz A, McMonagle P, Jesso S (2011) Extrapyramidal syndromes in frontotemporal degeneration. J MolNeurosci 45: 336-342.
- Rosso SM, DonkerKaat L, Baks T, Joosse M, de Koning I, et al. (2003) Frontotemporal dementia in the Netherlands: Patient characteristics and prevalence estimates from a population-based study. Brain 126: 2016-2022.
- Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, et al. (2005) Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 65: 1817-1819.
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, et al. (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72: 245-256.
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, et al. (1998) Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393: 702-705.
- Goldman JS, Rademakers R, Huey ED, Boxer AL, Mayeux R, et al. (2011) An algorithm for genetic testing of frontotemporal lobar degeneration. Neurology 76: 475-483.
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, et al. (2005) Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 37: 806-808.
- Van Langenhove T, van der Zee J, Sleegers K, Engelborghs S, Vandenberghe R, et al. (2010) Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology 74: 366-371.
- Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12: 609-622.
- Wszolek ZK, Tsuboi Y, Ghetti B, Pickering-Brown S, Baba Y, et al. (2006) Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Orphanet J Rare Dis 1: 30.
- Reed LA, Wszolek ZK, Hutton M (2001) Phenotypic correlations in FTDP-17. Neurobiol Aging 22: 89-107.
- Soliveri P, Rossi G, Monza D, Tagliavini F, Piacentini S, et al. (2003) A case of dementia parkinsonism resembling progressive supranuclear palsy due to mutation in the tau protein gene. Arch Neurol 60: 1454-1456.
- Baba Y, Tsuboi Y, Baker MC, Uitti RJ, Hutton ML, et al. (2005) The effect of tau genotype on clinical features in FTDP-17. Parkinsonism RelatDisord 11: 205-208.
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3: 519-526.
- Shahani N, Brandt R (2002) Functions and malfunctions of the tau proteins. Cell Mol Life Sci 59: 1668-1680.
- vanSwieten J, Spillantini MG (2007) Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol 17: 63-73.
- Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA (1989) Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: Differential expression of tau protein mRNAs in human brain. EMBO J 8: 393-399.
- Irwin DJ, Cohen TJ, Grossman M, Arnold SE, McCarty-Wood E, et al. (2013) Acetylated tau neuropathology in sporadic and hereditary tauopathies. Am J Pathol 183: 344-351.
- Dickson MME, Lee VMY, Trojanowski JQ (2004) Sporadic Tauopathies: Pick's disease, corticobasal degeneration, progressive supranuclear palsy and argyrophilic grain disease. The Neuropathology of Dementia, seconded, Cambridge University Press, NY.
- Höglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, et al. (2011) Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 43: 699-705.
- Marshall CR, Guerreiro R, Thust S, Fletcher P, Rohrer JD, et al. (2015) A novel MAPT mutation causing corticobasal syndrome led by progressive apraxia of speech. J Alzheimers Dis 48: 923-926.
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, et al. (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442: 916-919.
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, et al. (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442: 920-924.
- Daniel R, He Z, Carmichael KP, Halper J, Bateman A (2000) Cellular localization of gene expression for progranulin. J HistochemCytochem 48: 999-1009.
- Masellis M, Momeni P, Meschino W, Heffner R Jr, Elder J, et al. (2006) Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain 129: 3115-3123.
- Yu CE, Bird TD, Bekris LM, Montine TJ, Leverenz JB, et al. (2010) The spectrum of mutations in progranulin: A collaborative study screening 545 cases of neurodegeneration. Arch Neurol 67: 161-170.
- Kelley BJ, Haidar W, Boeve BF, Baker M, Shiung M, et al. (2010) Alzheimer disease-like phenotype associated with the c.154delA mutation in progranulin. Arch Neurol 67: 171-177.
- Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, et al. (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42: 234-239.
- van der Zee J, Van Langenhove T, Kleinberger G, Sleegers K, Engelborghs S, et al. (2011) TMEM106B is associated with frontotemporal lobar degeneration in a clinically diagnosed patient cohort. Brain 134: 808-815.
- van der Zee J, Van Broeckhoven C (2011) TMEM106B a novel risk factor for frontotemporal lobar degeneration. J MolNeurosci 45: 516-521.
- Wang WX, Wilfred BR, Madathil SK, Tang G, Hu Y, et al. (2010) miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol 177: 334-345.
- Jiao J, Herl LD, Farese RV, Gao FB (2010) MicroRNA-29b regulates the expression level of human progranulin, a secreted glycoprotein implicated in frontotemporal dementia. PloS one 5: e10551.
- Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, et al. (2010) Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68: 654-667.
- Carrasquillo MM, Nicholson AM, Finch N, Gibbs JR, Baker M, et al. (2010) Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet 87: 890-897.
- Bruni AC, Momeni P, Bernardi L, Tomaino C, Frangipane F, et al. (2007) Heterogeneity within a large kindred with frontotemporal dementia: A novel progranulin mutation. Neurology 69: 140-147.
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, et al. (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15: 2988-3001.
- Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, et al. (2012) Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet 90: 1102-1107.
- Benussi L, Rossi G, Glionna M, Tonoli E, Piccoli E, et al. (2014) C9ORF72 hexanucleotide repeat number in frontotemporal lobar degeneration: A genotype-phenotype correlation study. J Alzheimers Dis 38: 799-808.
- Benussi A, Padovani A, Borroni B (2015) Phenotypicheterogeneity of monogenic frontotemporaldementia. Front Aging Neurosci 7: 171.
- Smith BN, Newhouse S, Shatunov A, Vance C, Topp S, et al. (2013) The C9ORF72 expansion mutation is a common cause of ALS+/-FTD in Europe and has a single founder. Eur J Hum Genet 21: 102-108.
- Landqvist Waldo M, Gustafson L, Nilsson K, Traynor BJ, Renton AE, et al. (2013) Frontotemporal dementia with a C9ORF72 expansion in a Swedish family: Clinical and neuropathological characteristics. Am J Neurodegener Dis 2: 276-286.
- Simón-Sánchez J, Dopper EG, Cohn-Hokke PE, Hukema RK, Nicolaou N, et al. (2012) The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain 135: 723-735.
- Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, et al. (2012) Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 135: 765-783.
- Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, et al. (2011) Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. ActaNeuropathol 122: 673-690.
- Lindquist SG, Duno M, Batbayli M, Puschmann A, Braendgaard H, et al. (2013) Corticobasal and ataxia syndromes widen the spectrum of C9ORF72 hexanucleotide expansion disease. Clin Genet 83: 279-283.
- van der Zee J, Gijselinck I, Dillen L, Van Langenhove T, Theuns J, et al. (2013) A pan-European study of the C9orf72 repeat associated with FTLD: Geographic prevalence, genomic instability and intermediate repeats. Hum Mutat 34: 363-373.
- Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C (2013) Current insights into the C9orf72 repeats expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36: 450-459.
- Rohrer JD, Isaacs AM, Mizielinska S, Mead S, Lashley T, et al. (2015) C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 14: 291-301.
- Rademakers R, Neumann M, Mackenzie IR (2012) Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol 8: 423-434.
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, et al. (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339: 1335-1338.
- Mackenzie IR, Arzberger T, Kremmer E, Troost D, Lorenzl S, et al. (2013) Dipeptide repeat protein pathology in C9ORF72 mutation cases: Clinico-pathological correlations. ActaNeuropathol 126: 859-879.
- van der Zee J, Van Broeckhoven C (2014) Dementia in 2013: Frontotemporallobar degeneration-building on breakthroughs. Nat Rev Neurol 10: 70-72.
- van der Zee J, Urwin H, Engelborghs S, Bruyland M, Vandenberghe R, et al. (2008) CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum Mol Genet 17: 313-322.
- Cox LE, Ferraiuolo L, Goodall EF, Heath PR, Higginbottom A, et al. (2010) Mutations in CHMP2B in lower motor neuron predominant amyotrophic lateral sclerosis (ALS). PLoS One 5: e9872.
- Parkinson N, Ince PG, Smith MO, Highley R, Skibinski G, et al. (2006) ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67: 1074-1077.
- Stokholm J, Teasdale TW, Johannsen P, Nielsen JE, Nielsen TT, et al. (2013) Cognitive impairment in the preclinical stage of dementia in FTD-3 CHMP2B mutation carriers: a longitudinal prospective study. J NeurolNeurosurg Psychiatry 84: 170-176.
- Henne WM, Stenmark H, Emr SD (2013) Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring HarbPerspectBiol 5.
- Lu Y, Zhang Z, Sun D, Sweeney ST, Gao FB (2013) Syntaxin 13, a genetic modifier of mutant CHMP2B in frontotemporal dementia, is required for autophagosome maturation. Mol Cell 52: 264-271.
- Urwin H, Authier A, Nielsen JE, Metcalf D, Powell C, et al. (2010) Disruption of endocytic trafficking in frontotemporal dementia with CHMP2B mutations. Hum Mol Genet 19: 2228-2238.
- Clayton EL, Mizielinska S, Edgar JR, Nielsen TT, Marshall S, et al. (2015) Frontotemporal dementia caused by CHMP2B mutation is characterised by neuronal lysosomal storage pathology. ActaNeuropathol 130: 511-523.
- Chassefeyre R, MartÃnez-Hernández J, Bertaso F, Bouquier N, Blot B, et al. (2015) Regulation of postsynaptic function by the dementia-related ESCRT-III subunit CHMP2B. J Neurosci 35: 3155-3173.
- Jan YN, Jan LY (2010) Branching out: Mechanisms of dendritic arborization. Nat Rev Neurosci 11: 316-328.
- Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, et al. (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36: 377-381.
- van der Zee J, Pirici D, Van Langenhove T, Engelborghs S, Vandenberghe R, et al. (2009) Clinical heterogeneity in 3 unrelated families linked to VCP p.Arg159His. Neurology 73: 626-632.
- Chan N, Le C, Shieh P, Mozaffar T, Khare M, et al. (2012) Valosin-containing protein mutation and Parkinson's disease. Parkinsonism RelatDisord 18: 107-109.
- Yamanaka K, Sasagawa Y, Ogura T (2012) Recent advances in p97/VCP/Cdc48 cellular functions. BiochimBiophysActa 1823: 130-137.
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, et al. (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40: 572-574.
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, et al. (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319: 1668-1672.
- Mosca L, Lunetta C, Tarlarini C, Avemaria F, Maestri E, et al. (2012) Wide phenotypic spectrum of the TARDBP gene: Homozygosityof A382T mutation in a patient presenting with amyotrophic lateral sclerosis, Parkinson's disease, and frontotemporal lobar degeneration, and in neurologically healthy subject. Neurobiol Aging 33: 1846 e1-e4
- Quadri M, Cossu G, Saddi V, Simons EJ, Murgia D, et al. (2011) Broadening the phenotype of TARDBP mutations: the TARDBP Ala382Thr mutation and Parkinson's disease in Sardinia. Neurogenetics 12: 203-209.
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, et al. (2011) Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci 14: 452-458.
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, et al. (2011) Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci 14: 459-468.
- Kwiatkowski TJ Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, et al. (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323: 1205-1208.
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, et al. (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323: 1208-1211.
- Huey ED, Ferrari R, Moreno JH, Jensen C, Morris CM, et al. (2012) FUS and TDP43 genetic variability in FTD and CBS. Neurobiol Aging 33: 1016.
- Lagier-Tourenne C, Polymenidou M, Cleveland DW (2010) TDP-43 and FUS/TLS: Em erging roles in RNA processing and neurodegeneration. Hum Mol Genet 19: R46-64.
- Gitcho MA, Strider J, Carter D, Taylor-Reinwald L, Forman MS, et al. (2009) VCP mutations causing frontotemporal lobar degeneration disrupt localization of TDP-43 and induce
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 13491
- [From(publication date):
August-2016 - Dec 23, 2024] - Breakdown by view type
- HTML page views : 12694
- PDF downloads : 797