Review Article Open Access
Herpes Simplex Encephalitis in Childhood
| Bhagirathi Dwibedi* | |
| Regional Medical Research Centre Bhubaneswar, India | |
| *Corresponding Author : | Bhagirathi Dwibedi Department of Virology Chandrasekharpur Bhubaneswar, Odisha, India Tel: 916742300134 E-mail: bhagirathidwibedi@yahoo.com |
| Received: December 23, 2015 Accepted: March 02, 2016 Published: March 10, 2016 | |
| Citation: Dwibedi B (2016) Herpes Simplex Encephalitis in Childhood . J Infect Dis Ther 4:273. doi:10.4172/2332-0877.1000273 | |
| Copyright: © 2016 Dwibedi B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Acute Encephalitis Syndrome (AES) is one of the important health problems in childhood that leads to severe morbidity, mortality and sequelae. Herpes Simplex Virus (HSV) attributing towards such viral encephalitis carries importance with respect to its global prevalence challenging pathogenesis and morbidity outcomes. This report provides a review on several studies on epidemiology, pathogenesis (molecular and immune mechanism), clinical presentation, diagnosis and treatment related to Herpes simplex encephalitis (HSE) in children. It highlights wide spread distribution of HSE in children throughout the globe with mortality upto 70% in spite of availability of effective antiviral agent. In this context early diagnosis is a challenge as early CSF studies and imaging may not provide confirmatory diagnosis so an empirical treatment of Acyclovir will be of value. The review pointed that LATs play an important role in molecular modulation but it is still challenging to explain the exact cellular mechanism. The current antivirals available have shown to be potentially useful in curing symptomatic illness but eliminating neuronal carrier stage is still an issue of research for drug development. Better understanding of biochemical and immune response if available can open the scope for use of immunomodulators that can prevent reactivation. This review also focuses upon challenges in immunopathogenesis and treatment and vaccine development.
| Keywords |
| Herpes simplex virus; Infection; Viral DNA; Lesions; Encephalitis |
| Introduction and Epidemiology |
| Infection due to Herpes Simplex Virus (HSV) was first documented in Greece where Hippocrates used the term herpes which mean to creep or cranial herpetic infection defining its spreading lesion. It was reported by Vidal in 1893 as transmissible from person to person [1]. HSV is a member of Herpesviridae whose genome is made up of single large double stranded DNA molecule. The virus is made up of four components; Viral DNA (152kbp), Icosahedral capsid, Tegument and Envelope. There are two HSV types manifesting with neurological symptoms in humans; one is HSV-1 which generally causes orofacial lesions and other is HSV-II that causes genital lesions. Human being acts as the only reservoir for HSV infection. Following entry to the host, these viruses enter into the corresponding neuronal ganglia (trigeminal ganglia in HSV-I and lumbosacral ganglia in HSV-II) and establish a latent infection for the whole life [2]. The primary infection may or may not have symptomatic presentation, but manifests as reactivations from time to time depending upon stress (e.g. fever) and hormonal imbalance in the host [3]. |
| HSV infection is endemic throughout the globe and its widespread distribution has been evidenced by serological investigation indicating high prevalence of antibody against HSV. Prevalence of HSV-I and HSV-II has also been observed to vary with geographical location, socioeconomic status and age [4]. Two to four individuals per million populations per year are affected by HSV, of which more than 90% cases are due to HSV-I [5]. HSV-I has a varied clinical presentation; they are herpes labialis, keratitis, gingivostomatitis, meningoencephalitis, encephalitis and sometimes herpes genitalis [4]. |
| Central nervous system (CNS) infections due to HSV are less common but they are associated with significant morbidity and mortality even after antiviral therapy. Neurological involvement in HSV infections can be categorized as neonatal HSV CNS disease when it affects neonates and as Herpes Simplex Encephalitis (HSE) in individuals after the neonatal period. |
| The incidence of HSE due to confirmed HSV-1 and HSV-2 infections were reported as 1.2 cases per 100,000 inhabitants per year [5] and is considered the most common cause of sporadic, fatal encephalitis [6]. The incidence of HSE peaks between the age of 6 month and 3 years, a period during which vast majority of cases are consequences of primary infection with HSV-1 [5]. HSE can occur as a primary infection (30%) or it can be caused by a recurrent infection (70%) and virtually all cases of HSE are caused by HSV-1 [5]. If untreated, HSE mortality approaches 70% and of those patients who do survive, significant neurologic morbidity occurs in nearly 97% [7]. More than 90% cases are due to HSV-1 and rests are caused by HSV-2 [8]. After the introduction of Acyclovir (ACV) for the treatment of HSV the mortality has been reduced but still 20% deaths and permanent disability like cognitive and memory impairment is common [8]. HSE is manifested with an acute or sub-acute illness that causes both general and focal signs of cerebral dysfunction. Fever, headache, confusion, behavioral changes, focal neurologic findings are major clinical manifestations during HSE [9]. Only 10% of the childhood HSE cases have a prior history of mucosal symptoms like cold sores or conjunctivitis [10]. |
| Childhood encephalitis due to HSV is rare and its prevalence varies with geographic location. In an investigation from Japan during 1984-1993, HSV was the third most predominant causative agent of acute encephalitis [11]. HSV was also the most common cause in an observational prospective multicentric study conducted in Spain [12]. A Swedish study during 1992-93 on rapid diagnostic identification in viral CNS infection indicated HSV-1 as the most prevalent viral agent [13]. Similarly, in a retrospective study of 170 children aged 1 month to 15 years over a period of 13 years (1979-1991) showed that HSV-1 was the most common virus (10%) in Slovenia [14]. In China and Thailand, cases of HSE have been reported to be comparatively less prevalent [15,16]. Recently, a study of encephalitis in Australia reported HSV as the dominant cause of infectious encephalitis [17]. By definition, neonatal herpes simplex disease presents in the first 4 weeks of life and is almost always acquired by perinatal exposure to HSV. HSV infection of the neonate is rare with an estimated rate of 1 in 3,200 deliveries which is one-third of cases of neonatal herpes disease [18]. Long term neurological sequlae in most of the cases and 4%-14% mortality rate has been observed even after treatment in HSE [19]. |
| Pathogenesis |
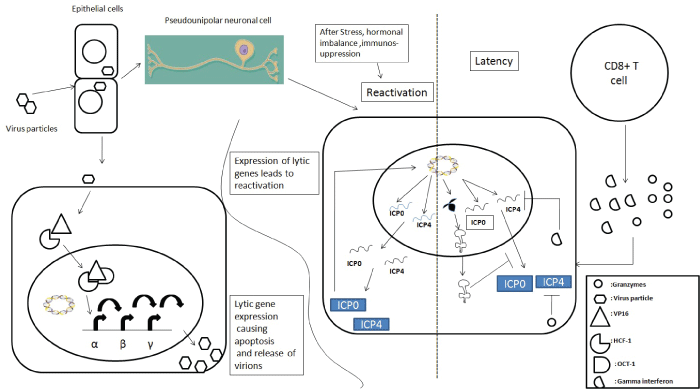
| The HSV-1 cycle starts when the virion gets attached to the epithelial cells through glycoprotein B (gB) and glycoprotein C (gC) by interacting with heparin sulphate proteoglycan (HSPG) [20]. Virus enters by fusing with plasma membrane or formation of endocytic vesicles. Fusion is mediated through glycoprotein D (gD) and interaction with cellular receptors. Interaction of cellular receptors causes conformational changes in gD which results in formation of multi protein fusion complex of gD and gB along with gH and gL so as to achieve fusion [21]. Nectin-1 is responsible for entry into neuronal cell population [22,23]. After fusion of HSV-1 envelope with plasma membrane, the tegument and nucleocapsid is delivered inside the cytosol. Tegument proteins here regulate cell process, escape from immune system and promote viral gene transcription. Tegument protein VP16 associates with host cell factor-1(HCF-1) in cytosol and enters into nucleus where VP16/HCF-1 complex binds with octamer binding transcription factor-1(Oct-1) to form transcription factor complex which starts transcription of immediate early or α gene [24]. Immediate early (IE) gene product activates transcription of early or β genes that encode for protein necessary for replication of viral DNA. Structural components of virions are synthesized from expression of ϒ or late genes after completion of viral DNA replication [25]. Different units of virions are then assembled and released to infect nearby cells. |
| Virions of HSV-1 enter the neuron through axon and travel in retrograde direction towards cell body [26]. After reaching nucleus, viral genome is released into nucleus and circularize so that DNA synthesis can be started. Such circular DNA has been observed during viral latency of HSV-1 [27,28]. Inside neuron the virus may follow one or the other pathway; (a) genomic IE gene may be activated and leads to productive infection or (b) productive infection may be checked by the presence of neuronal factor and let the virus enter into latent state [29-31]. Neuronal susceptibility or resistance to more productive infection is influenced by presence of different regulatory RNA or protein [32-35]. |
| Latency and reactivation can be possibly explained by molecular mechanism as following |
| The only transcripts readily detectable during latency are the latency associated transcripts (LATs) [36]. The mechanism behind the latency is inconclusive. One of the possible mechanisms has been attributed to the greater distance travelled by virus leading to less availability of tegument protein at the responding site [37,38]. Another theory proposes that HCF-1 is sequestered in cytoplasm so that it would not interact with VP16 inside nucleus [39]. Thus, a combined viral and host cell factors are probably responsible for establishment of latent infection of neurons. With some specific stimuli viral gene expression occurs in ganglia and later in peripheral sites. LATs are a set of colinear RNAs transcribed from a locus within the repeat regions flanking the unique long region of the viral genome [40]. Their transcription leads to the production of an 8.3 kb minor LAT primary transcript which is then spliced to produce an unusually stable 2.0 kb intron which is further spliced to produce an additional stable 1.5 kb intron [41].Together, these two RNA species are termed the major LATs. A number of recent studies have identified the presence of primary micro RNA (miRNA) sequences throughout the HSV-1 and HSV-2 genomes with the vast majority localized to the LAT locus [40-46]. LATs are proposed to be important though, are not essential for latency establishment, maintenance or reactivation [47,48]. The most consistent in vivo phenotype associated with LAT-negative virus mutants has been associated with reduction in the efficiency of virus reactivation. LATs may repress the lytic gene expression which was demonstrated in two studies that shown increased abundance of lytic gene transcripts during acute [49] and latent infection [50] of murine trigeminal ganglia with LAT deletion mutant. However, within this region two sRNA species (sRNA 1 & sRNA2) have recently been discovered [51] and both of these sRNAs were found capable of inhibiting productive infection in tissue culture but to different extents. Despite showing only weak repression of virus replication in comparison with sRNA1, sRNA2 exhibited down-regulation of ICP4 protein accumulation whilst not affecting cognate mRNA levels [52]. Another major observation attributed to the expression of the LATs is the inhibition of cell death in response to virus infection. A number of studies have attributed anti-apoptotic functions to the LATs [53]. These studies demonstrate a functional role in cell survival but a mechanism by which LAT could exactly produce this outcome, is still lacking. |
| Role of immune system in infection and disease |
| Replication in peripheral epithelial cells leads to production and release of type 1 interferon which activates cells of the innate immune system. Recruited neutrophils induce apoptosis in infected cells and phagocytose dying cells. Activated natural killer (NK) cells release interferon-γ and granzymes A and B that induce apoptosis in infected cells. Dendritic cells (DCs) secrete proinflammatory cytokines; take up antigen and present viral peptides to cells of the adaptive immune system. The innate immune response limits viral replication in the periphery and presents antigen to the naive lymphocytes to activate the adaptive immune response. It appears that the principal role of B cells in the immune response to HSV-1 infection is not to produce neutralizing antibodies but instead to present antigen and secrete cytokines [54]. Activated CD4+T and CD8+T cells play a pivotal role in clearing the primary infection. Additionally, CD8+ T cells are also thought to be important for maintaining the virus in the latent state. |
| Neuronal invasion and maintenance of infection in nerve cells |
| Before invading the nervous system, HSV typically infects and replicates in somatic cells (such as epithelia). To invade the nervous system of the host, viral particles enter nerve termini of the PNS and undergo retrograde transport towards the cell body, where they initiate a lifelong latent infection [55]. Targeted directional transport of viral particles in neurons is an integral part of the viral lifecycle and it is achieved by bidirectional transport of viral particles along axons and dendrites [56,57] due to polarized nature of neurons [58,59]. In vivo , directional spread is likely dependent upon the structure and connectivity of the neuron cell types that are typically infected. HSV-1 and HSV-2 often establish latent infections in sensory neurons of the dorsal root and trigeminal ganglia [60]. These neurons are mostly pseudounipolar and have a bifurcated axon, such that one branch projects towards the periphery while the other synapses with neurons of the CNS. Axonal sorting and anterograde transport of newly replicated particles is thought to proceed down both branches of these axons [61,62]. Trans synaptic movement of viral particles occurs routinely from infected PNS neurons into higher order CNS neurons [63-65]. As evidence of this, HSV genomes are readily detectable in the CNS of infected rodents and humans [57,66]. Furthermore, Chen et al. demonstrated that latent HSV-1 and HSV-2 genomes could be reactivated from mouse brain stem indicating that the CNS can be a latency site for HSV with the potential to cause recurrent disease [52]. Since HSV-1 gains access to the body of their natural hosts by infecting epithelial surfaces of the oronasal region, it may also invade the CNS directly via the olfactory routes [67]. In brains of patients suffering from herpes simplex encephalitis, immunohistochemical analysis showed an enrichment of viral antigen mainly in the medial and inferior temporal lobes, hippocampus, amygdaloid nuclei, olfactory cortex, insula and cingulate gyrus [68]. This localization pattern would be consistent with entry of the virus via the olfactory pathway with spread along the base of the brain to the temporal lobes. However another possibility is that herpes simplex encephalitis may result from viral spread from the trigeminal ganglia to the temporal and frontal cortex which would be consistent with the known site of HSV-1 latency. Further work is needed to understand the molecular factors that determine the route of HSV neuroinvasion. Casrouge et al. previously reported that autosomal recessive mutations in UNC-93B, a transmembrane protein located within the ER, render children more susceptible to herpes simplex encephalitis by disrupting the interferon (IFN) response pathway [69]. UNC-93B functions by delivering TLR3, TLR7 and TLR9 from the ER to the endosome. Apart from this, single gene inborn errors/mutation in TLR 3 dependent induction of IFN pathway has also been implicated for cause of childhood HSE. These mutations are either autosomal dominant (AD); UNC93B, TLR3, TRAF3, TRIF, partial TBK1deficiencies or autosomal recessive AR16; TLR3, TRIF [70] deficiencies. Few cases having AR STAT1 deficiency affecting cellular response to IFN [71] and X linked recessive NEMO deficiency with impaired TLR3 dependent IFN production also been demonstrated [58]. |
| Clinical presentation |
| Herpes simplex encephalitis is manifested with acute focal, necrotizing inflammation and swelling of the brain tissue. In one study HSV was reported to be the cause of 52% of the focal encephalitis cases, 25% of the mild cases and none of the diffuse cases. Thus HSV was majorly associated with the development of focal encephalitis but is less likely to produce diffuse or non-focal disease. Apart from that, HSV infection should still be ruled out in patients with mild or atypical presentations of encephalitis [72]. Patients suffering from meningitis typically had fever, headache, neck rigidity, altered sensorium but not focal neurological findings which is generally overlapping with encephalitis [73]. Range of clinical presentations of encephalitis in a case series of encephalitis of different aetiology was assessed in a PCR confirmed study [72] where patients were grouped into those with focal, diffuse and mild encephalitis. Patients manifested with focal disease had weakness, sensory abnormalities, aphasia, visual field defects or cranial nerve palsies and/or focal abnormalities on neuroimaging studies. Patients with diffuse disease were reported to have a decreased level of consciousness and neurobehavioural changes, but did not have focal abnormalities on neurological examination. Patients with mild encephalitis had preserved level of consciousness (Glasgow Coma Scale >13) predominantly in comparison to focal and diffuse groups. HSV was the major cause of encephalitis in this study being responsible for approximately 37% of cases [72]. |
| Source of HSE in neonatal disease could be difficult to identify as a history of known maternal HSV disease (genital or occasionally oral) is not universal and maternal disease may be asymptomatic [10]. Lethargy, poor feeding, irritability, temperature instability or seizures may be observed in neonates but the diagnosis is made by brain MRI and Lumber puncture. CSF findings includes pleocytosis (CSF WBC count >25 cells/ microliter in infants younger than 28 days old and >10 cells/microliter inpatients aged 29-90 days [74]; in conjunction with positive surface cultures and/or a finding of HSV DNA in the CSF. Sixty eight percent of these neonates also have skin manifestation (Figure 1). |
| Diagnosis |
| Clinical presentation of HSE is similar to a case of acute encephalitis syndrome and there is no such clear cut clinical presentation or a group of symptoms for which it can be attributed to HSV-1. This is an ever persisting enigma and any case of AES is first supposed to be due to HSV-1 in the absence of any vector borne encephalitis outbreak or any history pointing toward other viruses. |
| Mild atypical meningoencephalitis is a syndrome characterized by meningeal and neurological signs caused by HSV infection/ reactivation. It resolves without sequelae [73,75,76]. PCR has been used to detect HSV DNA in the CSF of patients who present with such cases. Patients with radiculomyelitis had also been reported to contain HSV DNA in their CSF [77,78]. Bell’s palsy [79,80], cluster headaches [81,82], migraines [83] and even common recurrent headaches [84] had been suggested to have HSV infection or reactivation as a cause. There is chance that treated cases of HSE have been observed with chronic granulomatous herpes encephalitis which was misinterpreted as neoplasm [85]. In addition, patients at risk for chronic HSV encephalitis could be identified through anti-viral immunity specifically to HSV-1 with TLR3. |
| Examination of the CSF |
| CSF analysis generally had elevated mononuclear cells and protein. Lymphocytic pleocytosis between 10-500 cells/mm3 was reported in 85% of HSE patients; less than 8% of patients with biopsy or PCRproven disease had normal CSF cell count [73,86,87]. Elevated protein (60-700 mg/dl) was found in 80% of patients whereas protein >800 mg/dl was uncommon [86]. In some patients, RBCs (10-500 cells/ mm3) were present or there may be a mild hypoglycorrhachia (30-40 mg/dl glucose). HSV culture from CSF was reported to be very difficult [88]. |
| Neuro-diagnostic tests |
| Magnetic resonance imaging (MRI) and EEG are the common neuroimaging tests used for the diagnosis of HSV infections. Normal MRI findings had been observed in 10% of PCR-positive patients but the remainder had temporal lobe abnormalities. In the initial stage of HSE, MRI generally had abnormal reports showing damage to the blood–brain barrier, oedema and necrosis in the temporal lobe [72,89]. In addition to that, high signal intensity lesions on T2-weighted images in the medial and inferior temporal lobe extending upto insula were also reported. Abnormalities may be seen on diffusion-weighted or FLAIR images in case of normal images in conventional MRI [90]. Lesions were rarely been observed in the basal ganglia, brainstem or cerebellum. Electroencephalography had only limited diagnostic utility as it shows non-specific slow-wave activity during the first 5-7 days of illness followed by paroxysmal sharp waves or triphasic complexes with temporal predominance [91,92]. Some patients did show periodic lateralizing epileptiform discharges (PLEDs) at a rate of 2-3 Hz originating from the temporal lobe [93]. PLEDs do not define but they can be suggestive of diagnosis of HSE. PLEDs may be absent at very early stage of disease and their absence does not exclude or significantly reduce the likelihood of HSE. Resolution of EEG abnormalities does not correlate well with clinical recovery [94]. |
| Virological diagnosis of HSE |
| The widespread use of PCR amplification of HSV DNA has improved our understanding of the signs and symptoms of HSE. It has also expanded the spectrum of diagnosis in HSV-related infections of the CNS. Brain biopsy along with virus isolation which has a specificity of 100% is the accepted standard for diagnosis before the use of PCR. In most cases of HSE, HSV DNA is present in CSF and can be detected by PCR [95-97]. In two very important studies to date, PCR of CSF was compared with brain biopsy (98) and brain biopsy was compared with intrathecal HSV antibody assay [96]. The first investigation shown CSF PCR was positive in 53 of 54 patients (98%) with biopsy-proven HSE and negative in 94% of biopsy-negative cases. Based on this study, PCR has a sensitivity of 98%, specificity of 94%, a positive predictive value of 95% and a negative predictive value of 98% [98]. In other study, a series of 43 HSE cases were tested by HSV in brain biopsy or necropsy and/or intrathecal HSV IgG antibody production and in 60 patients with acute febrile focal encephalopathy (first suspected to be HSE but excluded by the absence of intrathecal HSV antibody synthesis), 238 CSF samples were tested. HSV DNA was detected in 42 of the 43 patients with HSE, all but one was positive in the first CSF and all the control subjects were PCR negative [96]. The results of CSF HSV PCR should always be evaluated with pre-test likelihood that a patient has HSE. In patients with a low prior probability of HSE, a negative CSF HSV PCR result reduces disease likelihood to <1%. However, in patients with a high prior probability of HSE (e.g. an individual with typical EEG and MR findings and a CSF pleocytosis), a negative test only reduces disease likelihood to ~5%, but does not exclude it completely. It is therefore important to consider these issues carefully when deciding continuation of antiviral therapy based on PCR results. It has recently been reported that false-negative CSF PCR tests may occur when PCR is performed within 72 h after onset of symptoms. These are cases where it may be justified to continue antiviral therapy. Porphyrin compounds derived from the degradation of haeme of erythrocytes may also cause false-negative CSF HSV PCR result [96]. As a result negative PCR results in bloody CSF specimens should be carefully interpreted. HSV DNA is detectable by PCR in the CSF for at least 1 week after symptom onset in most patients with HSV-related infection of the CNS (99). In one study, 100% PCR positivity was reported when CSF was collected within 10 days after onset of symptoms, 30% positivity for samples collected between 11-20 days and 19% for samples collected between 21-40 days [99]. The European guidelines suggested that isolation of virus from brain biopsy tissue should be replaced with PCR of CSF and subsequently by antibody assay for diagnosis of HSE [100]. Though HSV PCR is sensitive and specific but should be done in the late phase as early CSF collection has led to negative result as reported in one of the study [101]. The real-time PCR assay (TaqMan) is more sensitive, specific, fast and eliminates the hybridization steps usually required after PCR [97]. However, it requires the expensive instrumentation for the sole purpose of specifically testing specimens by a PCR-based assay. On the other hand microchip-based technologies can identify a variety of fluorescently labeled clinical markers and have the potential to integrate additional laboratory functions other than separation and detection of clinical markers [102]. |
| Treatment and Prevention |
| ACV therapy in paediatric patients |
| Fifty percent of patients died by the age of one year due to CNS neonatal HSV disease in the pre-antiviral era [103]. Evaluations of two different doses of vidarabine and of a lower dose of acyclovir (30 mg/kg/day for 10 days) reported that both of these antiviral drugs reduce mortality to 14% [104]. The lower dose of acyclovir superseded vidarabine as the better treatment option for neonatal HSV disease due to its favorable safety profile and its ease of administration. Higher dose of acyclovir (60 mg/kg/day or 21 days) brought down the mortality to 4% for CNS disease [105]. Acyclovir therapy should be stopped when there is a negative CSF HSV PCR and very low cell count (<5 cells/mm3), normal MRI neuroimaging, normal EEG, a full and quick recovery with normal level of consciousness or an alternative diagnosis is proved [10]. |
| The improvements in mortality and morbidity achieved with the use of higher-dose acyclovir support the dose of acyclovir at 60 mg/kg/day delivered intravenously in three divided daily doses and it is currently recommended [105,106]. The dosing interval of intravenous acyclovir may have to be increased in premature infants, based on their creatinine clearance [107]. As noted above, all patients with CNS HSV involvement should have a repeat lumbar puncture at the end of intravenous acyclovir therapy to rule out PCR positivity [108]. Persons who remain PCR positive should continue to receive intravenous antiviral therapy until PCR negativity is achieved [108,109]. Neutrophil counts should be monitored at least twice in a week throughout the course of intravenous acyclovir therapy and decreasing the dose of acyclovir or administering granulocyte colony-stimulating factor if the absolute neutrophil count remains below 500/l for a prolonged period [105]. |
| Decompressing hemicraniectomy has been used to treat life threatening HSE. Though it is exceptional option for management but has been opted as salvation therapy in life threatening situation, with intractable high intracranial pressure (ICP) and brain herniation. It was suggested in patients with high ICP not responding to medical treatment [6]. |
| Passive immunotherapy as an adjuvant to active antiviral interventions could be the future therapeutic options for improvement in the management of neonatal HSV disease. Both human and humanized monoclonal antibodies directed against gB or gD were reported to be beneficial in animal models of HSV disease [110,111]. Studies with humans have documented the protective effects of high titers of neutralizing antibodies in neonates [112] and they are more likely to have localized disease (and less likely to have disseminated disease) once they are infected [113,114]. |
| Outcome |
| It is difficult to assess the clinical outcome of young children. Therefore one year follow up should be done. Adverse outcome was age related and reported in younger children below 11 months. It was also reported that patients aged between 3-35 months were more likely to be severely disabled [115]. The clinical outcome generally included developmental delay (both motor and cognitive), body convulsion, behavioral problem, hemiplegia and sometimes need of rehabilitation centers. Children less than one year were at high risk [115]. The incidence of disability after HSE was high with 50% cases showing complete recovery after one year [6]. |
| Limitations of antiviral therapy for HSV CNS infections |
| In patients affected by HSV, the best outcomes are seen when appropriate antiviral therapy is given prior to significant viral replication within the CNS or widespread dissemination throughout the body [116]. Theoretically if clinicians are able to initiate appropriate therapy earlier, it may be possible to improve current outcomes. The authors of this study highlight the need for clinicians to have an increased awareness of HSV infections in an effort to decrease the time for diagnostic evaluation and treatment. Though characteristic findings on magnetic resonance imaging (MRI) of the brain or electroencephalography (EEG) may be helpful, the current gold standard for diagnosis of HSV infection with CNS involvement is the detection of HSV DNA in the CSF by polymerase chain reaction (PCR). Other causes of encephalitis can mimic the MRI findings of HSV CNS infections [117] and though the EEG findings of affected patients often have the classic periodic spike and slow-wave patterns early in the course of disease, this is nonspecific [118,119]. HSV PCR is more specific and provides rapid diagnosis. However, some laboratories are unable to perform the test in a timely fashion and so prompt diagnosis remains a limitation to optimal therapy in many cases. It should also be recognized that negative CSF HSV PCR has been documented early in the course of illness in cases later confirmed to be HSV infection [120]. |
| Other antivirals in clinical trials for therapy HSE |
| Valacyclovir (VCV) is being used as it is absorbed rapidly and is converted to ACV by the herpetic enzyme VCV hydrolase. Approximately 55% of an orally administered dose of VCV is available as ACV (a three to fivefold increase in bioavailability). Thus, VCV is preferred in most settings. Thrombotic microangiopathy has been reported in immune-compromised HIV-infected patients with CD4+ cell counts of less than 50 cells/μl receiving high doses of VCV over long periods of time (median: 54 weeks; range: 8-77 weeks) [121,122]. Famciclovir is another drug which rapidly metabolized to the active drug penciclovir, which is a synthetic acyclic guanine derivative with a spectrum of activity and a mechanism of action similar to that of ACV but it is 100 times less potent inhibitor of viral DNA replication. Ganciclovir and its prodrug, valganciclovir, differ from ACV and famciclovir because they are efficiently phosphorylated but are associated with a greater risk of neutropenia and renal toxicity [123]. |
| Helicase/Primase inhibitor has shown promising result in animal model but resistant virus easily obtained by serial passage and these are unable to revert back to wild form in the absence of drug [124,125]. TLR-3 agonist like polyinosinic acid: polycytidilic acid (Poly I:C) has been tested in animal model survival rate was significantly higher in comparison to lipopolysaccharide which is a TLR-4 agonist. This may also provide neuro-protection [126]. |
| Vaccine development |
| The two major strategies in vaccine development are subunit vaccines and live-attenuated viral vaccines. One live-attenuated vaccine candidate was R7020, which was constructed on an HSV-1 background genetically deleted in some of the loci responsible for neurovirulence inserted with HSV-2 sequences to broaden the immune response and with the TK gene added to retain susceptibility to ACV for safety [127]. However, results of clinical trials were disappointing and two doses of the vaccine failed to deliver enough antigens to induce a sufficient immune response [128]. More promising results have been obtained with subunit vaccines. While the gD2gB2-MF59 vaccine developed by Chiron was found to be ineffective in randomized, double-blind, placebo controlled studies [129]. The recently completed studies with a gD-2 vaccine from GlaxoSmithKline show greater potential [129]. The differences between these vaccine candidates may relate to the adjuvant; the adjuvant for the Chiron vaccine was MF-59, a potent inducer of Th2 responses whereas the adjuvant for the gD-2 vaccine contains alum plus monophosphoryl lipid A, a potent inducer of Th1 responses. Two double-blind randomized trials of the gD-2–alum monophosphoryl lipid vaccine demonstrated efficacy against genital herpes in women who are seronegative for both HSV-1 and HSV-2 at baseline but failed to demonstrate efficacy in men regardless of their HSV serologic status [130]. |
| Future Perspectives |
| Child hood HSE can also be due to TLR3 deficiency in fraction of children and it should be taken seriously when recurrence of HSE is observed. In addition to that genes in TLR3 pathway and TLR3 mutation should be searched and patients with TLR3 deficiency should be followed up carefully. Investigation based on differential response of TLR and other pattern recognition receptors need to be searched for, so that its effect on neonatal HSV encephalitis can be found. Difference in T cell response to HSV by neonates and older children can open up new avenue of research where more complete knowledge of immune response could lead to formulation of intervention. Neonatal HSV encephalitis is an important clinical problem as well as a fascinating example of age dependent immunity. By designing rational clinical and biomedical experiment to understand the dynamic relation between host and virus safe and effective therapies to treat or prevent neonatal HSV encephalitis can be done. |
| Vaccine development against HSV-1 ad 2 is difficult and it is due to complex life cycle, dormant or silent (latent) infection in most of the time and it is represented by absence of vaccine against ocular or orofacial herpes beyond phase I. There is only single vaccine with glyB and glyD, tested in last 18 years in human to prevent herpes genitalis. Recently T cell epitope based HSV vaccine development has been advocated and need to be considered by majority of the scientific community so that challenges can be sorted out and opportunities can be explored. |
| Till now genetic cause of HSE in childhood has been attributed to five aetiologies; UNC-93B deficiency, Autosomal dominant TLR3 deficiency, Autosomal recessive TLR3 deficiency, Autosomal dominant TRAF-3 deficiency, TRIF deficiency and TBK-1 deficiency. Recent studies have shown that only a small portion of children with HSE carry mutations of TLR3-IFN pathway. But larger portion of patients display a impaired production of IFN I & II after TLR-3 activation, so proteomic study can help to understand the susceptibility to HSE. Presence of anti NMDAR antibodies in HSE positive cases have been reported and that subgroup of patient will be benefitted from immunotherapy which may sometimes be ignored continuing treatment based on HSV positivity. It should be investigated in children so that children with HSE may be benefitted. Human and humanized monoclonal antibodies directed against gB and gD are beneficial in animal model of HSV disease but studies in human are yet to be carried out. |
| Conclusion |
| The review gives an impression that LATs play an important role in molecular modulation but it is still challenging to explain the exact cellular mechanism. The current antivirals available have shown to be potentially useful in curing symptomatic illness but eliminating neuronal carrier stage is still an issue of research for drug development. Better understanding of biochemical and immune response if available can open the scope for use of immunomodulators that can prevent reactivation hence severe illness. Further to this, challenges in the area of vaccine development, laboratory diagnosis and early clinical suspicion of severe manifestations need to be resolved for optimal reduction in morbidity and mortality due to HSE in children. |
| References |
References
- Wildy P (1973) Herpes: history and classification. In: Kaplan AS, ed. The herpes-viruses. New York: Academic Press: 1-25.
- Roizman B, Sears AE (1996) Herpes simplex viruses and their replication. In: Fields BN, Knipe DM, Howley PM, eds. Fields virology. Philadelphia: Lippincott-Raven: 2231-2295.
- Whitley RJ, Kimberlin DW, Roizman B (1998) Herpes simplex viruses. Clin Infect Dis 26: 541-553.
- Hjalmarsson A, Blomqvist P, Sköldenberg B (2007) Herpes simplex encephalitis in Sweden, 1990-2001: incidence, morbidity, and mortality. Clin Infect Dis 45: 875-880.
- James SH, Kimberlin DW, Whitley RJ (2009) Antiviral Therapy for Herpesvirus Central Nervous System Infections: Neonatal Herpes Simplex Virus Infection, Herpes Simplex Encephalitis, and Congenital Cytomegalovirus. Antiviral Res 83: 207-213.
- Jouan Y, Grammatico-Guillon L, Espitalier F, Cazals X, François P,et al. (2015) Long-term outcome of severe herpes simplex encephalitis: a population-based observational study. Crit Care 19: 345.
- Kimberlin DW (2007) Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes 14: 11-16.
- Aurelius E, Johansson B, Sköldenberg B, Forsgren M (1993) Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J Med Virol 39: 179-186.
- Sköldenberg B, Aurelius E, Hjalmarsson A, Sabri F, Forsgren M, et al. (2006) Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 253: 163-170.
- Le Doare K, Menson E, Patel D, Lim M, Lyall H, et al. (2015) Fifteen minute consultation: managing neonatal and childhood herpes encephalitis. Arch Dis Child EducPract Ed 100: 58-63.
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, et al. (2003) Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 289: 203-209.
- Corey L, Wald A (2009) Maternal and neonatal herpes simplex virus infections. N Engl J Med 361: 1376-1385.
- Kuriki M, Fujihra N, Hasegawa S, Fujioka M (1995) Acute encephalitis and bacterial meningitis in children in Aichi Prefecture (1984-1993). Nihon KoshuEiseiZasshi 42: 338-345.
- Flores-Gonzalez JC, Jordan-Garcia I, Turon-Vinas E, Montero-Valladares C, Tellez-Gonzalez C, et al. (2015) Etiology, clinical presentation and outcome of severe viral acute childhood encephalitis (ECOVE study). Rev Neurol 61: 7-13.
- Bergström T, Olofsson S, Studahl M, Kyllerman M, Darin N, et al. (1995) Gene amplification in viral CNS infections. Rapid diagnostic identification of herpesviruses. Lakartidningen 92: 427-432.
- Cizman M, Jazbec J (1993) Etiology of acute encephalitis in childhood in Slovenia. Pediatr Infect Dis J 12: 903-908.
- Xu Y, Zhaori G, Vene S, Shen K, Zhou Y, et al. (1996) Viral etiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Pediatr Infect Dis J 15: 1018-1024.
- Chokephaibulkit K, Kankirawatana P, Apintanapong S, Pongthapisit V, Yoksan S, et al. (2001) Viral etiologies of encephalitis in Thai children. Pediatr Infect Dis J 20: 216-218.
- Britton PN, Khoury L, Booy R, Wood N, Jones CA (2016) Encephalitis in Australian children: contemporary trends in hospitalisation. Arch Dis Child 101: 51-56.
- Shukla D, Spear PG (2001) Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest 108: 503-510.
- Campadelli-Fiume G, Amasio M, Avitabile E, Cerretani A, Forghieri C, et al. (2007) The multipartite system that mediates entry of herpes simplex virus into the cell. Rev Med Virol 17: 313-326.
- Shukla ND, Tiwari V, Valyi-Nagy T (2012) Nectin-1-specific entry of herpes simplex virus 1 is sufficient for infection of the cornea and viral spread to the trigeminal ganglia. Mol Vis 18: 2711-2716.
- Simpson SA, Manchak MD, Hager EJ, Krummenacher C, Whitbeck JC, et al. (2005) Nectin-1/HveC Mediates herpes simplex virus type 1 entry into primary human sensory neurons and fibroblasts. J Neurovirol 11: 208-218.
- Gerster T, Roeder RG (1988) A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl AcadSci U S A 85: 6347-6351.
- Roizman B, Zhou G, Du T (2011) Checkpoints in productive and latent infections with herpes simplex virus 1: conceptualization of the issues. J Neurovirol 17: 512-517.
- Smith G (2012) Herpesvirus transport to the nervous system and back again. Annu Rev Microbiol 66: 153-176.
- Mellerick DM, Fraser NW (1987) Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology 158: 265-275.
- Su YH, Moxley MJ, Ng AK, Lin J, Jordan R, et al. (2002) Stability and circularization of herpes simplex virus type 1 genomes in quiescently infected PC12 cultures. J Gen Virol 83: 2943-2950.
- Bloom DC, Giordani NV, Kwiatkowski DL (2010) Epigenetic regulation of latent HSV-1 gene expression. BiochimBiophysActa 1799: 246-256.
- Cliffe AR, Garber DA, Knipe DM (2009) Transcription of the herpes simplex virus latency-associated transcript promotes the formation of facultative heterochromatin on lytic promoters. J Virol 83: 8182-8190.
- Knipe DM, Cliffe A (2008) Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol 6: 211-221.
- Camarena V, Kobayashi M, Kim JY, Roehm P, Perez R, et al. (2010) Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell Host Microbe 8: 320-330.
- Kim JY, Mandarino A, Chao MV, Mohr I, Wilson AC (2012) Transient reversal of episome silencing precedes VP16-dependent transcription during reactivation of latent HSV-1 in neurons. PLoSPathog 8: e1002540.
- Kim M, Osborne NR, Zeng W, Donaghy H, McKinnon K, et al. (2012) Herpes simplex virus antigens directly activate NK cells via TLR2, thus facilitating their presentation to CD4 T lymphocytes. J Immunol 188: 4158 -4170.
- Thompson RL, Sawtell NM (2011) The herpes simplex virus type 1 latency associated transcript locus is required for the maintenance of reactivation competent latent infections. J Neurovirol 17: 552-558.
- Wagner EK, Bloom DC (1997) Experimental investigation of herpes simplex virus latency. ClinMicrobiol Rev 10: 419-443.
- Kristie TM, Roizman B (1988) Differentiation and DNA contact points of host proteins binding at the cis site for virion-mediated induction of alpha genes of herpes simplex virus 1. J Virol 62: 1145-1157.
- Kristie TM, Vogel JL, Sears AE (1999) Nuclear localization of the C1 factor (host cell factor) in sensory neurons correlates with reactivation of herpes simplex virus from latency. Proc Natl AcadSci U S A 96: 1229-1233.
- Kolb G, Kristie TM (2008) Association of the cellular coactivator HCF-1 with the Golgi apparatus in sensory neurons. J Virol 82: 9555-9563.
- Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT (1987) RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235: 1056-1059.
- Zabolotny JM, Krummenacher C, Fraser NW (1997) The herpes simplex virus type 1 2.0-kilobase latency-associated transcript is a stable intron which branches at a guanosine. J Virol 71: 4199-4208.
- Cui C, Griffiths A, Li G, Silva LM, Kramer MF, et al. (2006) Prediction and identification of herpes simplex virus 1-encoded microRNAs. J Virol 80: 5499-5508.
- Tang S, Bertke AS, Patel A, Wang K, Cohen JI et al.(2008) An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor. P Nat AcadSci 105: 10931-10936.
- Tang S, Patel A, Krause PR (2009) Novel less-abundant viral microRNAs encoded by herpes simplex virus 2 latency-associated transcript and their roles in regulating ICP34.5 and ICP0 mRNAs. J Virol 83: 1433-1442.
- Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, et al. (2008) MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454: 780-783.
- Umbach JL, Nagel MA, Cohrs RJ, Gilden DH, Cullen BR (2009) Analysis of human alphaherpesvirus microRNA expression in latently infected human trigeminal ganglia. J Virol 83: 10677-10683.
- Umbach JL, Wang K, Tang S, Krause PR, Mont EK, et al. (2010) Identification of viral microRNAs expressed in human sacral ganglia latently infected with herpes simplex virus 2. J Virol 84: 1189-1192.
- Jurak I, Kramer MF, Mellor JC, van Lint AL, Roth FP, et al. (2010) Numerous conserved and divergent microRNAs expressed by herpes simplex viruses 1 and 2. J Virol 84: 4659-4672.
- Javier RT, Stevens JG, Dissette VB, Wagner EK (1988) A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology 166: 254-257.
- Sedarati F, Izumi KM, Wagner EK, Stevens JG (1989) Herpes simplex virus type 1 latency associated transcription plays no role in establishment or maintenance of a latent infection in murine sensory neurons. J Virol 63: 4455-4458.
- Garber DA, Schaffer PA, Knipe DM (1997) A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol 71: 5885 –5893.
- Chen SH, Kramer MF, Schaffer PA, Coen DM (1997) A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J Virol 71: 5878 –5884.
- Peng W, Vitvitskaia O, Carpenter D, Wechsler SL, Jones C (2008) Identification of two small RNAs within the first 1.5-kb of the herpes simplex virus type 1-encoded latency-associated transcript. J Neurovirol 14: 41-52.
- Deshpande SP, Kumaraguru U, Rouse BT (2000) Dual role of B cells in mediating innate and acquired immunity to herpes simplex virus infections. Cell Immunol 202: 79-87.
- Antinone SE, Smith GA (2010) Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J Virol 84: 1504-1512.
- Ekstrand MI, Enquist LW, Pomeranz LE (2008) The alpha-herpesviruses: molecular pathfinders in nervous system circuits. Trends Mol Med 14: 134-140.
- Cabrera CV, Wohlenberg C, Openshaw H, Rey-Mendez M, Puga A, et al. (1980) Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature 288: 288-290.
- Rasband MN (2010) The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11: 552-562.
- Moughamian AJ, Holzbaur EL (2012) Synaptic vesicle distribution by conveyor belt. Cell 148: 849-851.
- Enquist LW (2012) Five questions about viral trafficking in neurons. PLoSPathog 8: e1002472.
- Smith G (2012) Herpesvirus transport to the nervous system and back again. Annu Rev Microbiol 66: 153-176.
- Topp KS, Meade LB, LaVail JH (1994) Microtubule polarity in the peripheral processes of trigeminal ganglion cells: Relevance for the retrograde transport of herpes simplex virus J Neurosci 14: 318-325.
- Chen SH, Yao HW, Huang WY, Hsu KS, Lei HY, et al. (2006) Efficient reactivation of latent herpes simplex virus from mouse central nervous system tissues. J Virol 80: 12387-12392.
- Tyler KL (2004) Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret's. Herpes 11 Suppl 2: 57A-64A.
- Smith C, Lachmann RH, Efstathiou S (2000) Expression from the herpes simplex virus type 1 latency-associated promoter in the murine central nervous system. J Gen Virol 81: 649-662.
- Fraser NW, Lawrence WC, Wroblewska Z, Gilden DH, Koprowski H (1981) Herpes simplex type 1 DNA in human brain tissue. Proc Natl AcadSci U S A 78: 6461-6465.
- Mettenleiter TC (2003) Pathogenesis of neurotropic herpesviruses: role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res 92: 197-206.
- Esiri MM (1982) Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J NeurolSci 54: 209-226.
- Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, et al. (2006) Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314: 308-312.
- Heininger U, Seward JF (2006) Varicella. Lancet 368: 1365-1376.
- Kost RG, Straus SE (1996) Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med 335: 32-42.
- Domingues RB, Tsanaclis AM, Pannuti CS, Mayo MS, Lakeman FD(1997) Evaluation of the range of clinical presentations of herpes simplex encephalitis by using polymerase chain reaction assay of cerebrospinal fluid samples. Clin Infect Dis 25:86–91.
- Whitley RJ, Gnann JW (2002) Viral encephalitis: familiar infections and emerging pathogens. Lancet 359: 507-513.
- De Tiège X, Rozenberg F, Héron B (2008) The spectrum of herpes simplex encephalitis in children. Eur J PaediatrNeurol 12: 72-81.
- Fodor PA, Levin MJ, Weinberg A, Sandberg E, Sylman J, et al. (1998) Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology 51: 554-559.
- Klapper PE, Cleator GM, Longson M (1984) Mild forms of herpes encephalitis. J NeurolNeurosurg Psychiatry 47: 1247-1250.
- Färkkilä M, Koskiniemi M, Vaheri A (1993) Clinical spectrum of neurological herpes simplex infection. ActaNeurolScand 87: 325-328.
- Shyu WC, Lin JC, Chang BC, Harn HJ, Lee CC, et al. (1993) Recurrent ascending myelitis: an unusual presentation of herpes simplex virus type 1 infection. Ann Neurol 34: 625-627.
- Coker NJ (1998) Bell palsy: a herpes simplex mononeuritis? Arch Otolaryngol Head Neck Surg 124: 823-824.
- Spruance SL (1994) Bell palsy and herpes simplex virus. Ann Intern Med 120: 1045-1046.
- Graham JR (1981) The migraine connection. Headache 21: 243-250.
- Hardebo JE (1986) An association between cluster headache and herpes simplex. N Engl J Med 314: 316.
- Sureda B, Villalobos F, Gil-Néciga E, Martínez ML, Bautista J (1987) [Migraine and cerebrospinal fluid alterations: are they an expression of viral meningoencephalitis?]. Neurologia 2: 54-56.
- Nielsen PG (1992) Recurrent HSV-1 virus infection complicated with recalcitrant headache treated with acyclovir. ActaDermVenereol 72: 236.
- Adamo MA, Abraham L, Pollack IF (2011) Chronic granulomatous herpes encephalitis: a rare entity posing a diagnostic challenge. J NeurosurgPediatr 8: 402-406.
- Whitley RJ, Soong SJ, Linneman C Jr, Liu C, Pazin G, et al. (1982) Herpes simplex encephalitis. Clinical Assessment. JAMA 247: 317-320.
- Sköldenberg B (1991) Herpes simplex encephalitis. Scand J Infect Dis Suppl 80: 40-46.
- Nahmias AJ, Whitley RJ, Visintine AN, Takei Y, Alford CA Jr(1982) Herpes simplex virus encephalitis: laboratory evaluations and their diagnostic significance. J Infect Dis 145 :829–836.
- Domingues RB, Fink MC, Tsanaclis AM, de Castro CC, Cerri GG, et al. (1998) Diagnosis of herpes simplex encephalitis by magnetic resonance imaging and polymerase chain reaction assay of cerebrospinal fluid. J NeurolSci 157: 148-153.
- McCabe K, Tyler K, Tanabe J (2003) Diffusion-weighted MRI abnormalities as a clue to the diagnosis of herpes simplex encephalitis. Neurology 61: 1015-1016.
- Lai CW, Gragasin ME (1988) Electroencephalography in herpes simplex encephalitis. J ClinNeurophysiol 5: 87-103.
- Misra UK, Kalita J (1998) A comparative study of Japanese and herpes simplex encephalitides. ElectromyogrClinNeurophysiol 38: 41-46.
- Ch'ien LT, Boehm RM, Robinson H, Liu C, Frenkel LD (1977) Characteristic early electroencephalographic changes in herpes simplex encephalitis. Arch Neurol 34: 361-364.
- Misra UK, Kalita J (1998) Neurophysiological studies in herpes simplex encephalitis. ElectromyogrClinNeurophysiol 38: 177-182.
- Puchhammer-Stöckl E1, Popow-Kraupp T, Heinz FX, Mandl CW, Kunz C (1990) Establishment of PCR for the early diagnosis of herpes simplex encephalitis. J Med Virol 32: 77-82.
- Aurelius E, Johansson B, Sköldenberg B, Staland A, Forsgren M (1991) Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 337: 189-192.
- Anderson NE, Powell KF, Croxson MC (1993) A polymerase chain reaction assay of cerebrospinal fluid in patients with suspected herpes simplex encephalitis. J NeurolNeurosurg Psychiatry 56:520-525.
- Lakeman FD, Whitley RJ (1995) Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis;171: 857-863.
- Revello MG, Baldanti F, Sarasini A, Zella D, Zavattoni M, et al. (1997) Quantitation of herpes simplex virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis by the polymerase chain reaction. ClinDiagnVirol 7: 183-191.
- Klapper PE, Cleator GM (1998) European guidelines for diagnosis and management of patients with suspected herpes simplex encephalitis. ClinMicrobiol Infect 4: 178-180.
- De Tiège X, Héron B, Lebon P, Ponsot G, Rozenberg F (2003) Limits of early diagnosis of herpes simplex encephalitis in children: a retrospective study of 38 cases. Clin Infect Dis 36: 1335-1339.
- Hofgärtner WT, Hühmer AF, Landers JP, Kant JA (1999) Rapid diagnosis of herpes simplex encephalitis using microchip electrophoresis of PCR products. ClinChem 45: 2120-2128.
- Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, et al. (1980) Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics 66: 495-501.
- Whitley RJ, Arvin A, Prober C, Burchett S, Corey L, et al. (1991) A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection . Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med 324: 444-449.
- Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L et al. (2001) Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 108: 230-238.
- Herpes simplex (2000) In:L. K. Pickering (eds.), Red Book: report of the Committee on Infectious Diseases(25thedn.) American Academy of Pediatrics, Elk Grove Village, Ill. pp. 309-318.
- Englund JA, Fletcher CV, Balfour HH Jr (1991) Acyclovir therapy in neonates. J Pediatr 119: 129-135.
- Kimberlin DW,Lin CY, Jacobs RF, Powell DA, Frenkel LM, et al. (2001) Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics 108: 223-229.
- Kimberlin DW, Lakeman FD, Arvin AM, Prober CG,Corey L, et al.(1996) Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. J Infect Dis 174: 1162-1167
- Baron S, Worthington MG, Williams J, Gaines JW (1976) Postexposure serum prophylaxis of neonatal herpes simplex virus infection of mice. Nature 261: 505-506.
- Bravo FJ, Bourne N, Harrison CJ, Mani C, Stanberry LR, et al. (1996) Effect of antibody alone and combined with acyclovir on neonatal herpes simplex virus infection in guinea pigs. J Infect Dis 173: 1-6.
- Prober CG, Sullender WM, Yasukawa LL, Au DS, Yeager AS, et al. (1987) Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med 316: 240-244.
- Kohl S, West MS, Prober CG, Sullender WM, Loo LS et al(1989) Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis 160: 770–776.
- Sullender WM, Miller JL, Yasukawa LL, Bradley JS, Black SB, et al. (1987) Humoral and cell-mediated immunity in neonates with herpes simplex virus infection. J Infect Dis 155: 28-37.
- Ward KN, Ohrling A, Bryant NJ, Bowley JS, Ross EM, et al. (2012) Herpes simplex serious neurological disease in young children: incidence and long-term outcome. Arch Dis Child 97: 162-165.
- Whitley RJ, Corey L, Arvin A, Lakeman FD, Sumaya CV, et al. (1988) Changing presentation of herpes simplex virus infection in neonates. J Infect Dis 158: 109-116.
- Whitley RJ, Cobbs CG, Alford CA Jr, Soong SJ, Hirsch MS, et al. (1989) Diseases that mimic herpes simplex encephalitis. Diagnosis, presentation, and outcome. NIAID Collaborative Antiviral Study Group. JAMA 262:234-239.
- Mizrahi EM, Tharp BR (1982) A characteristic EEG pattern in neonatal herpes simplex encephalitis. Neurology 32: 1215-1220.
- Sainio K, Granstrom ML, Pettay O, Donner M (1983) EEG in neonatal herpes simplex encephalitis. Electroencephalography & Clinical Neurophysiology 56:556-561.
- Fonseca-Aten M, Messina AF, Jafri HS, Sánchez PJ (2005) Herpes simplex virus encephalitis during suppressive therapy with acyclovir in a premature infant. Pediatrics 115: 804-809.
- Misra UK, Kalita J (1998) A comparative study of Japanese and herpes simplex encephalitides. ElectromyogrClinNeurophysiol 38: 41-46.
- Ch'ien LT, Boehm RM, Robinson H, Liu C, Frenkel LD (1977) Characteristic early electroencephalographic changes in herpes simplex encephalitis. Arch Neurol 34: 361-364.
- Tyler KL (2004) Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret's. Herpes 11 Suppl 2: 57A-64A.
- Betz UA, Fischer R, Kleymann G, Hendrix M, Rubsamen-Waigmann H (2002) Potent In vivo antiviral activity of the herpes simplex virus primase–helicase inhibitor BAY 57-1293. Antimicrob. Agents Chemother 46: 1766-1772.
- Biswas S, Jennens L, Field HJ (2007) The helicase primase inhibitor, BAY 57-1293 shows potent therapeutic antiviral activity superior to famciclovir in BALB/c mice infected with herpes simplex virus type 1. Antiviral Res 75: 30-35.
- Boivin N, Sergerie Y, Rivest S, Boivin G (2008) Effect of pretreatment with toll-like receptor agonists in a mouse model of herpes simplex virus type 1 encephalitis. J Infect Dis 198: 664-672.
- Meignier B, Longnecker R, Roizman B (1988) In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis 158: 602-614.
- Cadoz M, Micoud M, Seigneurin JM (1992) Phase I trial of R7020: a live attenuated recombinant HSV candidate vaccine. Presented at: 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Anaheim 11-14.
- Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, et al. (1999) Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 282: 331-340.
- Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, et al. (2002) Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347: 1652-1661.
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 13929
- [From(publication date):
April-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 12744
- PDF downloads : 1185
