Research Article Open Access
Herbal Hair Gel Formulation having 5α-Reductase Inhibitory Activity and its Standardization by HPTLC
Arpan Chakraborty1, Arka Bhattacharjee2, Akshay Sodani3, Dixita Jain3, Goutam Mukhopadhyay3* and Nasim Sepay4
1Department of Pharmaceutical Technology, Jadavpur University, Kolkata, West Bengal, India
2Department of Pharmaceutical Sciences and Technology, Birla Institute of Technology, Mesra, Ranchi, Jharkhand, India
3BCDA College of Pharmacy and Technology, Kolkata, West Bengal, India
4Department of Chemistry, Presidency University, Kolkata, India
- *Corresponding Author:
- Goutam Mukhopadhyay
BCDA College of Pharmacy and Technology
Kolkata, West Bengal-700 127, India
Tel: +919836333563
E-mail: gmukhopadhyay8@gmail.com
Received date: December 17, 2016; Accepted date: December 28, 2016; Published date: December 30, 2016
Citation: Chakraborty A, Bhattacharjee A, Sodani A, Jain D, Mukhopadhyay G, et al. (2016) Herbal Hair Gel Formulation having 5 α-Reductase Inhibitory Activity and its Standardization by HPTLC. J Anal Bioanal Tech 7: 341. doi: 10.4172/2155-9872.1000341
Copyright: © 2016 Chakraborty A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The present work has been undertaken with the aim to formulate hair growth gel formulation containing extracts of Hibiscus rosa sinensis flower 1%, Eclipta alba whole plant 1% and Solanum nigrum plant berries 0.5% which are preferably used in case of Alopecia, i.e., baldness pattern as an effective herbal therapy showing 5 α-reductase inhibitory activity. The formulated gel was evaluated for parameters such as pH which was found to be 6.68, viscosity 4731 cps, spreadability 11.05 (g-cm/sec) whereas consistent homogeneity was found with no skin irritation. Simultaneous quantification of bioactive markers was done by HPTLC. Quercetin, β-sitosterol and linoleic acid were selected as bioactive markers for quantification of Hibiscus rosa sinensis flower, Eclipta alba whole plant and Solanum nigrum plant berries extract respectively in the formulation. The aforementioned markers have 5 α-reductase inhibitory activity. β-sitosterol, quercetin and linoleic acid was found to be 0.1377, 0.120 and 0.379% w/w respectively in the formulated gel.
Keywords
Quercetin; β-sitosterol, Linoleic acid; Fingerprint; HPTLC; Polyherbal hair gel
Abbreviations
HPTLC: High Performance Thin Layer Chromatography; μg: Microgram; mL: Milliliter; mg: Milligram; g: Gram; Rf: Retention Factor; nm: Nanometer; μL: Microlitre; TLC: Thin Layer Chromatography; °C: Degree Centigrade.
Introduction
5 α-reductase, also known as 3-oxo-5α-steroid 4-dehydrogenases is involved in steroid metabolism. The principal role of 5 α-reductase being conversion of testosterone to dihydrotestosterone (DHT). Inhibition of DHT formation serves as a strategy for the treatment of alopecia and prostate hyperplasia [1,2]. There are synthetic medicines, such as finasteride, dutasteride, alfatradiol but these compounds have been reported to have adverse effects which necessitate the interest in finding better 5 α-reductase inhibitors from natural resources.
Solanum nigrum (Black nightshade) is a medicinal plant member belonging to the family Solanaceae. S. nigrum has been extensively used traditionally to treat various ailments. The berries of Solanum nigrum used as medicine for alopecia [3,4]. Linoleic acid is a polyunsaturated omega-6 fatty acid. It is reported to have a number of useful physiological activities including 5 α-reductase inhibitor; antianaphylactic; antiarthritic; antiatherosclerotic; anticoronary; anticancer; antieczemic; antifibrinolytic; antigranular; antihistaminic; antiinflammatory; antimenorrhagic; antiprostatitic; hepatoprotective; hypocholesterolemic; immunomodulatory; insectifuge; metastatic; nematicide [5]. Solanum nigrum oil is reported to have linoleic acid as the most abundant unsaturated fatty acid found in it [6,7].
Eclipta alba is a small branched perennial herbaceous plant belonging to the family Asteraceae. This plant has a huge number of medicinal properties. Eclipta alba (Bhringaraj) oil is a famous hair tonic for maintaining dark hair and reversing baldness. It is often translated as ‘king of the hair’. It is decocted in coconut oil and as this is a ‘cooling’ oil it is used externally for ‘hot’ and inflammatory head problems such as headaches, sinusitis and ear infections. The herb also benefits heat problems [8]. β-sitosterol is one of several phytosterols. It is reported to have a number of useful physiological activities including 5 α-reductase reductaseinhibitor; androgenic; anorectic; antiadenomic; antiandrogenic; antibacterial; anticancer; antiestrogenic; antiedemic; antifeedant; antifertility; antigonadotropic; antiinflammatory; antileukemic; antilymphomic; antimutagenic [5]. Eclipta alba is reported to have β-sitosterol as the most abundant phytoconstituent [9].
Hibiscus rosa-sinens is a medicinal plant member belonging to the family Malvaceae. Hibiscus rosa-sinens is is used for the treatment of various diseases including alopecia [10,11,12]. Traditionally, apart from this it is also used for anti-dandruff agent [13]. Quercetin is a flavonoid found in H. rosa-sinensis flower. In flowers (per g fresh tissues) included quercetin 7 mg [14]. It can be used as an ingredient in supplements, beverages, or foods. Quercetin supplements have been used for prevention and treatment of cancer. It is a potent 5α-reductase inhibitor [15]. Moreover it is useful for hair growth promoting activity, by stimulating hair follicles and improves the blood flow of the hair [16,17]. Positive results were obtained from study done on mouse for evaluation of hair growth promoting activity [18].
Quercetin, β-sitosterol and linoleic acid can be selected as bioactive markers for quantification of Hibiscus rosa sinensis flower, Eclipta alba whole plant and Solanum nigrum plant berries extract respectively. Standardization of Solanum nigrum plant berries by selecting linoleic acid as active biomarker has already being reported [19]. Simultaneous quantification of the three aforesaid biomarkers in a polyherbal gel has not being reported earlier.
Materials and Methods
Materials
Instrumentation and reagents: CAMAG HPTLC system consisting of WINCATS software, LINOMAT V automatic sample applicator, automatic development chamber, scanning densitometer CAMAG scanner 3 and photo documentation apparatus CAMAG Reprostar3 were used. Stationary phase used was aluminum based silica gel plate 60 F254 (Merck, Mumbai) with 20 cm × 10 cm in a particle size of 5-10 μm. 100 μL syringe (HAMILTON, Switzerland) was used for sample application on HPTLC plates. pH meter, Brookfield viscometer were also used for measuring the physical parameters of gel formulation.
All the solvents used were of analytical grade. β-sitosterol and Quercetin was purchased from Sigma Aldrich. Linoleic acid was purchased from Sisco Research Laboratories (SRL). Methanol, toluene, ethyl acetate of analytical grade was purchased from Merck (Mumbai, India). Whatman’s syringe filter (NYL 0.45 μm) was used for the filtration of samples. The plant material was collected from local area of Kolkata, West Bengal, India and the identity was confirmed by taxonomist. The shade dried powdered berries of Hibiscus rosa- sinensis, Solanum nigrum were extracted with methanol by cold maceration. The shade dried powdered berries of Eclipta alba were extracted with petroleum ether extract by cold maceration. The extract solution was dried under reduced pressure with the help of rotary evaporator. Carbopol 940, propylene glycol, polyvinyl pyrrolidine, DMDM hydration, sodium benzoate, triethanolamine, EDTA disodium, igsurf 1540 were gifted by Drakt International Pvt. Ltd., Kolkata.
Methods
The ranges of concentration of herbal extracts used in formulation are based on literature survey and closely related to or amount kept above label claims from established marketed herbal formulations such Alova Hibiscus Gel (Care Cosmetics), Anti Hair Fall Cream (Himalaya). Hibiscus rosa sinensis flower 1% w/w, Eclipta alba whole plant 1% w/w and Solanum nigrum plant berries 0.5% w/w extract were used as active herbal ingredients in the gel [4,9,12]. The detail composition being reported in Table 1.
| Steps | Ingredients | Quantity used on 100 g basis |
|---|---|---|
| Mixture I | Carbopol 940 | 1.0 g |
| EDTA Disodium | 0.5 g | |
| Purified water | 40.0 g | |
| Mixture II | Hibiscus rosa sinensisflower methanolic extract | 1.0 g |
| Eclipta albapet. Ether extract | 1.0 g | |
| Solanum nigrumberries methanolic extract | 0.5 g | |
| DMDM Hydration | 0.3 g | |
| Sodium benzoate | 0.3 g | |
| Propylene glycol | 15 g | |
| Igsurf 1540 | 0.5 g | |
| Kollidon 30 | 0.2 g | |
| Purified water | 38 g | |
| For pH adjustment | Triethanolamine | Q.S. |
Table 1: Composition of herbal hair growth gel.
Manufacturing method
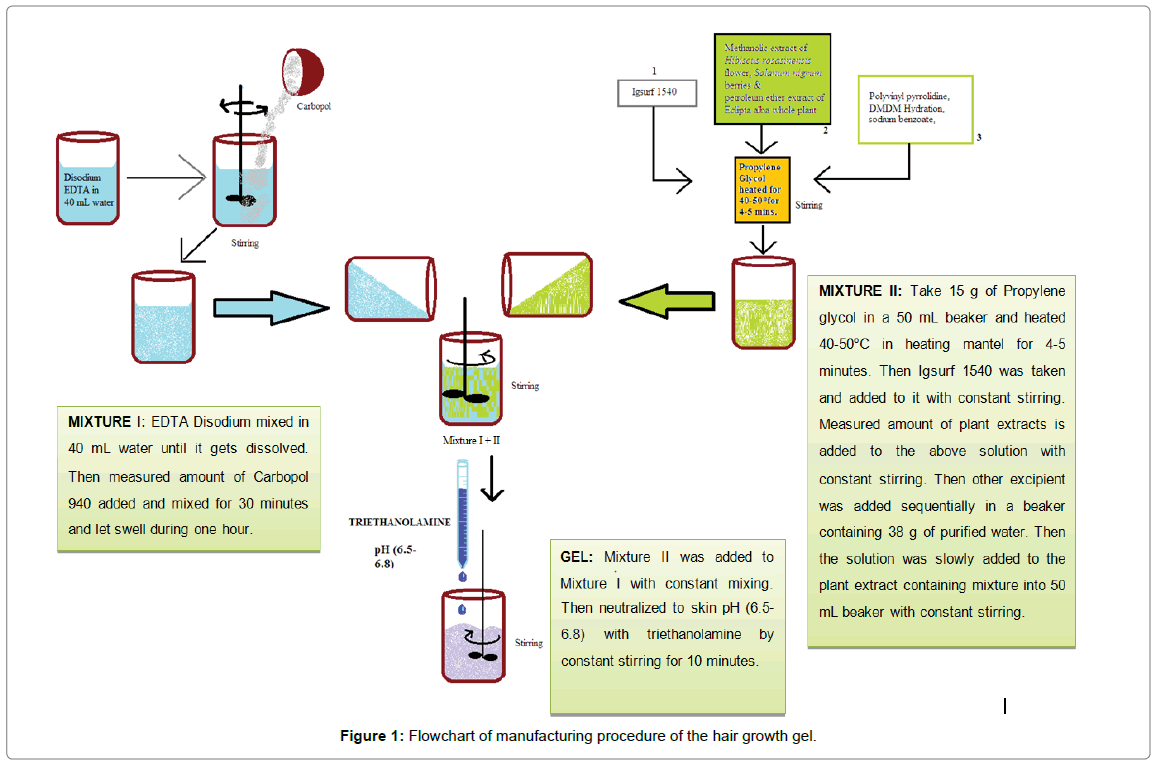
The gel was formulated in three steps, mixture I and II were prepared as given below and finally the aforesaid two were mixed to get the final gel. Flowchart of the manufacturing procedure is given in Figure 1.
Mixture I: 0.5 g EDTA disodium was dissolved in 40 mL water. Then measured amount of carbopol 940 (gelling agent) was added and mixed for 30 minutes and allowed to swell for an hour.
Mixture II: 15 g of propylene glycol (cosolvent) was taken in a 50 mL beaker and heated at 40-50°C in heating mantel for 4-5 minutes. Then igsurf 1540 (non-ionic solubilizer and emulsifying agent) was added to it with constant stirring. Measured amount of Hibiscus rosasinensis flower methanolic extract, Eclipta alba whole plant petroleum ether extract, Solanum nigrum berries methanolic extract were added to the above mixture with constant stirring. Polyvinyl pyrrolidine (binder or adhesive), DMDM hydantoin, sodium benzoate (preservatives) was added sequentially in a beaker containing 38 g of purified water. Then the solution was slowly added to the plant extract containing mixture into 50 mL beaker with constant stirring.
Gel: Mixture II was added to Mixture I with constant mixing. The final mixture so obtained was subjected to pH adjustment to skin pH (6.5-6.8) by slow addition of triethanolamine and constant stirring for 10 minutes. A brown coloured gel was obtained as shown in Figure 2.
Evaluation of hair gel formulation
pH: The pH of the gel formulation was determined by using digital pH meter. One gram of gel was taken and dissolved in 100 mL distilled water and measurement of pH was done in triplicate and average value was calculated.
Viscosity: Brookfield viscometer was used for the measurement of viscosity of the prepared gel. The Brookfield viscometer was rotated at 100 rpm, spindle no.6. Each reading was taken after equilibrium was attained by the sample at the end of two minutes. The study was repeated three times and average value is given in Table 2.
| Parameters | Results |
|---|---|
| pH | 6.68 |
| Viscosity | 4731 cps |
| Drug content by HPTLC | β-sitosterol, quercetin and linoleic acid was found to be 0.1377, 0.120 and 0.379% w/w respectively. |
| Physical appearance | Brownish yellow colour, odourless gel found. |
| Homogeneity | Homogeneous, smooth and consistent. No aggregates found. |
| Skin irritation test | Skin compatible, no irritation found. |
| Spredibility | 11.05 (g-cm/sec) |
Table 2: Evaluation parameters of hair gel formulations.
Spreadability: It was determined by wooden block and glass slide apparatus. Weights of about 20 g was added to the pan and the time was noted for upper slide (movable) to separate completely from the fixed slides. Spreadability was then calculated by using the formula,
S=M.L/T
Where, S=Spreadability, M=Weight tide to upper slide, L=Length of glass slide, T=Time taken to separate the slide completely from each other. The therapeutic efficacy of a formulation also depends upon its value.
Physical appearance: The gel formulation was evaluated in terms of physical character like phase separation and change in colour, odour and rheological parameters.
Homogeneity: Developed gel was tested for homogeneity by visual inspection after the gel was set in the container. It was tested for appearance and presence of any aggregates.
Skin irritation test: Test for irritation was performed on human volunteers with their consent. Five volunteers were selected and 1.0 g of formulated gel was applied on an area of 2 square inch to the back of hand. The volunteers were observed for lesions or irritation.
Drug content determination, standardization of herbal gel by HPTLC
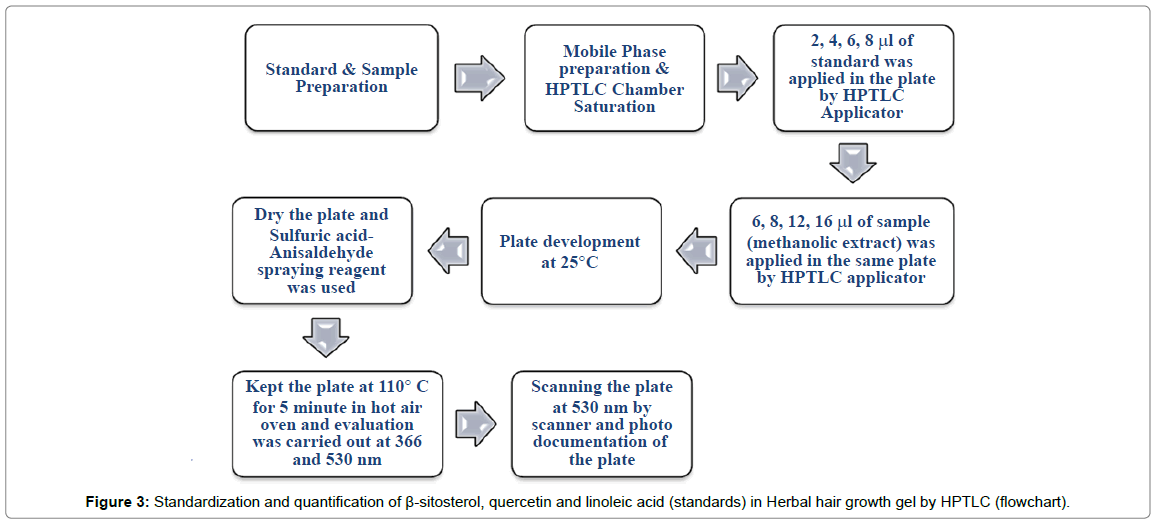
In this method 1 g of prepared gel was mixed with 10 mL of solvent suitable for extracting the biomarker. Then HPTLC was carried out. Drug content was calculated by the equation obtained by calibration curve. The flowchart of the procedure is depicted in Figure 3 and described below.
Preparation of standard solution: About 1 mg of β-sitosterol, linoleic acid, quercetin standard was weighed and put into separate 1 mL eppendorf tubes. 1.0 mL of methanol was added in each aforesaid 1 mL eppendorf tube and content was mixed in vortex mixture till the materials got completely dissolved.
Preparation of sample solution: About 1 g of gel formulation was weighed and taken in a centrifuge tube and 10 mL methanol was added to each. The sample so prepared ws subjected to mixing in vortex mixture for 15 minutes and then put to ultrasonication bath for 30 minutes. It was then filtered through 0.45 μ syringe filter and kept it in 1 mL eppendorf tube for further study.
Chromatographic conditions
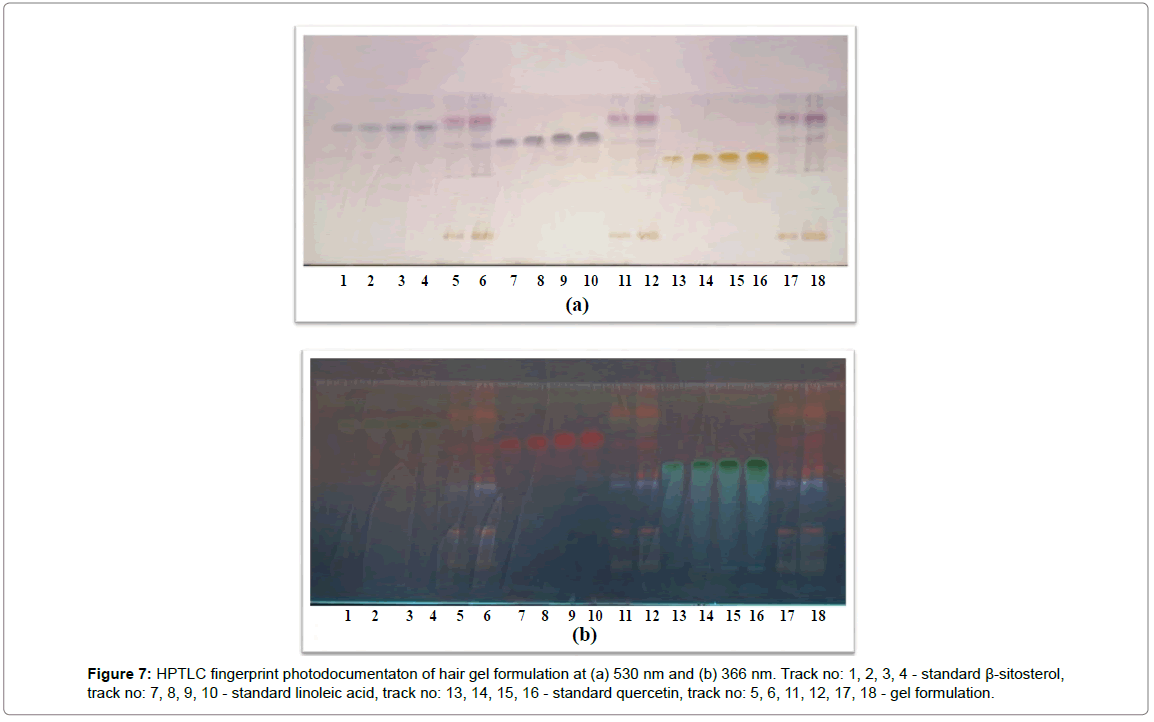
HPTLC analysis was performed using isocratic technique. Composition of mobile phase was toluene: ethyl acetate: methanol in a ratio of 5:3:2 v/v. The temperature was kept at 25°C and mobile phase was developed in a twin trough glass chamber. Standard stock solution of three biomarkers β-sitosterol, quercetin and linoleic acid was applied consequently in the range of 2-8 μL with 2 μL gradual increments. Sample solution was applied 6, 8, 12, 16 μL respectively. All total 18 tracks in HPTLC plate were used for standardization including standard and sample solution respectively in a band wise fashion. After development, plates were air dried. Colored bands were observed at 366 and 530 nm.
Results and Discussion
Evaluation
Reports of pH, viscosity, spreadability, physical appearance, homogeneity and skin irritation test are given in Table 2. It can be found from literature survey that pH mean value of female hair 6.784 ± 0.16, and that of male hair 5.604 ± 0.93 [20]. The pH of the formulated gel was found to be 6.68 which is close to the aforesaid values as well as skin pH. A good viscosity is required to have an acceptable formulation, too viscous gel may cause pourability problem whereas too low viscosity may cause settling of dispersed contents while storage. The gel was moderate viscous in appearance with 4731 cps viscosity. Application of a gel is comfortable if the base spreads easily, exhibiting maximum slip and drag. The prepared gel produces good spreadability 11.05 (g- cm/sec). In general, the gels that possess a high consistency index are less spreadable. The brownish yellow colour, odourless gel was found to be homogeneous, smooth, and consistent with no grittiness with compatible to skin compatible and no irritation found on application.
HPTLC analysis
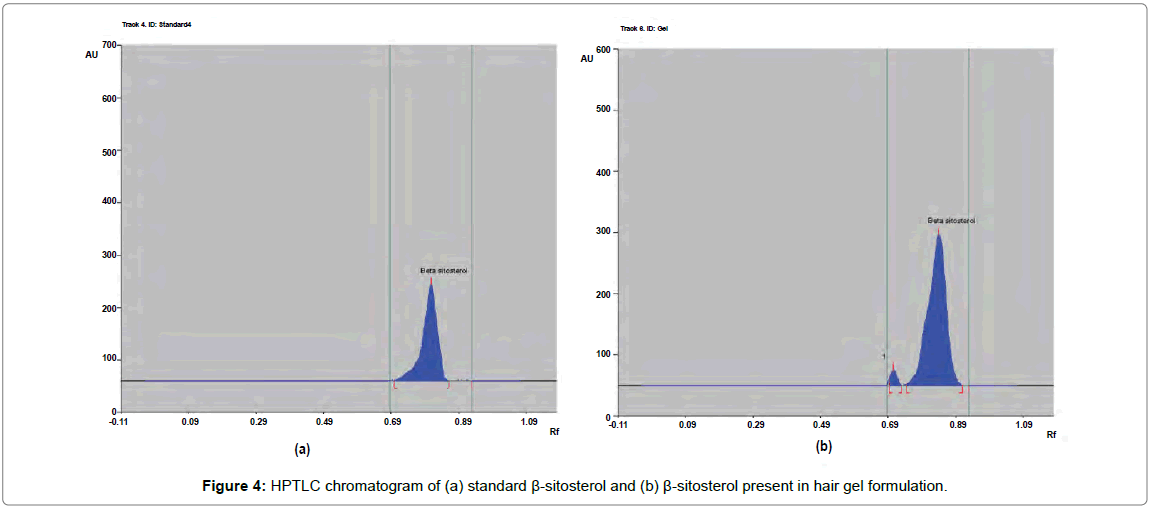
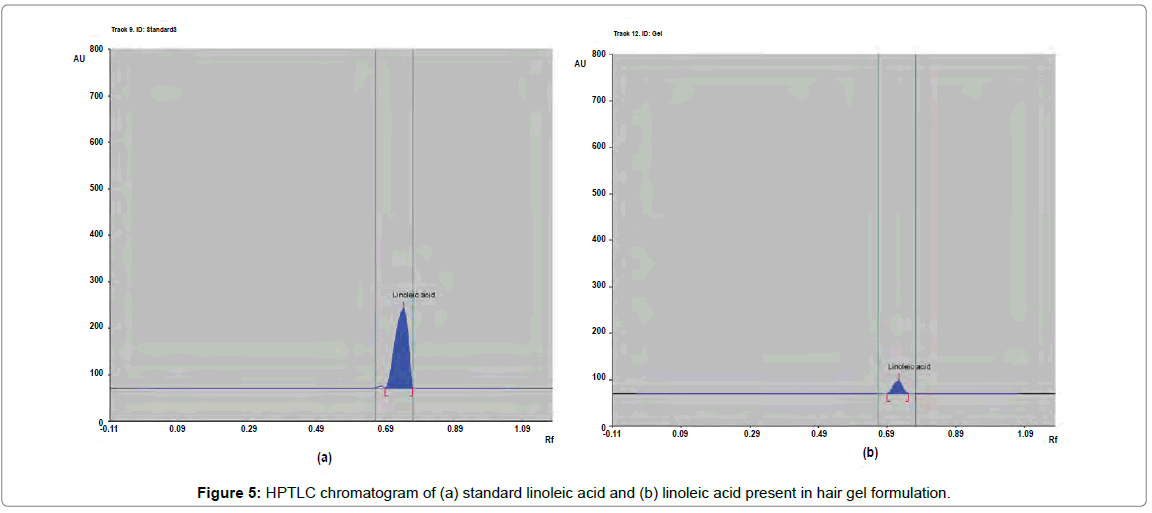
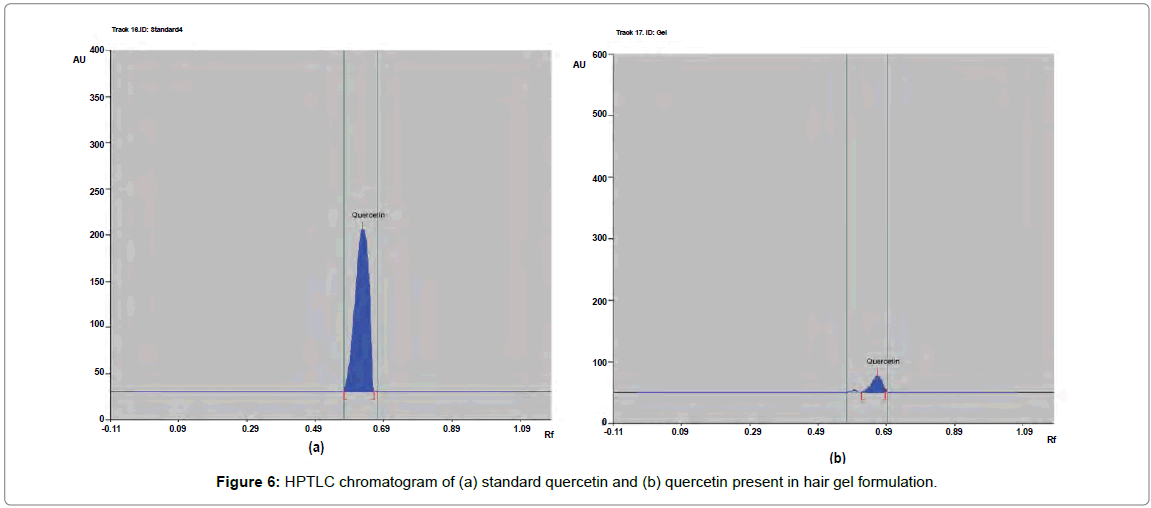
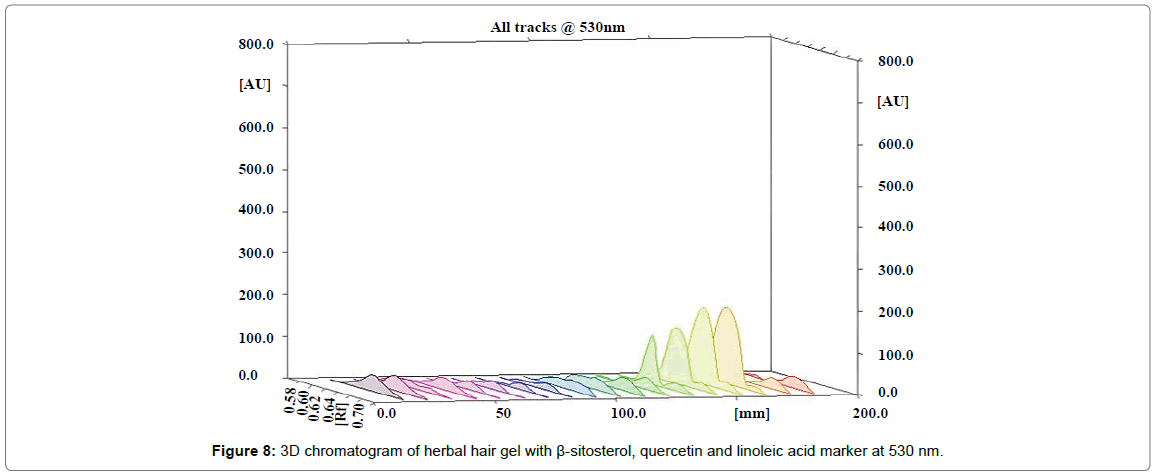
The percentage content of β-sitosterol, quercetin and linoleic acid in herbal hair gel methanolic extract was found to be 0.1377, 0.120 and 0.379% w/w respectively. Rf value of standard β-sitosterol, quercetin and linoleic acid was found to be 0.72, 0.49, 0.61 respectively. Specificity was confirmed by comparing the Rf of standard and sample (Figures 4-6). 3D chromatogram of herbal hair gel with β-sitosterol, quercetin and linoleic acid marker at 530 nm is provided in Figures 7 and 8. Literature survey reveals quantification of the standards by HPLC either as single entity or simultaneous detection with other moieties [21,22,23]. But simultaneous quantification of above three is mentioned nowhere. HPLC method may also be developed for this simultaneous quantification but HPTLC has advantages over HPLC in this aspect such as extreme flexibility for various steps i.e., selection of stationary phase, mobile phase, developing technique, detection with or without pre or post column derivatization. HPTLC is also technically simple and is of low cost.
Conclusion
In the present study, the gel was formulated successfully as well as the simultaneous quantification of the phytoconstituents by HPTLC technique resulted in a positive outcome. The topical application of this gel may have minimum side effects as compared to allopathic drugs which is needed to be established. Furthermore, the HPTLC method stated may be used for simultaneous quantification or standardization of β-sitosterol, linoleic acid and quercetin in other formulations too with necessary changes in the method as required.
References
- Raynaud JP, Cousse H, Martin PM (2002) Inhibition of type 1 and type 2, 5 alpha reductase activity by free fatty acids, active ingredients of Permixon (the lipido-sterolic extract of Serenoa repens). Journal of Steroid Biochemistry & Molecular Biology 82: 233-239.
- Roh ES, Kim CD, Lee MH, Hwang SL, Rang MJ, et al. (2002) The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. Journal of Dermatological Science 30: 43-49.
- Patel S, Sharma V, Chauhan SN, Thakur M, Dixit VK (2015) Hair Growth: Focus on Herbal Therapeutic Agent. Current drug discovery technologies 12: 21-42.
- Sudarshan SR (1985) Encyclopedia of Indian medicine. SKR Rao (ed.). Popular Prakashan 6: 171.
- Duke JA, Bogenschutz-Godwin MJ, Du-Cellier J, Duke PK (2000) CRC handbook of medicinal spices. CRC Press LLC, NW.
- Sarma H, Sarma A (2011) Solanum nigrum L, a nutraceutical enriched herb or invasive weed? International Conference on Environment and BioScience. IPCBEE 21: 105-109.
- Saleem TMS, Chetty CM, Ramkanth S, Alagusundaram M, Gnanaprakash K, et al. (2009) Solanum nigrum Linn. - A review. Pharmacognosy review 3: 342-345.
- Caldecott T, Tierra M (2006) Ayurveda: The divine science of life. Elsevier Health Sciences, pp: 177-178.
- Roy RK, Thakur M, Dixit VK (2008) Hair growth promoting activity of Eclipta alba in male albino rats. Archives of dermatological research 300: 357-364.
- Upadhyay S, Upadhyay P (2011) Hibiscus rosa-sinens is: pharmacological review. International Journal of Research in Pharmaceutical and Biomedical Sciences 2: 1449-1450.
- Upadhyay S, Upadhyay P, Vinode R, Dixit VK (2013) Effect of ethanolic fraction of Hibiscus rosa sinensis l., leaves in androgenic alopecia. Egyptian Dermatology Online Journal 9: 2.
- Adhirajan N, Kumar TR, Shanmugasundaram N, Babu M (2003) In vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinens is Linn. Journal of ethnopharmacology 88: 235-239.
- Vyjayanthi G, Kulkarni C, Abraham A, Kolhapure SA (2004) Evaluation of anti-dandruff activity and safety of polyherbal hair oil: An open pilot clinical trial. The Antiseptic 101: 368-372.
- Lim TK (2014) Edible Medicinal and Non Medicinal Plants. 1st edn. Springer, Netherlands 8: 309-310.
- Park Y, Kim Y, Han J (2014) Synthesis and Rat Prostate 5-Alpha Reductase Inhibitions of Methylated Quercetin Derivatives. KKU Res 19: 168-171.
- Brandon
- Thorat RM, Jadhav VM, Kadam VJ, Kamble SS, Salaskar KP (2009) Development of HPTLC method for estimation of Wedelolactone, Quercetin and Jatamansone in Polyherbal Formulation. International Journal of ChemTech Research 1: 1079-1086.
- Wikramanayake CT, Villasante AC, Mauro LM, Perez CI, Schachner LA, et al. (2012) Prevention and treatment of alopecia areata with quercetin in the C3H/HeJ mouse model. Cell Stress and Chaperones 1: 267-274.
- Chakraborty A, Bhattacharjee A, Dasgupta P, Manna D, Chun WOH, et al. (2016) Simple Method for Standardization and Quantification of Linoleic Acid in Solanum nigrum Berries by HPTLC. J Chromatogr Sep Tech 7: 342.
- Ping S, Xingquan Z (1993) Relationship between the elemental content and pH of human hair. Science of the total environment 128: 151-156.
- Ye JC, Chang WC, Hsieh DJY, Hsiao MW (2010) Extraction and analysis of β-sitosterol in Herbal Medicines. Journal of Medicinal Plants Research 4: 522-527.
- Ang LF, Yam MF, Fung YTT, Kiang PK, Darwin Y (2014) HPLC Method for Simultaneous Quantitative Detection of Quercetin and Curcuminoids in Traditional Chinese Medicines. Journal of Pharmacopuncture 17: 36-49.
- Tarola AM, Girelli AM, Lorusso S (2012) High performance liquid chromatography determination of fatty acids in drying oils following lipase action. Journal of Chromatographic Science 50: 294-300.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 9228
- [From(publication date):
December-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 8092
- PDF downloads : 1136