Hepatorenal Syndrome
Received: 22-Jun-2018 / Accepted Date: 30-Jun-2018 / Published Date: 05-Jul-2018 DOI: 10.4172/2161-069X.1000570
Keywords: Acute kidney injury; Cirrhosis; Pathophysiology; Biomarkers; Vasoconstrictors
Introduction
HRS is a life-threatening complication present in very advanced liver disease/cirrhosis or fulminant liver injury and represents an extreme circulatory dysfunction. It is characterized by an acute functional renal impairment with increased renal vasoconstriction, a reduced glomerular filtration rate (GFR) along with splanchnic arterial vasodilation [1]. The spectrum of etiologies for acute kidney injury (AKI) in cirrhosis includes 4 major causes: prerenal AKI (such as hypovolemia due to gastrointestinal (GI) bleeding, lactulose-induced diarrhea, diuretic overtreatment or infections); the HRS-type AKI (HRS-AKI); intrinsic causes such as acute tubular necrosis (ATN) and postrenal causes, which are the most uncommon ones [2]. The etiopathogenesis of renal vasoconstriction and the acute onset of kidney injury in chirrotics is complex and multifactorial with cardiac dysfunction also playing along [3].
HRS has an incidence of 10% in patients with cirrhosis and ascites without having a prevalence of sex. In patients with decompensated cirrhosis the probability per year of developing HRS is 8%-20% and 40% at 5 years. In end stage cirrhotics, from 30% to 40% will have HRS [4]. These numbers are probably not exact because HRS is likely to occur more commonly than expected, mostly because of the difficulties in assessing renal function in cirrhosis and the varying HRS diagnostic criteria [5].
HRS is a diagnosis of exclusion and its definition has been recently updated according to the AKI criteria [6]. In the past two decades, two different types of HRS have been distinguished. While HRS1 describes a rapidly progressive and marked loss of kidney function during a 2 weeks period in patients with advanced liver disease, HRS2 is defined as a moderated, steady renal failure in the setting of refractory ascites [7]. More recently the former HRS1 was reclassified as a special entity of AKI: the ‘HRS type of AKI’ (HRS-AKI); whereas HRS2 has been classified as a form of chronic kidney disease (CKD) in patients with cirrhosis, and is now referred as HRS-type of CKD (HRS-CKD) [2].
Treatment with vasoconstrictors and volume expansion is the current standard of care but it´s only effective in 40%-50% of patients [8]. Despite the recent pharmacological and other therapeutic innovations liver transplantation remains (LT) the only curative option and should be considered in all patients suitable for it.
Pathophysiology
The hallmark of HRS is a striking hemodynamic dysfunction that begins early in the course of the disease possibly even before ascites. This dysfunction evolves trough a pre-ascites stage (with progressive, marked renal cortical ischemia) to diuretic-sensitive ascites and diuretic-refractory ascites. This resumes a recognized continuum of renal dysfunction in cirrhosis [9].
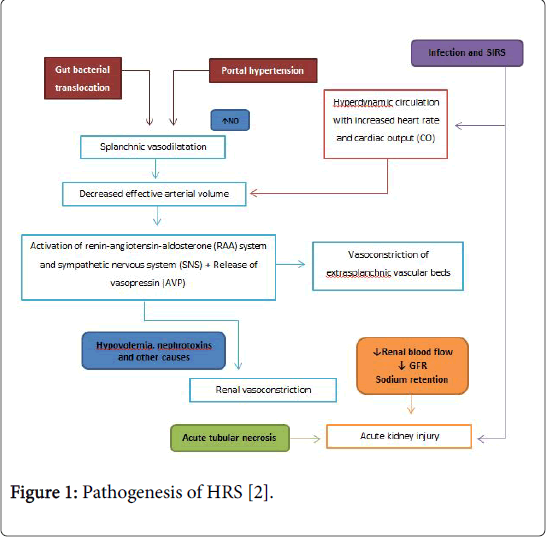
The hemodynamic pattern of patients with HRS involves several mechanisms which are characterized in Figure 1.
Figure 1: Pathogenesis of HRS [2].
The “classic vasodilation theory”
Marked increased arterial splanchnic vasodilatation, which is triggered by portal hypertension (PH), appears to be the most important patophysiological event in HRS. Hepatocytes and stellate cells produce several local acting vasodilators such as nitric oxide (NO), cannabinoids, substance P, calcitonin gene-related peptide (CGRP) among others which act on splanchnic circulation producing splanchnic arterial vasodilation [10]. L-Arginine (LArg) is the precursor for NO production by nitric oxide synthase (NOS). In patients with cirrhosis, hepatic clearance of amino acids, including LArg, is reduced, and this may help explain increased NO production due to increased levels of substrate. Systemic NO production occurs both due to up-regulation of endothelial NO synthase (eNOS) due to shear-stress as well as endotoxin-mediated eNOS activation. Furthermore increased inducible NOS (iNOS) activity has been recognized in these patients [11]. In cirrhosis both excessive (in systemic circulation) and insufficient (in renal vasculature) NO production leads to reduced renal blood flow (RBF).
Several studies found that elevated levels of dimethylarginines including ADMA and its isomer SDMA are associated with reduced NO production. ADMA is a natural inhibitor of NOS and is hydrolyzed by dimethylarginine dimethylaminohydrolase (DDAH). Because DDAH activity requires intact liver function [12] ADMA levels are elevated in advanced liver disease so inhibiting NOS, reducing NO synthesis and compromising RBF. In this scenery, plasma levels of SDMA are also increased due to compromised hepatic and renal clearance. Therefore it has been suggested that SDMA is a potential early marker of HRS [13]. Furthermore high ADMA levels in cirrhotics may be a prominent factor determining high intrahepatic vascular resistance and progression of the liver condition [14]. Splanchnic circulation represents an important part of the circulation of the body. Therefore, splanchnic vasodilation will decrease the mean arterial pressure (MAP), which triggers the activation of the sympathetic nervous system (SNS). As the disease progresses other vasoconstrictor systems get activated such as the renin-angiotensine angiotensinealdosterone system (RAAS) and vasopressin (AVP) release [15]. Aldosterone promotes retention of sodium and water by the kidneys contributing to the development of ascites. Vasopressin enhances retention of water which leads to hyponatremia. The splanchnic vascular bed is refractory to the action of all these vasoconstrictors because of the continuous production of local vasodilators. On the contrary other vascular beds such as femoral and brachial are sensible to these vasoconstrictor systems resulting in cramps and in the brain they may play a role in encephalopathy [16].
Other substances that may be involved in renal dysfunction include endothelin, adenosine and natriuretic hormones. Endothelin is a powerful vasoconstrictor and its plasma levels are increased in patients with HRS although its role in the pathogenesis of this syndrome has not yet been clarified. Adenosine is well known for its vasodilator function although it acts as a vasoconstrictor in the kidney. Elevated levels are more common in patients with heightened activity on the RAAS and may work in synergism with angiotensine II to induce renal vasoconstriction [17]. This effect as also been described with other potent vasoconstrictor, leukotriene E4 [18]. Vasodilators natriuretic hormones (atrial and brain natriuretic peptide) levels are elevated in patients with cirrhosis, act as counterbalance mechanisms but are overcome by vasoconstrictors [19,20]. Prostaglandins are also involved in renal dysfunction. They have protective effects in the kidney by compensating vasoconstriction but it’s known that some of their production is reduced in HRS. Loss of the medullary prostaglandin endoperoxide synthase may be the cause of diminished prostaglandin E2 production [21]. One of the main renal pronstaglandins (prostaglandin 12) is present in higher levels in cirrhosis and ascites. This possibly explains why the administration of non-steroidal antiinflammatory drugs (NSAIDs), which inhibit prostaglandin synthesis, frequently precipitates AKI in cirrhotics [22]. Additionally, it has been shown that non-selective betablockers can also trigger HRS-AKI due to their impact on the systemic circulation [2]. Due to its complexity, circulatory changes resulting from PH and kidney dysfunction cannot explain per se the HRS pathogenesis as well as the other phenotypes of AKI. Therefore new theories are emerging and challenging the vasodilatation theory.
HRS-AKI as part of a multiorgan failure syndrome/systemic inflammatory response syndrome (SIRS)-a new hypothesis
Decompensated cirrhosis leads to a systemic inflammatory state that enhances the already impaired circulatory function so it also plays an important role in the development of PH complications. This inflammatory state is characterized by an increase in serum levels of Creactive protein and proinflammatory cytokines such as tumor necrosis factor alpha and interleukin 6 [23,24]. Markers of oxidative stress such as oxidized albumin are also increased in HRS [25]. In cirrhosis, renal dysfunction is often triggered by bacterial infection. SIRS and sepsis apparently lead to renal blood flow (RBF) redistribution, resulting in ischemia and subsequent tubular injury [26]. Furthermore, special particles of the cell wall of gram-negative bacteria such as endotoxins or lipopolysaccharides (LPS) represent strong pro-inflammatory factors by inducing TNF-alpha, when linked to toll-like receptor 4 (TLR4) and this local inflammation also plays a role in kidney structural damage [27]. Several studies have showed that TLR4 is overexpressed in patients with cirrhosis and renal dysfunction [28,29] which helps supporting this inflammatory hypothesis.
Even in the absence of bacterial infection, cirrhosis is associated with a systemic inflammation state. The main mechanism is the translocation of bacteria and/or pathogen-associated molecular patterns (PAMPs) from the gut. Translocation induces a wide variety of genes that encode molecules responsible for inflammation via particular receptors (pattern recognition receptors-PRRs). These inflammatory molecules may migrate to the systemic circulation and organs leading to multiple organ dysfunction [30]. It is important to consider that most patients with HRS have SIRS and/or bacterial infection. However, about 30% of patients with HRS have SIRS without documented bacterial infection [31].
Role of cardiac dysfunction
Increased heart rate and cardiac output seen in cirrhosis initially compensates for the decline in effective circulating volume, but as hepatic function deteriorates, this mechanism ultimately fails resulting in cirrhotic cardiomyopathy. This condition is characterized by a blunted systolic and diastolic contractile response in addition to ventricular hypertrophy [32,33]. With this in mind, new studies are recommended in assessing the role of inotropes in HRS as cardiac dysfunction may be a key factor in its pathogenesis [34].
Role of adrenal insufficiency (AI)
Normal adrenal function is required for an appropriate response of arterial circulation to vasoconstrictive substances. The prevalence of AI in patients with advanced stage cirrhosis and severe sepsis is over 60%. AI may also develop in non-critically ill cirrhotic [35]. A study performed by Acevedo et al. 143 non-critically cirrhosis patients admitted for acute decompensation. AI was defined as a serum total cortisol increase <9 μg/dL after 250 μg of intravenous corticotropin from basal values <35 μg/dL. Patients were followed for 3 months. AI was detected in 26% of patients (n=37) so it’s frequent in non-critically ill patients with acute decompensated cirrhosis. During follow-up, patients with AI exhibited a higher probability of infection, severe sepsis, HRS1 and death.
Also Jang et al. [36] showed in a prospective study with 71 noncritically ill patients with liver cirrhosis (n=54) and chronic hepatitis (n=17) that AI was observed in 24.1% of patients with cirrhosis. The cortisol response to corticotropin was negatively correlated with the severity of cirrhosis (P<0.05). In addition, the mortality rate was higher in cirrhotic patients with RAI (69.2%) than in those without AI (4.9%; P<0.001) during the follow-up period of 20.1 ± 13.5 months (range, 5.8-51.1 months). The cumulative 1 year survival rates in cirrhotic patients with and without AI were 69.2% and 95.0%, respectively (P=0.05), while the corresponding cumulative 3-year survival rates were 0% and 95.0% (P<0.001). Concerning pathophysiology, the presence of AI with impaired cortisol production may play a role in HRS by endorsing the circulatory dysfunction. The mechanisms behind adrenal dysfunction in cirrhosis are possibly due to a reduced adrenal blood flow in result of local vasoconstriction.
Structural Changes in HRS-AKI
In patients with cirrhosis, several structural renal changes are frequently combined in a subgroup of patients. Glomerular, tubulointerstitial and vascular abnormalities were reported in patients with end stage liver diseases and the absence of significant proteinuria and haematuria did not rule out the presence of these lesions [37]. An important differential diagnosis for HRS-AKI is ATN. Next to prerenal azotemia, ATN is the most common cause of AKI in cirrhosis. ATN is mainly caused by ischemia following a hypotensive event, such as GI hemorrhage or sepsis. ATN clinical presentation is often very similar to HRS and biomarkers are frequently unable to accurately discriminate between these entities, especially in cirrhosis [38].
Definitions and Diagnosis
The criteria for HRS diagnosis were first given in 1996 by the International Club of Ascites (ICA) [39]. Since that time several updates have been made. One of the main changes on the new criteria was the removal of a rigid very high cut-off value of serum creatinine (sCr) (1.5 mg/dL) to define HRS. This alteration improves patient prognosis by facilitating earlier diagnosis and treatment. In addition, the use of urinary output as part of the diagnostic criteria was also eliminated, since many patients with cirrhosis maintain a conserved renal function despite being oliguric [40]. ICA defines AKI as the increase in sCr of at least 0.3 mg/dL and/or ≥ 50% from baseline, within 48 hour. AKI can also be categorized in three stages according to severity (Table 1). Nowadays HRS-AKI is defined as ≥ stage 2 ICAAKI that is diagnosed after other causes of renal failure have been excluded [6,41]. The appropriate diagnosis further requires the fulfillment of several specific criteria that are summarized in Table 2.
| AKI stages according to ICA criteria | |
|---|---|
| ICA-AKI Stage 1 | Increase in serum creatinine 0.3 mg/dl or Increase in serum creatinine by 50-100% from baseline |
| ICA-AKI Stage 2 | Increase in serum creatinine by 100-200% from baseline |
| ICA-AKI Stage 3 | Increase in serum creatinine by 200% from baseline or Increase in serum creatinine to 4 mg/dL with an acute increase by 0.3 mg/dL or need for renal replacement therapy |
Table 1: AKI stages according to ICA criteria.
| ICA-AKI criteria for the diagnosis of hepatorenal syndrome [6]: |
|---|
| 1. Diagnosis of ascites and cirrhosis. |
| 2. Diagnosis of AKI according to ICA-AKI criteria. |
| 3. No response after two consecutive days of diuretic withdrawal and plasma volume expansion with albumin (1 g/kg of body weight). |
| 4. Absence of shock. |
| 5. No current or recent use of nephrotoxic drugs (non-steroidal anti-inflammatory drugs - AINEs, aminoglycosides, iodinated contrast media, etc.). |
| 6. No macroscopic signs of structural kidney injury such as absence of proteinuria (>500 mg/d), absence of microhematuria (>50 red blood cells per high power field) and normal findings on renal ultrasound. |
| *Patients who fulfil these criteria may still have structural damage such as tubular damage. Urine biomarkers will become an important element in making a more accurate differential diagnosis between HRS and acute tubular necrosis, ICA. |
Table 2: AKI criteria for the diagnosis of hepatorenal syndrome.
From a clinical angle, HRS-AKI is characterized by a rapid increase in sCR and progressive oliguria in the absence of other recognizable causes of AKI [42]. Most forms of prerenal AKI improve with withdrawal of diuretics and plasma expansion using i.v. albumin. On the contrary renal function in HRS-AKI does not [43]. HRS2 is defined as a stable or slowly progressive impairment in renal function in patients with decompensated liver disease who suffer from refractory ascites [44]. Patients usually develop oliguria over a course of several weeks or months with a slow but steady rise in renal retention markers [45]. Apart from the evolution period, the same specific diagnostic criteria for HRS-AKI also apply for HRS-CKD [42]. However, HRSCKD is challenging to diagnose in clinical practice, as it is a diagnosis of exclusion since there are other potential causes for kidney insufficiency in cirrhosis. Some challenges in precisely assessing renal function in patients with cirrhosis remain due to the limitations of sCr alone in estimating GFR. Current models appear to overestimate renal function [46]. Newer ones incorporating renal biomarkers such as cystatin C appear to more accurately estimate GFR promising the possibility of earlier identification of renal dysfunction in patients with cirrhosis [5]. As seen above HRS diagnosis is based on clinical and laboratorial principles. However a clinical diagnosis does not exclude associated structural changes, in particular ATN. There is still no goldstandard for this differential diagnosis. In addiction this clinical entities may coexist or even represent a continuum in some patients [47].
Biomarkers
Several recent studies have shown that numerous biomarkers including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), liver-type fatty acid binding protein (L-FABP), endothelin-1 (ET-1), adrenomedullin, uromodulin (UMOD), epidermal growth factor (EGF), kidney injury molecule-1 (KIM-1), TLR4, π and α-glutathione S-transferase and other urine markers such as albuminuria and fractional excretion of sodium (FeNa) may be helpful in HRS differential diagnosis [48]. Some of these such as NGAL, KIM-1 and IL-18 may diagnose AKI earlier, differentiate ATN from HRS, and also help predict outcomes [22,48,49]. NGAL is a small protein produced by numerous organs including kidney, stomach, colon and lung. In animal models, NGAL kidneys expression and urinary secretion is significantly increased following nephrotoxic or ischemic insults. Urinary concentration increases very quickly (within 2 hour) after ischemia [50]. Human studies have shown that NGAL levels (either urine or serum) might be helpful to detect early stage AKI in numerous clinical situations (sepsis, polytrauma, cardiac surgery and hypothermia) [22]. Recent studies suggested that NGAL may help identify AKI etiology in patients with liver disease, especially in differentiating ATN from HRS [51]. NGAL was found to be significantly higher in those with ATN when compared to HRS-AKI, prerenal azotemia or CKD [52]. Further studies are needed to establish exact cut-offs to differentiate this conditions [53]. Additionally, two studies indicate that high urinary NGAL is predictive of early mortality in cirrhotics with AKI [54,55]. Therefore NGAL appears to be one of the most useful markers. However, initial enthusiasm has been tempered by its clinical use limitations as it levels also increase in other conditions (infections and other inflammatory states) [56].
Albuminuria, a marker of glomerular injury, is also significantly higher in ATN in comparison with HRS cases. Precise cutoff values are still needed and more studies should be made to better understand the interactions among albuminuria, liver dysfunction and glomerular changes in cirrhosis [57]. FeNa is reduced in HRS. Classic teaching has used FeNa cut-off of <1% or >1% to differentiate between HRS and intrinsic renal dysfunctions as in ATN. In a recent retrospective study the performance of FeNa <1% in diagnosing HRS was overall poor. Nevertheless the test had high sensitivity and high negative predictive value (both 100%), indicating that in patients with negative test results (i.e., FeNa >1%) HRS diagnosis could be excluded [58]. Adrenomedullin is a potent vasodilator peptide able to increase both NO and cGMP production. In advanced cirrhotic patients with HRS, adrenomedullin is significantly increased and contributes to vascular dysfunction [59]. Thromboxane A2 (TXA2) is a potent renal vasoconstrictor synthesized also by the kidney. It seems that the urinary excretion of TXB2, a stable metabolite of TXA2, is increased in cirrhotic patients with HRS or ascites. High urinary TXB2 seem to act as an independent predictor for HRS in decompensated cirrhosis. In a prospective cohort study adrenomedullin and urinary TXB2 levels appeared to be better predictors for early diagnosis of HRS in acute decompensated cirrhosis with AKI when compared to urinary NGAL and IL-18 [59].
It is known that HRS prevalence in patients with advanced liver diseases differs among patients. Cirrhotic patients with the same liver function level would not all have HRS as complication, so it can be inferred that gene alterations may constitute a risk factor for HRS. As referred on pathophysiology, vasopressin plays a role in renal vasoconstriction. Arginine vasopressin receptor 1a (AVPR1A) antagonists can block the vasoconstrictor effect. It has been found that AVPR1A gene promoter-6951 locus' single nucleotide polymorphisms (SNPs) in patients with primary hypertension are closely associated with a vasoconstrictor effect. A case control study showed that a polymorphism (T allele located at AVPR1A receptor promoter rs113481894 locus) may also be associated with HRS1 [60]. Other polymorphisms that can also be considered as risk factors are the ones occurring in NOS. As explained in pathophysiology iNOS and eNOS function is enhanced in splanchnic vasculature. On the contrary eNOS function is compromised in renal vasculature. In a prospective study it was demonstrated that the eNOS G894T homozygote mutant (TT) and heterozygote (GT) genotypes are associated with significantly increased risk of developing HRS in cirrhotic patients by declining kidney NO production [61].
Precipitating Factors and Prevention
HRS typically occurs after a precipitating factor, although it can also occur in the absence of identifiable triggers. HRS can develop in the setting of infection, mainly after spontaneous bacterial peritonitis (SBP), circulatory dysfunction after large volume paracentesis (LVP) in the absence of volume expanders, variceal bleeding, over diuresis or cholestasis [62]. SBP is an infection of the ascitic fluid due to bacterial translocation. This condition stimulates the release of inflammatory cytokines and vasodilators which play an important role in splanchnic vasodilatation. Clinical data supporting this hypothesis are that antibiotic use such as fluoroquinolones helps prevent HRS [63]. Three major interventions appear to reduce HRS incidence in cirrhotic patients. First, albumin administration (7-8 g per liter of fluid removed) during large-volume paracentesis (>5 L) prevents the precipitation of HRS after the procedure. Second, the administration of albumin (1.5 g/kg of body weight on diagnosis and 1.0 g/kg 48 hours later) in patients admitted with SBP reduces the incidence of HRS [64]. And finally, antibiotics given to selected patients with cirrhosis, PH, and low total protein in ascitic fluid reduces the incidences of both SBP and HRS [65].
Prognosis
Patients with HRS have an overall poor prognosis. The vasoconstrictive therapy has only allowed moderate improvement in overall survival rates and currently serves as bridge to LT. Several studies show a 2 week mortality rate as high as 80% in untreated HRS1 patients and 3 month survival in 10% of these cases. The prognosis is particularly poor in patients with potential precipitating factors. HRS2 has a lengthened survival as kidney dysfunction evolves more gradually. However, it is not that extensive with survival rates being somewhere between 3 and 6 months [16].
Treatment
Various HRS treatment regimens are available worldwide and their goal is to increase mean arterial pressure (MAP) by increasing the central blood volume and decreasing splanchnic vasodilation, serving as temporary modalities as bridge to liver transplant (LT) [66]. Evaluation for need of LT should be considered at the earliest. General measures (ideally in an intensive care or semi-intensive care unit in HRS1) include careful monitoring of blood pressure and urine output, daily liver and renal function tests, assessment for the presence of bacterial infection (including ascitic, urine and blood cultures), GI bleeding and hepatic encephalopathy [67]. A cautious review of all medications (e.g. NSAIDs need to be withdrawn) and the use of drugs that may induce or exacerbate arterial hypotension (e.g. beta-blockers or vasodilators) should be carefully assessed. In volume depletion conditions, lactulose and/or diuretics should be withdrawn and plasma volume should be expanded with albumin, or with blood transfusions in anemic patients due to GI bleeding [2]. Early mobilization and appropriate nutrition should also be concerned. Systemic vasoconstrictors have been established as first line therapy in both HRS1 and 2. The different vasoconstrictive treatment approaches are listed on Table 3 [68]. Dopamine given in low doses increases RBF but has no effects on GFR or HRS outcome. Therefore it’s not considered an appropriate treatment for this condition [69,70].
| Vasoconstrictors | Mechanism of action and final effect | Dose and administration | Time duration | Comments |
|---|---|---|---|---|
| Vasopressin | Vasopressin receptors (V1) on vascular smooth muscle cells including splanchnic vascular beds | Severe ischemic complications. No longer in use. | ||
| Terlipressin | Vasopressin analogue on V1 receptors. Increases vascular resistance in splanchnic circulation and redistributes blood flow to the kidneys | 0.5 - 2 mg every 4-6 hour or 2 - 12 mg IV every day in continuous infusion. | Up to 24 hour until sCr is <1.5 mg/dL or for a maximum of 15 days | Adverse effects include abdominal cramps, diarrhea, ischemia and arrhythmias. Several studies show it´s higher efficacy when administered in continuous infusion. |
| Octreotide | Somatostatin analogue (inhibits glucagon release) and increases vascular resistance in splanchnic circulation | 25-50 mcg/h maximal treatment for 15 days in continuous infusion; 100-200 mcg/8h subcutaneously. | Up to 24 hour until sCr is < 1.5 mg/dL or for a maximum of 15 days | Adverse effects include abdominal or stomach pain, blurred vision, constipation and depressed mood |
| Midrodine (Midrodrine and Octeotride may be used in association) | Alpha adrenergic agonist (alpha 1 receptors on smooth muscle cells including splanchnic vascular beds). Increases arterial pressure, induces RAAS system leading to renal volume and sodium excretion. | 7.5 mg to 15 mg every 8 hour. Oral administration. | Up to 24 hour until sCr is <1.5 mg/dL or for a maximum of 15 days | Adverse effects include blurred vision, cardiac awareness, headache and pounding in the ears. |
| Norepinephrine | Alpha adrenergic agonist. | 0.5 - 3.0 mg/h in continuous infusion. | Up to 24 hour until sCr is <1.5 mg/dL or for a maximum of 15 days | Used in countries where terlipressin is not available. Requires an ICU. More cost effective and with less side effects than terlipressin. |
Table 3: Vasoconstrictor therapy in HRS [68], all these compounds are used in association with an albumin infusion at the dosage of 1 g/kg of body weight on the first day, followed by 200-400 g daily.
Serelaxin is a recombinant form of the human peptide hormone relaxin-2 and it increases RBF. Its function has been explored in a pilot study on compensated cirrhotic patients: it increases renal perfusion but has no effect on systemic blood pressure. Data on this hormone is still scarce and new studies are necessary for better understand its role in treating HRS [71].
HRS-AKI (HRS1)
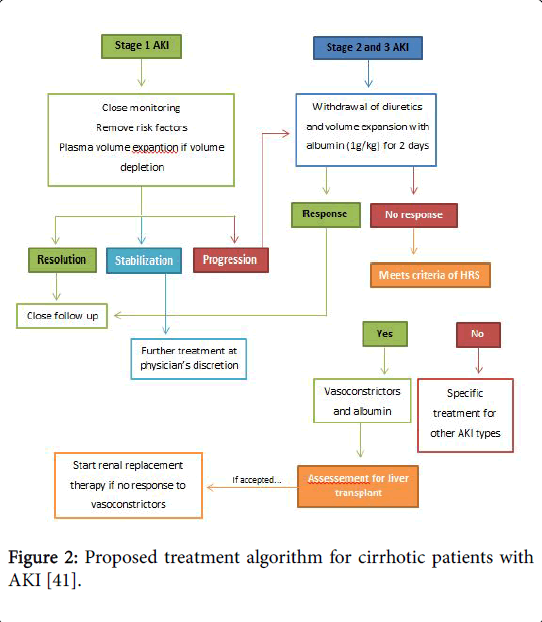
The suggested treatment algorithm for cirrhotic patients with AKI is present In Figure 2 [41]. As it has been said, concerning HRS, the initial management must identify and correct potential trigger events to prevent further hemodynamic decline.
Figure 2: Proposed treatment algorithm for cirrhotic patients with AKI [41].
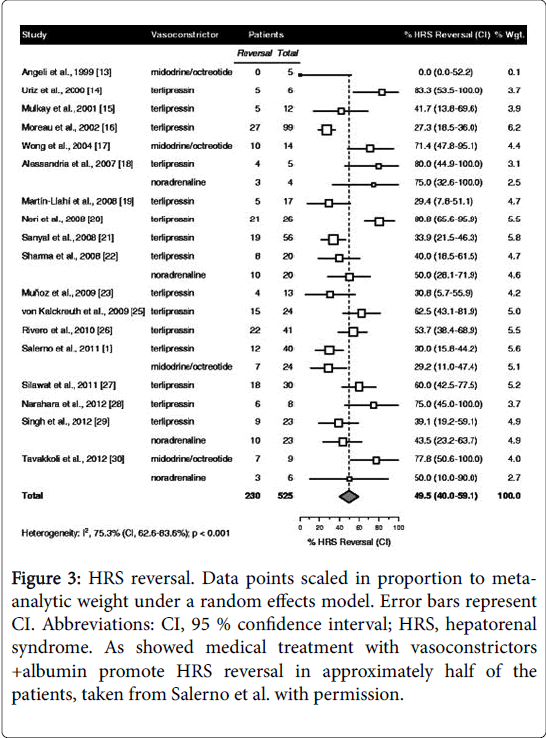
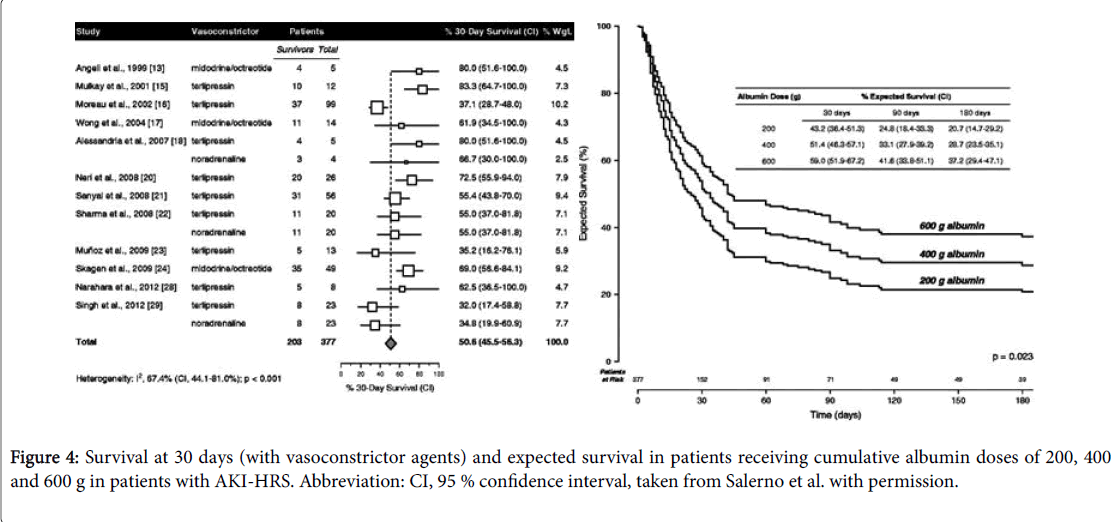
Patients should be carefully screened to rule in/out SBP or sepsis. Early empiric antibiotic treatment should be initiated if clinical suspicion and based on local epidemiology and resistance patterns [72]. Albumin is mainly beneficial in these patients, since it has antioxidant, scavenging and endothelial-stabilizing functions adding to its volume expansion effect [73]. Therapeutic response is defined as a decrease of sCr to a value within 0.3 mg/dL from baseline. If present, patients should be watchfully followed for early recognition of recurrent episodes of AKI. It is recommended to assess sCr levels every 2-4 days during hospitalization and every 2-4 weeks during the first 6 months after discharge [6]. In case of AKI progression to stage 2 or 3 patients should be assessed for the presence of HRS-AKI and diuretics immediately withdrawn. In addition, patients should receive vasoconstrictors and albumin for 2 consecutive days (1 g per kg of body weight, maximum 100 g/day). Hemodialysis may be necessary to correct electrolyte imbalance and azotemia, functioning as a bridge to LT [74]. Recently Salerno et al. [75] published the results of a metaanalysis including 19 studies and 574 patients with HRS-AKI treated with different doses of albumin and with vasoconstrictor agents (8 randomized controlled trials, 8 prospective studies and 3 retrospective studies) and the main results were: 49.5% of patients achieving HRS reversal (95% CI, 40.0-59.1%) (Figure 3); cumulative albumin dose (increments of 100 g) were related with significant increased survival (hazard ratio, 1.15; 95% CI, 1.02-1.31; p=0.023); expected survival rates at 30 days among patients receiving cumulative albumin doses of 200, 400 and 600 g were 43.2% (95% CI, 36.4-51.3%), 51.4% (95% confidence interval, 46.3-57.1%) and 59.0% (95% CI, 51.9-67.2), respectively.
Figure 3: HRS reversal. Data points scaled in proportion to metaanalytic weight under a random effects model. Error bars represent CI. Abbreviations: CI, 95 % confidence interval; HRS, hepatorenal syndrome. As showed medical treatment with vasoconstrictors +albumin promote HRS reversal in approximately half of the patients, taken from Salerno et al. with permission.
Neither survival nor HRS reversal was significantly affected by vasoconstrictor dose or type, treatment duration, age, baseline sCr, bilirubin or albumin, baseline MAP, or study design, size or time period. This study suggests a dose–response relation between infused albumin and survival in patients with HRS-AKI.
As can be seen on Figure 4, the previous study also revealed that survival rates and treatment outcomes significantly improved with albumin cumulative doses but without any influence from vasoconstrictor dose or type and treatment duration. On the basis of cumulative albumin doses, it is recommended to monitor central venous pressure with sonographic cava vein sign or catheterization, to avoid an excessive cardiac preload [76]. Additionally, it was also demonstrated a considerably greater efficiency of a treatment regimen based on terlipressin plus albumin in improving renal function [77]. There are still conflicting results on which is the best vasoconstrictor to combine with albumin in the management of HRS [78].
HRS-CKD (HRS2)
Patients with HRS2 can be managed in a non-intensive care setting. Diuretics should be withdrawal and albumin should be administered in all large volume paracenteses (>5 L, with 8 g/L of ascitic fluid removed), since it decreases the risk of renal malfunction, prevents post-paracentesis circulatory dysfunction and might even improve survival [79].
Other Non-Pharmacological Treatment Modalities
Transjugular intrahepatic portosystemic shunts (TIPS)
TIPS are effective in both HRS1 and 2 however its use is limited due to simultaneous presence of contraindications for its application (congestive heart failure, multiple hepatic cysts, sepsis, biliary obstruction and severe pulmonary hypertension) in a substantial proportion of patients. Heinzow HS et al. showed in his study that TIPS improve both renal function and survival in patients with severe/ refractory ascites [80]. It is important to have in mind that TIPS can increase the risk of hepatic encephalopathy as well as liver failure in some rare occasions [81].
Renal replacement therapy (RRT)
RRT is recommended in patients with volume overload, metabolic acidosis, hyperkalemia and at risk of elevated intracranial pressure [44]. It is mostly a consideration for patients who are candidates for LT and for patients who failed other therapies [82]. Extra-corporeal albumin dialysis using Molecular Absorbent Recirculating System (MARS) is a form of RRT and has improved survival compared to continuous RRT and intermittent hemodialysis [83]. However, this is not yet widely available and has been tried in a very small number of patients.
Recently Thorat and Jeng [82] published the results of a metaanalysis of nine different studies done in the last 2 decades that included 464 patients with end-stage liver disease and renal failure who received either pre-transplantation or post-transplantation continuous veno-venous hemodialysis (CVVHD). The results of this meta-analysis reveal the role of pretransplantation CVVHD in designated patients with HRS who could reach significantly higher survival rates compared with patients without any RRT or patients with post-transplantation CVVHD [84-86].
Liver transplantation (LT)
As LT remains the only definitive treatment for HRS, patients with HRS1 and no contraindications for LT should be invariably worked up and placed in a LT waiting list. As HRS is seen as a reversible condition, LT reverses both liver and kidney dysfunction [87]; it is important to refer that not all those who receive transplants achieve full resolution of kidney dysfunction and this can be due to a number of different factors such as age and duration of HRS [88]. After LT, HRS1 resolved in 47 of 62 patients (75.8%) at a mean time of 1362 days [89]. Patients without HRS reversal had significantly higher pretransplant sCr levels, a longer duration of pretransplant dialysis, a longer duration of HRS1 and increased posttransplant mortality in comparison with those with renal function recovery [89]. The only predictor of HRS1 nonreversal was the extent of pretransplant dialysis with a 6% bigger risk of nonreversal with each additional day of dialysis [88]. A recent study focusing on plasma biomarkers in liver transplant recipients has shown that increases in osteopontin and tissue inhibitor of metalloproteinase (TIMP-1) in pretransplantation patients are predictive of unlikely recovery of kidney function after LT [89].
Renal function is difficult to evaluate in HRS2 due to fluctuations in GFR and SCr. LT alone is usually recommended as small studies have shown reversal in the majority of patients [90,91]. Nonetheless, even if HRS reverses, more than 50-60% of patients develop stage 3 CKD on the first year after transplant, suggesting that sustained kidney ischemia caused permanent kidney changes [22].
Simultaneous liver-kidney (SLK)
SLK is the last therapeutic option. It remains controversial if it is better for HRS patients than isolated LT and there are still no standard guidelines for its appliance [92]. However In patients with AKI, it has been proposed criteria to implement SLK rather than LT alone based on a high likelihood of non-renal recovery after transplantation [93]. Either patients on dialysis or not, these criteria depend on the progression of AKI over a 4 weeks or more period. In these patients, increased baseline creatinine (before the onset of AKI), diabetes [89], advanced age, abnormal renal imaging [94], history of hypertension and proteinuria >2 g/day support for SLK. However, no exact cutoff has been confirmed yet 22. Patients on dialysis for at least 8-10 weeks are also suitable for SLK since they’ll likely develop ATN [95].
Future Directions
Further research focused on new diagnostic biomarkers and precise cutoff values is still needed but fortunately, there is increasing interest in this field. New data looking for novel treatments besides terlipressin and albumin are also crucial. Finally, identifying patients with low probability of responding to treatment is of major importance so that other alternative treatments can be early started as these patients potentially prioritize LT or SLT waiting lists.
Conclusion
HRS is a life threatening complication of liver cirrhosis with relatively grim prognosis and high morbidity and mortality rates associated. Its definition is constantly changing and is now based on clinical grounds which have many limitations. Therefore increased awareness, precise evaluation and early detection of renal dysfunction (new biomarkers) in patients with end stage liver disease is fundamental to allow optimization of the therapeutic algorithm and improve patient long-term outcomes.
Acknowledgments
The principle author gratefully acknowledges the cooperation and guidance from Prof. Manuel Vaz Silva.
References
- Wadei HM, Mai ML, Ahsan N, Gonwa TA (2006) Hepatorenal syndrome: Pathophysiology and management. Clin J Am Soc Nephrol 1: 1066-1079.
- Bucsics T, Krones E (2017) Renal dysfunction in cirrhosis: Acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep (Oxf) 5: 127-137.
- Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH et al. (1988) Peripheral arterial vasodilation hypothesis: A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 8: 1151-1157.
- Carvalho GC, Regis Cde A, Kalil JR (2012) Causes of renal failure in patients with decompensated cirrhosis and its impact in hospital mortality. Ann Hepatol 11: 90-95.
- Mindikoglu AL, Pappas SC (2017) New Developments in Hepatorenal Syndrome. Clin Gastroenterol Hepatol 16: 162-177.
- Angeli P, Ginès P, Wong F (2015) Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol 62: 968-974.
- Gines P, Schrier RW (2009) Renal failure in cirrhosis. N Engl J Med 361: 1279-1290.
- Arab JP, Claro JC, Arancibia JP (2016) Therapeutic alternatives for the treatment of type 1 hepatorenal syndrome: A Delphi technique-based consensus. World J Hepatol 8: 1075-1086.
- Mohindra S, Kumar K (2013) Hepatorenal syndrome. Clinical Queries: Nephrology 2: 205-211.
- Acevedo JG, Cramp ME (2017) Hepatorenal syndrome: Update on diagnosis and therapy. World J Hepatol 9: 293-299.
- Laffi, G, Foschi M, Masini E (1995) Increased production of nitric oxide by neutrophils and monocytes from cirrhotic patients with ascites and hyperdynamic circulation. Hepatology 22: 1666-1673.
- Nijveldt RJ, Teerlink T, van Leeuwen PA (2003) The asymmetrical dimethylarginine (ADMA)-multiple organ failure hypothesis. Clin Nutr 22: 99-104.
- Lluch, P, Mauricio MD, Vila JM (2006) Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp Biol Med (Maywood) 231: 70-75.
- Ferrigno A, Di Pasqua LG, Berardo C, Richelmi P, Vairetti M (2015) Liver plays a central role in asymmetric dimethylarginine-mediated organ injury. World J Gastroenterol 21: 5131-5137.
- Sola E, Gines P (2015) Challenges and management of liver cirrhosis: Pathophysiology of renal dysfunction in cirrhosis. Dig Dis 33: 534-538.
- Licata, A, Maida M, Bonaccorso A (2013) Clinical course and prognostic factors of hepatorenal syndrome: A retrospective single-center cohort study. World J Hepatol 5: 685-691.
- Gerbes AL, Gulberg V, Bilzer M (1998) Endothelin and other mediators in the pathophysiology of portal hypertension. Digestion 2: 8-10.
- Huber M, Kastner S, Scholmerich J, Gerok W, Keppler D (1989) Analysis of cysteinyl leukotrienes in human urine: Enhanced excretion in patients with liver cirrhosis and hepatorenal syndrome. Eur J Clin Invest 19: 53-60.
- La Villa G, Romanelli RG, Casini Raggi V (1992) Plasma levels of brain natriuretic peptide in patients with cirrhosis. Hepatology 16: 156-161.
- Gines P, Jimenez W, Arroyo V (1988) Atrial natriuretic factor in cirrhosis with ascites: Plasma levels, cardiac release and splanchnic extraction. Hepatology 8: 636-642.
- Govindarajan S, Nast CC, Smith WL (1987) Immunohistochemical distribution of renal prostaglandin endoperoxide synthase and prostacyclin synthase: Diminished endoperoxide synthase in the hepatorenal syndrome. Hepatology 7: 654-659.
- Francoz C, Nadim MK, Durand F (2016) Kidney biomarkers in cirrhosis. J Hepatol 65: 809-824.
- Cervoni JP, Thevenot T, Weil D (2012) C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol 56: 1299-1304.
- Mehta, G, Gustot T, Mookerjee RP (2014) Inflammation and portal hypertension: The undiscovered country. J Hepatol 61: 155-163.
- Jalan R, Schnurr K, Mookerjee RP (2009) Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 50: 555-564.
- Prowle JR, Bellomo R (2015) Sepsis-associated acute kidney injury: Macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin Nephrol 35: 64-74.
- Wang JB, Wang HT, Li LP (2015) Development of a rat model of D-galactosamine/lipopolysaccharide induced hepatorenal syndrome. World J Gastroenterol 21: 9927-9935.
- Shah N, Mohamed FE, Jover-Cobos M (2013) Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int 33: 398-409.
- Shah N, Dhar D, El Zahraa Mohammed F (2012) Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol 56: 1047-1053.
- Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V (2015) Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 63: 1272-1284.
- Thabut D, Massard J, Gangloff A (2007) Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology 46: 1872-1882.
- Moller S, JH Henriksen, F (2014) Bendtsen, Extrahepatic complications to cirrhosis and portal hypertension: haemodynamic and homeostatic aspects. World J Gastroenterol 20: 15499-15517.
- Bortoluzzi A, Ceolotto G, Gola E (2013) Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: Molecular mechanisms. Hepatology 57: 266-276.
- Mocarzel LO, Bicca J, Jarske L (2016) Cirrhotic cardiomyopathy: Another case of a successful approach to treatment of hepatorenal syndrome. Case Rep Gastroenterol 10: 531-537.
- Acevedo J, Fernandez J, Prado V (2013) Relative adrenal insufficiency in decompensated cirrhosis: Relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology 58: 1757-1765.
- Jang JY, Kim TY, Sohn JH (2014) Relative adrenal insufficiency in chronic liver disease: its prevalence and effects on long-term mortality. Aliment Pharmacol Ther 40: 819-826.
- Trawale JM, Paradis V, Rautou PE (2010) The spectrum of renal lesions in patients with cirrhosis: A clinicopathological study. Liver Int 30: 725-732.
- Garcia-Tsao G, Parikh CR, Viola A (2008) Acute kidney injury in cirrhosis. Hepatology 48: 2064-2077.
- Arroyo V, Gines P, Gerbes AL (1996) Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 23: 164-176.
- Angeli P, Gatta A, Caregaro L (1990) Tubular site of renal sodium retention in ascitic liver cirrhosis evaluated by lithium clearance. Eur J Clin Invest 20: 111-117.
- Wong, F (2016) Acute kidney injury in liver cirrhosis: New definition and application. Clin Mol Hepatol 22: 415-422.
- Salerno F, Gerbes A, Gines P (2008) Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J 84: 662-670.
- Bucsics T, Schwabl P, Mandorfer M (2016) The trigger matters-outcome of hepatorenal syndrome vs. specifically triggered acute kidney injury in cirrhotic patients with ascites. Liver Int 36: 1649-1656.
- Nadim MK, Kellum JA, Davenport A (2012) Hepatorenal syndrome: The 8th international consensus conference of the acute dialysis quality initiative (ADQI) Group. Crit Care 16: R23.
- Fagundes C, Gines P (2012) Hepatorenal syndrome: A severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis 59: 874-85.
- Francoz C, Glotz D, Moreau R, Durand F (2010) The evaluation of renal function and disease in patients with cirrhosis. J Hepatol 52: 605-613.
- Durand F, Graupera I, Gines P (2016) Pathogenesis of hepatorenal syndrome: Implications for therapy. Am J Kidney Dis 67: 318-328.
- Singal AK, Jackson B, Pereira GB (2017) Biomarkers of renal injury in cirrhosis: Association with acute kidney injury and recovery after liver transplantation. Nephron 138: 1-12.
- Belcher, JM, Garcia-Tsao G, Sanyal AJ (2014) Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol 9: 1857-1867.
- Devarajan P (2010) Neutrophil gelatinase-associated lipocalin: A promising biomarker for human acute kidney injury. Biomark Med 4: 265-280.
- Belcher JM, Sanyal AJ, Peixoto AJ (2014) Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 60: 622-632.
- Fagundes C, Pepin MN, Guevara M (2012) Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 57: 267-273.
- Haase-Fielitz A, Haase M, Devarajan P (2014) Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann Clin Biochem 51: 335-351.
- Barreto R, Elia C, Sola E (2014) Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol 61: 35-42.
- Verna EC, Brown RS, Farrand E (2012) Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 57: 2362-2370.
- Martensson J, Bellomo R (2014) The rise and fall of NGAL in acute kidney injury. Blood Purif 37: 304-310.
- Belcher JM (2015) Acute kidney injury in liver disease: Role of biomarkers. Adv Chronic Kidney Dis 22: 368-375.
- Alsaad AA, Wadei HM (2016) Fractional excretion of sodium in hepatorenal syndrome: Clinical and pathological correlation. World J Hepatol 8: 1497-1501.
- Liu CW, Yigit A, Yesilada E (2017) Serum adrenomedullin and urinary thromboxane B2 help early categorizing of acute kidney injury in decompensated cirrhotic patients: A prospective cohort study. Hepatol Res 48: E9-E21.
- Wang C, Yu Z, Luo X, Ye J, Liu S, et al. (2017) Association of arginine vasopressin receptor 1a gene polymorphisms with hepatorenal syndrome. J Pak Med Assoc 67: 577-579.
- Seckin Y, Yigit A, Yesilada E, Gulbay G, Furkan Y, et al. (2016) Association of eNOS gene polymorphisms G894T and T-786C with risk of hepatorenal syndrome. Gastroenterol Res Pract 2016: 2579626.
- Low G, Alexander GJ, Lomas DJ (2015) Hepatorenal syndrome: Aetiology, diagnosis, and treatment. Gastroenterol Res Pract 2015: 207012.
- Egerod IM, Gluud LL, Krag A (2015) Acute kidney injury and hepatorenal syndrome in cirrhosis. J Gastroenterol Hepatol 30: 236-243.
- European Association for the Study of the Liver (2010) EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 53: 397-417.
- Fernandez J, Navasa M, Planas R (2007) Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 133: 818-824.
- Glass L, Sharma P (2016) Evidence-based therapeutic options for hepatorenal syndrome. Gastroenterology 150: 1031-1033.
- Fleming JN, Abou AA (2010) Hepatorenal syndrome: A comprehensive overview for the critical care nurse. Crit Care Nurs Clin North Am 22: 351-368.
- Zardi EM, Zardi DM, Giorgi C, Chin D, Dobrina A (2017) Portopulmonary hypertension and hepatorenal syndrome. Two faces of the same coin. Eur J Intern Med 43: 22-27.
- Srivastava S, Shalimar, Vishnubhatla S (2015) Randomized controlled trial comparing the efficacy of terlipressin and albumin with a combination of concurrent dopamine, furosemide, and albumin in hepatorenal syndrome. J Clin Exp Hepatol 5: 276-285.
- Piano S, Angeli P (2015) Dopamine and furosemide for the treatment of hepatorenal syndrome: A reappraisal or just smoke and mirrors? J Clin Exp Hepatol 5: 273-275.
- Snowdon VK, Lachlan NJ, Hoy AM (2017) Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial. PLoS Med 14: e1002248.
- Jalan R, Fernandez J, Wiest R (2013) Bacterial infections in cirrhosis: A position statement based on the EASL Special Conference 2013. J Hepatol. 60: 1310-1324.
- Bernardi M, Ricci CS, Zaccherini G (2014) Role of human albumin in the management of complications of liver cirrhosis. J Clin Exp Hepatol 4: 302-311.
- Cavallin M, Fasolato S, Marenco S (2015) The treatment of hepatorenal syndrome. Dig Dis 33: 548-554.
- Salerno F, Navickis RJ, Wilkes MM (2015) Albumin treatment regimen for type 1 hepatorenal syndrome: A dose-response meta-analysis. BMC Gastroenterol 15: 167.
- Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, et al. (2011) Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J Hepatol 55: 315-321.
- Fabrizi F, Dixit V, Messa P, Martin P (2009) Terlipressin for hepatorenal syndrome: A meta-analysis of randomized trials. Int J Artif Organs 32: 133-140.
- Israelsen M, Krag A, Allegretti AS, Jovani M, Goldin AH, et al. (2017) Terlipressin versus other vasoactive drugs for hepatorenal syndrome. Cochrane Database Syst Rev 9: Cd011532.
- Bernardi M, Caraceni P, Navickis RJ, Wilkes MM (2012) Albumin infusion in patients undergoing large-volume paracentesis: A meta-analysis of randomized trials. Hepatology 55: 1172-1181.
- Heinzow HS, Lenz P, Kohler M, Reinecke F, Ullerich H et al. (2012) Clinical outcome and predictors of survival after TIPS insertion in patients with liver cirrhosis. World J Gastroenterol 18: 5211-5218.
- Berry K, Lerrigo R, Liou IW, Ioannou GN (2016) Association between transjugular intrahepatic portosystemic shunt and survival in patients with cirrhosis. Clin Gastroenterol Hepatol 14: 118-123.
- Thorat A, Jeng LB (2016) Management of renal dysfunction in patients with liver cirrhosis: Role of pretransplantation hemodialysis and outcomes after liver transplantation. Semin Vasc Surg 29: 227-235.
- Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, et al. (2000) Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: Results of a prospective, randomized, controlled clinical trial. Liver Transpl 6: 277-286.
- Gonwa TA, Mai ML, Melton LB (2001) Renal replacement therapy and orthotopic liver transplantation: The role of continuous veno-venous hemodialysis. Transplantation 71: 1424-1428.
- Ikegami T, Shirabe K, Soejima Y, Tanemura A, Murata Y, et al. (2012) The impact of renal replacement therapy before or after living donor liver transplantation. Clin Transplant 26: 143-148.
- Zand MS, Orloff MS, Abt P (2011) High mortality in orthotopic liver transplant recipients who require hemodialysis. Clin Transplant 25: 213-221.
- Modi RM, Patel N, Metwally SN, Mumtaz K (2016) Outcomes of liver transplantation in patients with hepatorenal syndrome. World J Hepatol 8: 999-1011.
- Wong F, Leung W, Al Beshir M, Marquez M, Renner EL (2015) Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl 21: 300-307.
- Levitsky J, Baker TB, Jie C, Ahya S, Levin M, et al. (2017) Plasma protein biomarkers enhance the clinical prediction of kidney injury recovery in patients undergoing liver transplantation. Hepatology 60: 2017-2026.
- Rodriguez E, Henrique PG, Sola E, Elia C, Barreto R, et al. (2015) Treatment of type 2 hepatorenal syndrome in patients awaiting transplantation: Effects on kidney function and transplantation outcomes. Liver Transpl 21: 1347-1354.
- Tan HK, Marquez M, Wong F, Renner EL (2015) Pretransplant type 2 hepatorenal syndrome is associated with persistently impaired renal function after liver transplantation. Transplantation 99: 1441-1446.
- Ruiz R, Kunitake H, Wilkinson AH, Danovitch GM, Farmer DG, et al. (2006) Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg 141: 735-741.
- Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, et al. (2012) Simultaneous liver-kidney transplantation summit: Current state and future directions. Am J Transplant 12: 2901-2908.
- Levitsky J, Baker T, Ahya SN, Levin ML, Friedewald J, et al. (2012) Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant 12: 2949-2957.
- Sung RS, Wiseman AC (2015) Simultaneous liver-kidney transplant: Too many or just enough? Adv Chron Kid Dis 22: 399-403.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5241
- [From(publication date): 0-2018 - Jan 27, 2025]
- Breakdown by view type
- HTML page views: 4466
- PDF downloads: 775