Research Article Open Access
Hepatic Arterial Anatomy Variations Evaluated by the Computed Tomography Angiography in the Patients with Hepatocellular Carcinoma: A Case-Control Study
Onashvili N*, Mizandari M, Kutateladze M and Tughushi NDepartment of Radiology, Tbilisi State Medical University, Tbilisi, Georgia
- Corresponding Author:
- Onashvili N
Department of Radiology, Tbilisi State Medical University, Tbilisi, Georgia
Tel: +995599434844
E-mail: nikaonashvili@mail.ru
Received Date: November 24, 2016; Accepted Date: December 06, 2016; Published Date: December 09, 2016
Citation: Onashvili N, Mizandari M, Kutateladze M, Tughushi N (2016) Hepatic Arterial Anatomy Variations Evaluated by the Computed Tomography Angiography in the Patients with Hepatocellular Carcinoma: A Case-Control Study. OMICS J Radiol 5:242. doi: 10.4172/2167-7964.1000242
Copyright: © 2016 Onashvili N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Radiology
Abstract
Background: Hepatic arterial anatomy is of major importance in performing many surgical and endovascular procedures on the liver. Hepatic artery anatomical variations are a common finding. Aim: The aim of our study was to estimate the prevalence of various hepatic arterial variants in HCC patients and compare it with that in individuals with no abdominal abnormality. Patients and methods: Total of 78 patients was included in the study. They were categorized into following groups: 1) Patients with HCC (n=46); 2) Control group (n=32). Prevalence of variant arterial anatomy in each group and subgroup was determined and compared to the values in Control Group. Results: Compared to the control group HCC group showed higher incidence of hepatic arterial anatomy variations (54.5% vs. 31.2%). The difference was statistically significant (p<0.05). Among the patients with HCC lesions larger than 10 cm the percentage of arterial variations was 81.25% which was very statistically significantly different from that of the control group (p<0.01). This subgroup of patients also showed higher incidence of SMA type RHA p<0.05). Among the patients with single HCC lesion the percentage of arterial variations was 76.92% which was very statistically significantly different from that of the control group (p<0.01). Among the patients with two HCC lesions the percentage of arterial variations was 62.5% which was not statistically significantly different from that of the control group. Groups of patients with HCC lesions less than 10 cm as well as the group with multiple HCC lesions did not show statistically significant difference of the hepatic arterial anatomy variation incidence compared to the control group either. Our study implies that there may be a difference in the incidence of hepatic arterial anatomical variations between HCC patients and healthy individuals. This issue is worth of conducting further research on large series.
Keywords
Hepatic arterial anatomy; Hepatocellular Carcinoma (HCC); Computed tomography; Carcinoma
Introduction
Hepatic arterial anatomy is of major importance in performing many surgical and endovascular procedures on the liver. Hepatic artery anatomical variations are a common finding; according to publications, normal variant is encountered in 25-80% to individuals [1,2]. One of the earliest publications regarding liver vascular anatomy belongs to Michels, who divided the variations into ten variants [3]. Later Hiatt proposed modified classification [4].
Hepatocellular Carcinoma (HCC) is the most common primary liver malignancy and one of the major reasons for performing percutaneous endovascular procedures or liver surgery. Thus assessment of hepatic arterial anatomy variations in patients with HCC is of importance in successful interventional management of these patients.
According to publications Computed Tomography (CT) angiography is the most reliable non-invasive tool to assess arterial anatomy of the liver [5-12].
Materials and Methods
The study was approved by the ethics committee of the Tbilisi State Medical University. Total of 78 patients were included in the study. They were categorized into following groups:
1. The first group (HCC Group) consisted of all the HCC patients who had undergone abdominal CT angiography (CTA) scan from 2011 to 2015 at the department of radiology. The number of these individuals was 46 (38 men and 8 women, age range 39-79 years, average age 56 years). HCC was diagnosed either with the pathological analysis of the biopsy specimen or combination of the clinical, laboratory and radiological data. Diagnostic criteria for the non-invasive diagnosis of the HCC were hyper vascular lesion with early contrast wash-out combined with cirrhosis and AFT level above 400.
2. Individuals for the second group (Control Group) were recruited from the database of CT examinations. These patients had undergone abdominal CTA for various reasons and had no known or visible abdominal disorder. The number of cases in the control group was 32 (19 men and 13 women, age range 24-72 years, average age 50 years).
HCC group was further divided into 5 subgroups:
• Patients with HCC lesions larger than 10 cm.
• Those less than 10 cm.
• Patients with single HCC lesion.
• Patients with two HCC lesions.
• Patients with multiple HCC lesions.
The numbers of patients in each subgroup are given in Table 1.
| Size of the Lesions | Number of the Lesions | ||||
|---|---|---|---|---|---|
| >10cm | <10cm | 1 | 2 | Multiple | |
| Number of Patients | 16 | 30 | 13 | 8 | 25 |
Table 1: Subgroups of HCC group.
Abdominal CT scan was done using 16 and 64 slice scanner using multiphase CT protocol.
Analysis of the CT scans assumed the assessment of hepatic arterial anatomy. Evaluation of anatomical variations was made using Michels’ classification.
Prevalence of variant arterial anatomy in each group and subgroup was determined and compared to the values in Control Group. Statistical significance of the difference was estimated. The p value calculation was made using two tiled Fisher’s Exact Test.
Results
Anatomical variants and their distribution in both groups are summarized in Table 2.
| Michel’s Type | Number of Cases in HCC Group | Number of Cases in Control Group | Number of Cases in Both Groups | ||
|---|---|---|---|---|---|
| Standard Anatomy | I | 20 (43.5%)* | 22 (68.8%)* | 41 (52.6%) | |
| Variant Anatomy | II | 5 (10.9%) | 1 (3.1%) | 6 (7.7%) | |
| III | 9 (19.6%) | 4 (12.5%) | 13 (16.7%) | ||
| IV | - | - | - | ||
| V | 4 (8.7%) | - | 4 (5.1%) | ||
| VI | 3 (6.5%) | 4 (12.5%) | 7 (9%) | ||
| VII | - | - | - | ||
| VIII | 2 (4.3%) | - | 2 (2.6%) | ||
| IX | 1 (2.2%) | - | 1 (1.3%) | ||
| X | - | - | - | ||
| Unclassified | double hepatic artery | 1 (2.2%) | 1 (3.1%) | 2 (2.6%) | |
| double hepatic artery and anastomosis between PDA and SMA | 1 (2.2%) | - | 1 (1.3%) | ||
| Total | 46 (100%) | 32 (100%) | 78 (100%) | ||
Table 2: Hepatic arterial anatomy in both groups.
Arterial Anatomy among the Patients in the HCC Group
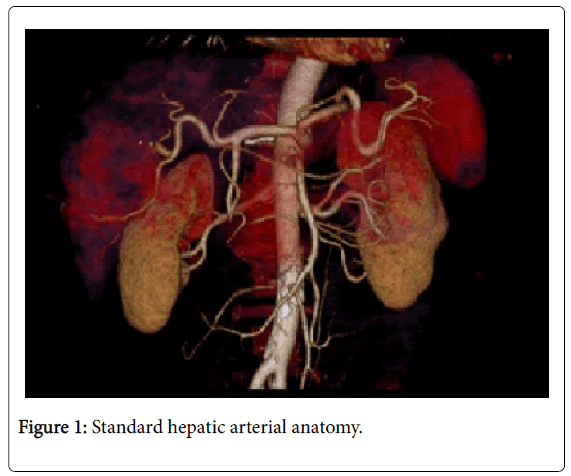
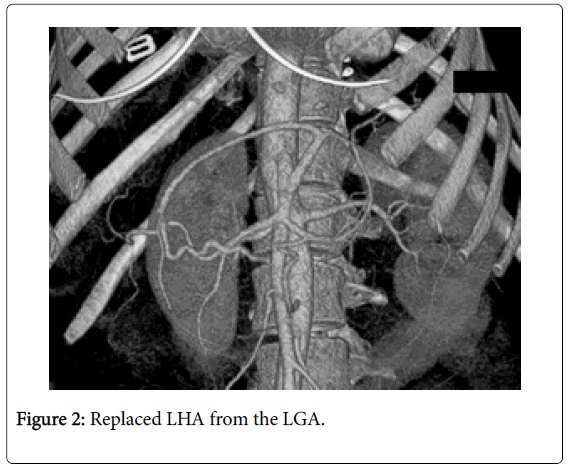
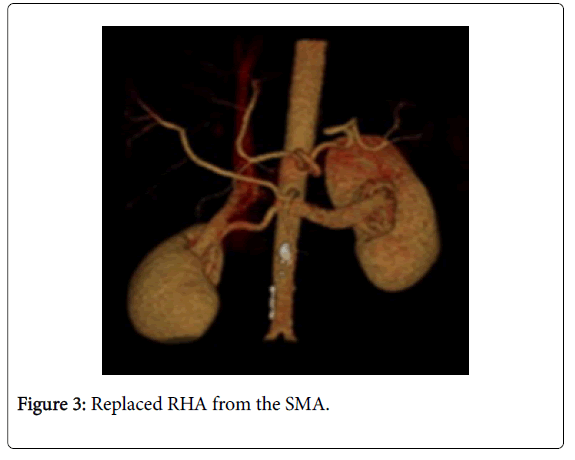
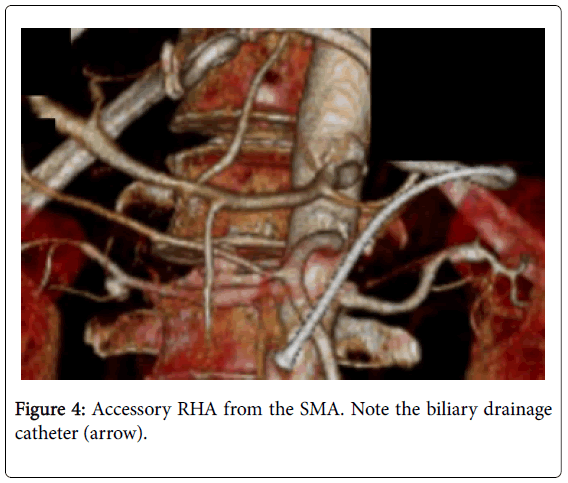
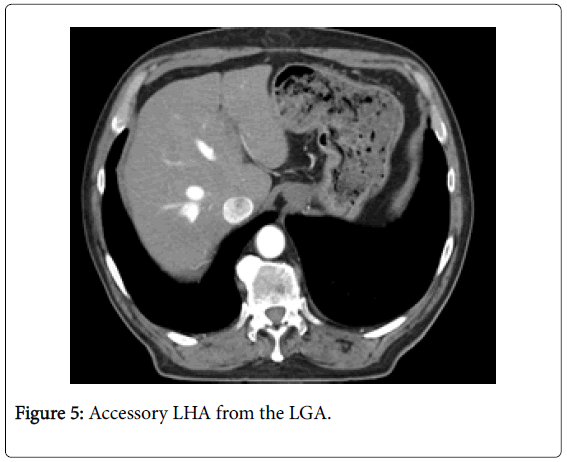
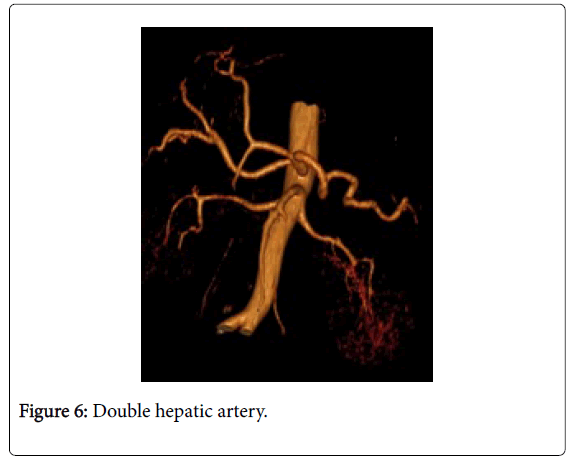
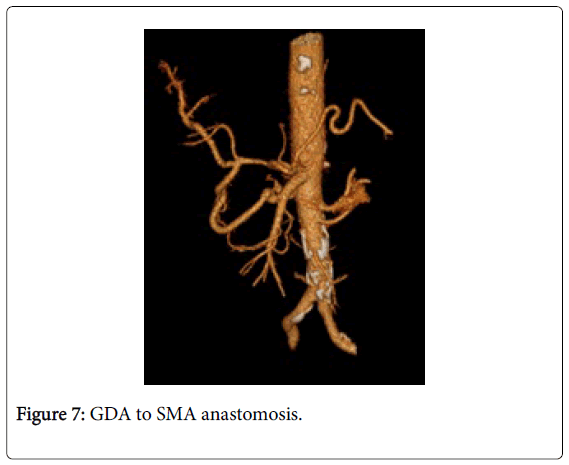
Among 46 patients with HCC standard anatomy (Michels’ type I) was seen in 20 (43.5%) patients (Figure 1). The rest 26 patients (56.5%) had anatomical variations. Among those 5 patients had left hepatic artery (LHA) replaced to the left gastric artery (LGA) (Figure 2). 9 patients were found to have replaced right hepatic artery (RHA) from the superior mesenteric artery (SMA) (Figures 3 and 4). Accessory LHA originating from the left gastric artery was found in 4 patients (Figure 5). Accessory RHA arising from the SMA was seen in 3 patients. Simultaneous existence of the replaced RHA and accessory LHA was encountered in 2 cases. There was 1 case of the common hepatic artery (CHA) arising from the SMA. Besides, double hepatic artery was found in 2 patients, one of which was combined with the anastomosis between the GDA and the SMA (Figures 6 and 7). GDA to SMA anastomosis was found in 5 patients, 3 of them had otherwise standard anatomy and were regarded as Michels’ type I anatomical variant, and 1 had coexistent replaced LHA.
Arterial anatomy among the subgroups of HCC group is summarized in Table 3.
| Michels’ Type | Number of Cases >10cm Subgroup | Number of Cases in <10cm Subgroup | Number of Cases in 1 Lesion Subgroup | Number of Cases in 2 Lesion Subgroup | Number of Cases in Multiple Lesion Subgroup | |
|---|---|---|---|---|---|---|
| Standard Anatomy | I | 3 (18.75%)** | 17 (56.67%) | 3 (23.08%)** | 3 (37.5%) | 14 (56%) |
| Variant Anatomy | II | 3 (18.75%) | 2 (6.67%) | 2 (15.38%) | 1 (12.5%) | 2 (8%) |
| III | 7 (43.75%)* | 3 (10%) | 6 (46.15%)* | 2 (25%) | 2 (8%) | |
| IV | - | - | - | - | - | |
| V | 2 (12.5%) | 1 (3.33%) | 1 (7.69%) | - | 2 (8%) | |
| VI | 1 (6.25%) | 2 (6.67%) | - | - | 3 (12%) | |
| VII | - | - | - | - | - | |
| VIII | - | 2 (6.67%) | - | 1 (12.5%) | 1 (4%) | |
| IX | - | 1 (3.33%) | 1 (7.69%) | - | - | |
| X | - | - | - | - | - | |
| unclassified | - | 2 (6.67%) | - | 1 (12.5%) | 1 (4%) | |
| Total | 16 | 30 | 13 | 8 | 25 |
Table 3: Arterial variations among the subgroups of HCC group.
Among 16 patients with HCC lesion larger than 10 cm standard hepatic arterial anatomy (Michels’ type I) was seen in 3 (18.75%) patients. The rest 13 patients (81.25%) had anatomical variations. Among those 3 patients had LHA replaced to the LGA. 7 patients were found to have replaced RHA from the SMA. Accessory LHA originating from the LGA was found in 2 patients. Accessory RHA arising from the SMA was seen in 1 patient.
Among 30 patients with HCC lesion smaller than 10 cm standard hepatic arterial anatomy (Michels’ type I) was seen in 17 (56.67%) patients. The rest 13 patients (43.33%) had anatomical variations. Between those 2 patients had LHA replaced to the LGA. 6 patients were found to have replaced RHA from the SMA. Accessory LHA originating from the LGA was found in 1 patient. Accessory RHA arising from the SMA was seen in 2 patients. Simultaneous existence of the replaced RHA and accessory LHA was encountered in 2 cases. The case of the common hepatic artery (CHA) arising from the SMA, also 2 cases with double hepatic artery were included in this subgroup.
Among 13 patients with single HCC lesion standard hepatic arterial anatomy (Michels’ type I) was seen in 3 (23.08%) patients. The rest 10 patients (76.92%) had anatomical variations. Among those, 2 patients had LHA replaced to the LGA. 6 patients were found to have replaced RHA from the SMA. Accessory LHA originating from the LGA was found in 1 patient. The case of the common hepatic artery (CHA) arising from the SMA was included in this subgroup.
Among 8 patients with two HCC lesions standard hepatic arterial anatomy (Michels’ type I) was seen in 3 (37.5%) patients. The rest 5 patients (62.5%) had anatomical variations. Among those, 1 patient had LHA replaced to the LGA. 2 patients were found to have replaced RHA from the SMA. Simultaneous existence of the replaced RHA and accessory LHA was encountered 1 patient.
Among 25 patients with multiple HCC lesions standard hepatic arterial anatomy (Michels’ type I) was seen in 14 (56%) patients. The rest 11 patients (44%) had anatomical variations. Among those 2 patients had LHA replaced to the LGA. 2 patients were found to have replaced RHA from the SMA. Accessory LHA originating from the LGA was found in 2 patients. Accessory RHA arising from the SMA was seen in 3 patients. Simultaneous existence of the replaced RHA and accessory LHA was encountered in 2 cases. One patient with double hepatic artery was also found belong to this subgroup.
Arterial Anatomy among the Patients in the Control Group
22 individuals in the control group had standard anatomy. Replaced LHA arising from the LGA and double hepatic artery were found each in single cases. Replaced and accessory RHA from the SMA was seen in 8 cases (4 cases each).
Data Analysis
Compared to the control group HCC group showed higher incidence of hepatic arterial anatomy variations (54.5% vs. 31.2%). The difference was statistically significant (p<0.05) (Table 3).
Among the subgroups of HCC group statistically significant difference was found in the following subgroups (Table 4).
Among the patients with HCC lesions larger than 10 cm the percentage of arterial variations was 81.25% which was very statistically significantly different from that of the control group (p<0.01). This subgroup of patients also showed higher incidence of SMA type RHA (p<0.05).
Among the patients with single HCC lesion the percentage of arterial variations was 76.92% which was very statistically significantly different from that of the control group (p<0.01).
Among the patients with two HCC lesions the percentage of arterial variations was 62.5% which was not statistically significantly different from that of the control group. Groups of patients with HCC lesions less than 10 cm as well as the group with multiple HCC lesions did not show statistically significant difference of the hepatic arterial anatomy variation incidence compared to the control group either.
Discussion
Our study implies that there may be a difference in the incidence of hepatic arterial anatomical variations between HCC patients and healthy individuals, particularly in those with single tumor lesions larger than 10 cm. However, we did not get statistically significant increase in arterial variations in the subgroup of patients with tumor lesion less than 10 cm. Thus the difference between HCC and Control Groups may be due to very high percentage of variant anatomy among the patients with larger tumors (87.5%) and in fact the variant anatomy is linked with HCC size only and not the existence of the tumor itself. Anyway, it is above the capabilities of our study and requires further research. Perhaps larger series will show it better.
To our knowledge this is a pilot study comparing hepatic arterial anatomy in HCC patients in case-control fashion. Publications estimating hepatic arterial variations do exist [1-6,13-15], but those determining the prevalence in HCC patients are rare [16-19]. Their results are summarized and compared to our data in Table 4.
| Arterial Anatomy | Our Study | Nikolopoulos et al. | Jiang | Rawat |
|---|---|---|---|---|
| Standard | 19 (41.3%) | 22 (66.7%) | 413 (82.6%) | 49 (39.2%) |
| Variant | 27 (58.7%) | 11 (33.3%) | 87 (17.4%) | 76 (60.8%) |
| Total | 46 | 33 | 500 | 125 |
Table 4: Hepatic arterial anatomy variants among liver cancer patients in various studies and those reported by us.
The data is confusing and ambiguous with considerable diversity between the authors. This might be due to the difference in the patients among various studies (patient age, nationality, other genetic factors etc.). By including control group in our study, we tried to minimize these differences between HCC and Control groups.
Some anatomical variants are identified that do not fit into any variant proposed by Michels (Figure 6).
We also report 5 cases of PDA to SMA anastomosis which was more prevalent in HCC group (though not statistically significantly different). 3 of these anastomoses coexisted with the standard arterial anatomy and were regarded as Michels’ type I configuration. Though in some circumstances (probably increased demand of the liver for the blood) it may serve (via retrograde flow) as an alternative arterial supply to the liver. Conditions that increase the arterial blood demand of the liver include cirrhosis. In our study minimum 35 patients (76%) in HCC group had concomitant liver cirrhosis. As far as our study was retrospective it was not possible to determine the direction of the blood flow in these anastomoses, but the issue is undoubtedly interesting.
Finally, it is worth of noting that we also had cases of the HCC fed by alternative nonstandard sources. We did not consider those vessels hepatic arterial variations. These alternative sources included GDA, LGA and inferior phrenic artery.
We also understand the limitations of our study which includes first of all small sample size (number of individuals). Our effort to compensate this limitation was inclusion of the Control Group in our study. Another limitation of our study is the fact that HCC group was inhomogeneous by including newly diagnosed HCC-s as well as post-embolization CT scans. The number of post-embolization patients was 4. Evidence supports the fact that embolization can change the arterial supply of the HCC [20-27].
In conclusion, higher prevalence of the hepatic arterial anatomy variations raises the suspect of possible increased risk of locally rapidly growing HCC in the individuals with nonstandard arterial anatomy. This issue is worth of conducting further research on large series. Also, this fact favours performing pre-embolization and pre-surgical CTA for the mapping of the hepatic arteries and is in consensus with other authors [22-24].
References
- Nelson TM, Pollak R, Jonasson O, Abcarian H (1988) Anatomic variants of the celiac, superior mesenteric and inferior mesenteric arteries and their clinical relevance. ClinAnat1:75-91.
- SabaL (2012) CT Imaging of Hepatic arteries, computed tomography - clinical applications.
- Michels NA (1966) Newer anatomy of the liver and variant blood supply and collateral circulation. Am J Surg112: 337-347.
- Hiatt JR, Gabbay J, Busuttil RW (1994) Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg220:50-52.
- Winter TC, Nghiem HV, Freeny PC, Hommeyer SC, Mack LA (1995) Hepatic arterial anatomy: demonstration of normal supply and vascular variants with three-dimensional CT angiography. Radiographics 15: 771-780.
- Ugurel MS, Battal B, Bozlar U, Nural MS, Tasar M, et al. (2014) Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Brit J Radiol 83:661-667.
- Furuta T, Maeda E, Akai H, Hanaoka S, Yoshioka N, et al. (2009) Hepatic Segments and Vasculature: Projecting CT Anatomy onto Angiograms. RadioGraphics29:e37.
- Duran C, Uraz S, Kantarci M, Ozturk E, Doganay S, et al. (2009) Hepatic arterial mapping by multidetector computed tomographic angiography in living donor liver transplantation.J Comput Assist Tomogr 33:618-625.
- Perez-Johnston R, Lenhart DK, Sahani DV (2010) CT angiography of the hepatic and pancreatic circulation. RadiolClin North Am 48:311-330.
- CoŇ?kun M, Kayahan EM, Ozbek O, Cakir B, Dalgiç A, et al. (2005) Imaging of hepatic arterial anatomy for depicting vascular variations in living related liver transplant donor candidates with multidetector computed tomography: comparison with conventional angiography. Transplantation Proceedings 37: 1070-1073.
- Saba L, Mallarini G (2008) Multidetector row CT angiography in the evaluation of the hepatic artery and its anatomical variants. ClinRadiol 63:312-321.
- Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, et al. (2007) CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol 189:W13-W19.
- Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT (2002) Variant Hepatic Arterial Anatomy Revisited: Digital Subtraction Angiography Performed in 600 Patients.Radiology 224:542-547.
- Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, et al. (2010) Celiac Axis and Common Hepatic Artery Variations in 5002 Patients: Systematic Analysis with Spiral CT and DSA.Radiology 255:278-288.
- De Santis M, Ariosi P, Calo GF, Romagnoli R (2000) Hepatic arterial vascular anatomy and its variants. Radiol Med 100:145-151.
- Triantopoulou C (1970) Transcatheter arterial chemoembolization of hepatocellular tumors in patients with variant hepatic arterial anatomy. European Congress of Radiology 2014.
- Jiang L (2010) The Hepatic Arterial Anatomy Variations and Classifications. Guangxi Medical University.
- Rawat KS (2006) CT angiography in evaluation of vascular anatomy and prevalence of vascular variants in upper abdomen in cancer patients. Indian J RadiolImag16: 457-461.
- Huang Y, Liu C, Lin JL (2013) Clinical significance of hepatic artery variations originating from the superior mesenteric artery in abdominal tumor surgery. Chin Med J 126:899-902.
- Mine T, Murata S, Ueda T, Takeda M, Onozawa S, et al. (2014) Contribution of extrahepatic collaterals to liver parenchymal circulation after proper hepatic artery embolization. J GastroenterolHepatol 29: 1515-1521.
- Gao S, Yang RJ, Dong JH (2012) Hepatocellular carcinoma with blood supply from parasitized omental artery: Angiographic appearance and chemoembolization. Chinese J Cancer Res 24: 207-212.
- Apisarnthanarak P, Raman SS, Ghobrial RM, Busuttil RW, Saab S, et al. (2011) Efficacy of CT angiography for preoperative vascular mapping in adult to adult living related liver transplant donors. J Med Assoc Thai 94:98-105.
- Kim I, Kim DJ, Kim KA, Yoon SW, Lee JT (2014) Feasibility of MDCT angiography for determination of tumor-feeding vessels in chemoembolization of hepatocellular carcinoma. J Comput Assist Tomogr 38:742-746.
- Ahmed S, Johnson PT, Fishman EK (2013) Defining vascular signatures of benign hepatic masses: role of MDCT with 3D rendering. AbdomImag 38:763-773.
- Bolton RP, Mairiang E, Parkin A, Ware F, Robinson P, et al. (1988) Dynamic liver scanning in cirrhosis. Nucl Med Commun 9:235-247.
- Baer JW (1976) The arterial blood supply to the liver in cirrhosis. Gastrointestinal radiology 1: 209-213.
- Zipprich A, Steudel N, Behrmann C, Meiss F, Sziegoleit U, et al. (2003) Functional significance of hepatic arterial flow reserve in patients with cirrhosis. Hepatology 37: 385-392.
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 4259
- [From(publication date):
December-2016 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 3312
- PDF downloads : 947