Hemostasis with Woundclot Hemostatic Gauze in Orthopedic Oncology Surgery
Received: 12-Feb-2019 / Accepted Date: 19-Mar-2019 / Published Date: 26-Mar-2019 DOI: 10.4172/2472-016X.1000129
Abstract
The subspecialty of orthopedic oncology is concerned with the diagnosis and treatment of musculoskeletal tumors, both primary and secondary, both benign and malignant. Nearly 50 types of procedures, ranging from short biopsies to lengthy 12 hour hemi-pelvectomies, are performed by orthopedic oncologists. They can require significant neurovascular dissection, removal of substantial bone and muscle, replacing large sections of bone with cement or 3D-printed prostheses, or total joint replacement. Patients who undergo orthopedic oncologic surgery often have received substantial medical treatment prior to surgery. Chemotherapy or radiation therapy can result in anemia and thrombocytopenia. In addition to these side effects, there may be significant co-morbidities.
Keywords: Hemostasis; Orthopedic oncology; Surgery; Woundclot
Introduction
In general, because tumors are fairly vascular structures, they are susceptible to bleed throughout the surgery. Systemic effects of the tumor and chemotherapy and radiotherapy also contribute to the preponderance for the bleeding [1]. In particular, renal cell and thyroid metastases, with marked neovascularization to affected areas, frequently hemorrhage considerably during surgery, more so than other forms of osseous metastasis.
60% of spinal metastasis, 40% of benign primary spinal neoplasms, and 85% of all malignant primary spinal neoplasms are hypervascular. In addition to renal and thyroid metastasis, other examples of highly vascular tumors prone to bleeding include breast carcinoma, prostate carcinoma, hemangiomas, aneurysmal bone cysts, melanomas, osteoblastomas, osteosarcomas, and giant cell tumors [2]. Some independent indicators of major bleeding include the procedure type, cancer type, female patients and co-existing coronary artery disease or chronic obstructive pulmonary disease [3].
A potentially large blood loss significantly complicates surgery for patients with hypervascular tumors [4]. Management of patients undergoing surgery for tumors involving the spine or pelvis is especially problematic due to the potential for massive and ongoing hemorrhage. Pelvic metastases often hemorrhage significantly regardless of histological subtype, and should be considered for preoperative embolization, especially when lesions are large [5].
Embolization involves intentional blockage of a vessel to prevent blood flow into that vessel. Preoperative tumor embolization may be considered in order to prevent or reduce large blood loss when operating on a highly vascular tumor. Embolization can facilitate the surgery by reducing intraoperative hemorrhage, permitting better visualization of the surgical field, and facilitating more optimal tumor resection [2-4]. Futhermore, it can help prevent life-threatening hemorrhage and the need for transfusion, as well as to potentially decrease surgical time and related complications [2]. Embolization can also contribute to promoting ischemia in the tumor, leading to necrosis. A chemotherapeutic medication can be added to the embolic agent, to further enhance its effect [4].
Notwithstanding the benefits of embolization, there are complications with this procedure. A commonly occurring complication of trans-catheter arterial chemoembolization is 'Postembolization syndrome'. Reported in up to 86% of patients, it involves abdominal pain, nausea, vomiting and fever for 2-3 days post-surgery. With large hepatic tumors, acute liver failure becomes likely, as well as biliary sepsis, gallbladder infarction and hepatic abscess. Embolization in renal cell carcinoma may result in inadvertent embolization of nontarget vessels, renal failure, renal abscess, and post-embolization syndrome [4].
A 2009 study by Moreira, Teixeria et al. examining the treatment of orthopedic metastasis, found a nearly 27% incidence of complications; furthermore, the most frequent surgical complication was bleeding, followed by superficial infection [1].
Patients demonstrating bleeding as a complication of their surgery can expect a hospital stay that is double in duration to those without this complication [6]. Additionally, intra- and perioperative hemorrhage can require transfusions, resulting in increased costs, and raising the potential for reoperation [2]. A further noted consequence of transfusions is a reported decrease in disease-free survival after surgery; this relates to the negative effect transfusion, either autologous or allogenic, has on immunomodulation [2].
Perioperative blood loss during orthopedic surgery is often treated with antifibrinolytic drugs, which prevent blood clots from breaking down. Some which are commonly used are Tranexamic Acid (TXA) and Epsilon-Aminocaproic Acid (EACA) [7]. There is some debate about their use in cancer patients, however, due to the frequent hypercoagulability present in this population. Aprotinin, another such drug, had demonstrated some initial blood loss decrease as well, but was removed from the market for causing renal failure [5].
Tranexamic acid reduces blood loss and the need for intraoperative transfusion in spine cancer patients [7]. However, it cannot be used if the patient is hypersensitive to it, or if there is a subarachnoid hemorrhage.
On the other hand, a serious postoperative complication is venous thromboembolism. Perioperative thrombotic complications are a major cause of morbidity and mortality, occurring in up to 53% of cases depending on type of surgery and comorbidities present [8].
There are numerous pharmacological agents that provide thromboprophylaxis, including warfarin, low-dose heparin, low molecular-weight-heparins, and aspirin [5].
Comorbidities also play a significant role. Nearly one quarter of patients in the aging population who undergo orthopedic surgery have concomitant coronary artery disease [9-11]. It is well known and accepted that coronary artery disease is a risk factor for thrombosis and bleeding complications; cancer also has a similar role as a risk factor [3]. It would be valuable to have a hemostatic agent that could be used regardless of any comorbidities.
Patients further compromised by cancer are also at increased risk for complications involving both thrombosis and bleeding. The possible benefit of decreasing thrombotic events with antiplatelet therapy has to be weighed against the higher chance of perioperative hemorrhage [12]. An optimal solution would be an effective hemostat that halts bleeding without increasing the potential for thrombosis.
Case Reports
Surgery was performed by Dr. Ortal Segal, senior surgeon in the National Unit for Orthopedic Oncology at Tel Aviv Souraski Medical Center, Tel Aviv-Yafo, Israel.
Case 1
A 53 year-old male presented with a small benign but painful bone tumor in the right elbow, having had symptoms for a year. On CT scan the lesion measured 1 cm in diameter, and was located at the distal anterior aspect of the humerus, close to the neurovascular bundle. Clinical presentation and imaging supported the diagnosis of osteoid osteoma.
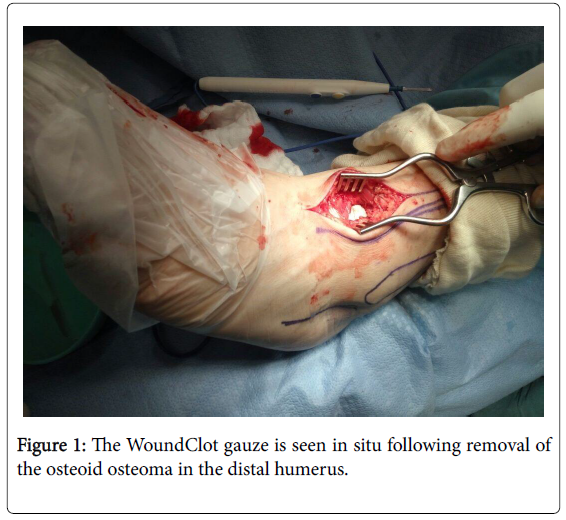
With the assistance of an advanced surgical navigation system, the tumor was isolated, and excision was made. A focused excavation of the affected cortex was conducted. The diseased cells were scraped out. The exposed bone demonstrated moderate bleeding. A 5 x 7.5 cm section of WoundClot gauze was placed directly against the bleeding bone surface (Figure 1).
No manual pressure was required. The WoundClot formed a completely stable gel, and was not removed from the surgical site. No re-bleeding was observed. The site was sutured. No hematomas resulted. The patient was released from the hospital the next day with no pain or complications.
Case 2
A 30 year-old female presented with a painful growth on her sacrum, diagnosed as a giant cell tumor (GCT) which was unresectible. It was decided to treat the tumor with cryoablation. Once the lesion was exposed, needles were inserted and the tumor was frozen. When the GCT cryo-ablation was stopped, the exposed surface demonstrated oozing bleeding. 2 patches of 5 x 7.5 cm WoundClot were applied and its semi-gel formation provided good hemostasis. No manual pressure was required. The product was not removed from the surgical site. No re-bleeding was observed. A drain was inserted for a short time. The patient reported pain relief; no neurological deficiencies ensued.
Discussion and Conclusion
WoundClot Surgical is a Class III bio-absorbable, noncompressional hemostatic dressing made by non-oxidative chemical reaction using cellulose as a substrate to build upon advanced functional groups. Those Functional Molecular Groups (FMGs) were specifically designed to provide special physical and performance properties. They increase the blood’s affinity for the product while enhancing intermolecular forces between the polymeric chains in order to maintain product stability. These additional interactions inhibit stability. These additional interactions inhibit rapid dissociation when exposed to an aqueous environment and preserve the stable gel state for an extended period of time.
A key to WoundClot’s effectiveness is its non-oxidative production method. Other hemostatic products on the market utilize an oxidative process, which accelerates their decomposition, therefore limiting the crucial initial duration of product activity.
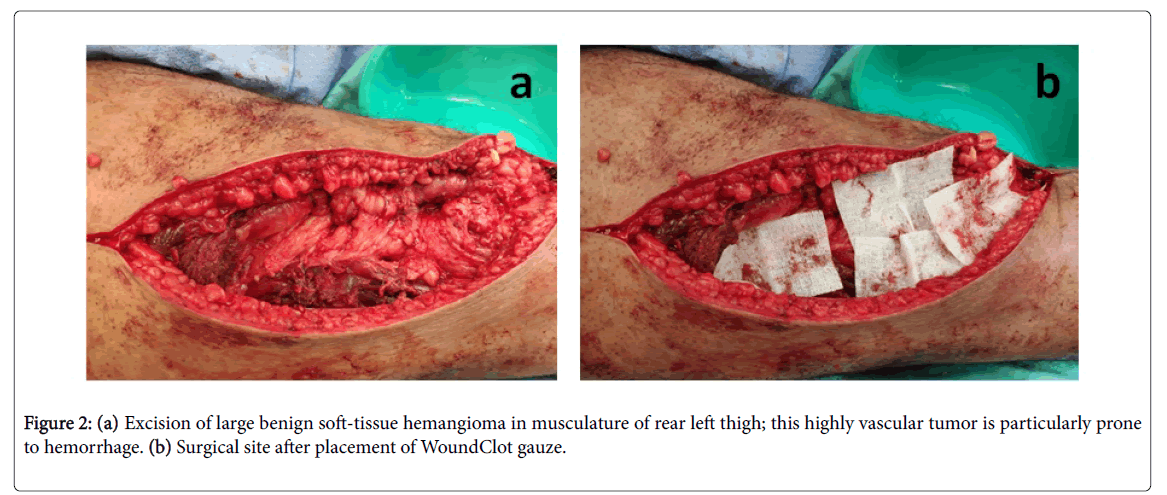
Upon placement of WoundClot dressing on the bleeding site, the product's unique gel utilizes the hydration forces between the soft tissue layer and itself, which results in a strong physical attraction that leads to the adherence of the product to the tissue. The stronger the bleeding pressures in the wound, the stronger the attraction (Figure 2).
For this to happen, the gel formed is designed to be relatively slow in forming, with a vast ability to absorb bleeding without breaking down. This ability to absorb and maintain a stable membrane allows for the pooling of active coagulation factors in significant quantities to be sequestered in the membrane. Membrane flexibility on the molecular gel structure allows a hemodynamic environment in which rapid coagulation cascades are created, because of the large amount of coagulation factors present due to the product’s high absorption capability.
The coagulation factors attach to the surface tissue and the membrane in the presence of a patented molecular group incorporated into the product. The coagulation process initiated is much more rapid, by enhancing the presence of platelets, multiple coagulation factors, and amino acids. This dramatically reduces the flow of blood from the wound.
In turn, this process also increases adherence of the membrane to the tissue, ensuring its stability on the wound site.
Once bleeding has stopped and the clot has formed, the capillary and hydration physical forces are reduced, allowing for the easy removal of WoundClot, if desired, in one piece, without clot disruption.
The liquid absorption capability of WoundClot is over 2500 times its own weight. When exposed to blood, the formed 3D matrix entraps platelets and coagulants in a hemodynamic environment. This allows for an increase in concentration of blood components at the wound site, while retaining their mobility and activity in order to form a clot. Moreover, its Functional Molecular Groups (FMGs) are designed to have a strong impact on the natural biological clotting process.
WoundClot initially forms a mechanical plug by adhering to the cavity, slowing down the blood flow, promoting massive absorption of platelets, and accelerating the plug formation. Once the platelets interact with WoundClot, the FMG promotes platelet activation, initiating the intrinsic clotting pathway. WoundClot affects the coagulation process by transforming Hageman factor (factor XII) from inactive to active (XIIa), and activating plasma thromboplastin antecedent (factor XI) for a longer duration when compared to other common hemostats.
WoundClot is compatible with a variety of relevant clinical considerations, including oncology, orthopedics, cardiovascular, endoscopic, obstetric/gynecologic, bariatric/gastrointestinal surgery, trauma resulting in many types of bleeding, as well as a valuable adjunct for dialysis patients.
WoundClot provides numerous and significant advantages to both doctor and patient. It is versatile, adjustable and flexible. WoundClot is therefore extremely user friendly; it can be cut to custom size or rolled for optimal wound insertion; it does not stick to gloves or instruments. Importantly, it uniquely involves a non-pressure application, allowing for effective use in situations where maintained pressure is contraindicated. Although bio-absorbable, it also possesses ease of removal. There are no active ingredients, and the product even lends itself to use by coagulopathic patients.
WoundClot hemostatic gauze comprises a complete new hierarchy of material engineering from its molecular level, through optimization of its physical properties, interaction with blood and maximization of its clotting promotion capabilities. Its unique biocompatibility effectively solves the major thromboembolism problem demonstrated by the other major hemostatic dressings over long term use.
As a simple to use, versatile, non-compressional hemostat, WoundClot finally provides a new and improved solution to the clinical problem of uncontrolled hemorrhage for virtually all prehospital civilian and military hemorrhaging trauma, as well as in the operating room and post-surgical applications. Additionally, WoundClot is a cost-effective solution to the many applications just mentioned; its low cost point is comparable or better than other hemostatic products.
Conflict of Interest
The authors declare no conflict of interest in the preparation of this manuscript, and they received no funding from the manufacturer of WoundClot surgical hemostatic gauze, Core Scientific Creations.
References
- Teixeria LEM, Miranda RH, Ghedini DF, Aguilar RB, Novais ENV, et al. (2009) Early complications in the orthopedic treatment of bone metastases. Rev Bras Ortop 44: 519–523.
- Peabody TD, Attar S (2014) Orthopaedic Oncology: Primary and Metastatic Tumors of the Skeletal System. Springer International Publishing, Switzerland.Â
-  Oberweis BS,Nukala S, Rosenberg A, Guo Y,  Stuchin S (2013) Thrombotic and Bleeding Complications Following Orthopedic Surgery. Am Heart J 165: 427–433.
- Goode JA, Matson MB (2002) Embolisation of cancer: what is the evidence?. Cancer Imaging 4: 133-141.
- Weber RS, Jabbour N, Martin RC (2008) Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol 15: 34–45.
- Dalmau A, Sabaté A, Acosta F, Garcia-Huete L, Koo M, et al. (2000) Tranexamic acid reduces red cell transfusion better than epsilon-aminocaproic acid or placebo in liver transplantation. Anesth Analg 91: 29–34.
- Vera-Llonch M, Hagiwara M, Oster G (2006) Clinical and economic consequences of bleeding following major orthopedic surgery. Thromb Res 117: 569–577.
-  Ausset S, Auroy Y, Lambert E, Vest P, Plotton C, et al. (2008) Cardiac troponin I release after hip surgery correlates with poor long-term cardiac outcome. Eur J Anaesthesiol 25: 158–164.
- Salerno SM, Carlson DW, Soh EK, Lettieri CJ (2007) Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med 120: 185.
- Ackland GL, Harris S, Ziabari Y, Grocott M, Mythen M (2010) Revised cardiac risk index and postoperative morbidity after elective orthopaedic surgery: a prospective cohort study. Br J Anaesth 105: 744–752.
- Lanes S, Fraeman K, Meyers A, Ives JW, Huang HY (2011) Incidence rates for thromboembolic, bleeding and hepatic outcomes in patients undergoing hip or knee replacement surgery. J Thromb Haemost 9: 325–332.
Citation: Segal O (2019) Hemostasis with WoundClot Hemostatic Gauze in Orthopedic Oncology Surgery. J Orthop Oncol 5: 129. DOI: 10.4172/2472-016X.1000129
Copyright: © 2019 Segal O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3722
- [From(publication date): 0-2019 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 2874
- PDF downloads: 848