Research Article Open Access
Hemostasis in Erysipelas-The Reason for Considering Antithrombotic Therapy
| Fokina EG* | |
| Department of Epidemiology, Federal Budget Institution of Science, Central Research Institute of Epidemiology of the Federal Service on Customers' Rights Protection and Human Well-being Surveillance, Moscow, Russia | |
| Corresponding Author : | Elena G Fokina Department of Epidemiology Federal Budget Institution of Science Central Research Institute of Epidemiology of the Federal Service on Customers' Rights Protection and Human Well-being Surveillance Moscow, Russia Tel: +79032277979 E-mail: e-fokina@yandex.ru |
| Received: October 07, 2015 Accepted: November 06, 2015 Published: November 12, 2015 | |
| Citation: Fokina EG (2015) Hemostasis in Erysipelas-The Reason for Considering Antithrombotic Therapy. J Infect Dis Ther 3:252. doi:10.4172/2332-0877.1000252 | |
| Copyright: © 2015 Fokina This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
The indices of external (prothrombin time, INR) and internal (activated partial prothrombin time (aTpp) coagulation pathways, the degree of disfibrinogenemia (thrombin time, functional fibrinogen activity and D-dimer level), the amount and functional activity of the platelets (aggregation with ADP) and the erythrocytes (aggregation with lanthanoid and protamine sulfate) were studied in 60 patients with erysipelas. Also, we have studied endothelial dysfunction manifested in the decrease of athrombogenicity of vascular wall endothelium (antithrombin III and protein C) and in the increase of adhesive properties of the endothelium (von Willebrand factor).
The comparison groups comprised normal volunteers (n=32) and patients with focus of inflammation localized on the face (n=24), and the legs (n=36) at various stages of the disease (day 1–3; 4-6; 7-10; and 11-15 from the onset of the disease), undergoing in hospital treatment in Moscow 2nd Clinical Hospital for the Infectious Diseases.
The thesis, according to which the rate of hemorrhagic complications in leg erysipelas is by 3,9 times higher than in facial erysipelas, was confirmed by laboratory findings. In particular, a significant decrease of protein C was noted in patients with leg erysipelas and concomitant chronic venous insufficiency. We have found increased D-dimer and decreased α2-macroglobulin levels, suggesting potent activation of proteolytic enzymes (plasmin, matrix metalloproteinases, neutrophil elastase), which can be one of the causes of bullae, erosion and ulceration formation in the erysipelas focus on the lower limb.
The signs of intravascular (latent) hemolysis - the decrease of haptoglobin concentration and the increase of indirect bilirubin and LDH blood level; the changes of rheological properties of erythrocytes – the increase of deformability (aggregation with lanthanum chloride) and the decrease of elasticity (aggregation with protamine sulfate) – have been identified as one of the main factors for DIC-like syndrome in erysipelas.
The comparison groups comprised normal volunteers (n=32) and patients with focus of inflammation localized on the face (n=24), and the legs (n=36) at various stages of the disease (day 1-3, 4-6, 7-10 and 11-15 from the onset of the disease), undergoing in hospital treatment in Moscow 2nd Clinical Hospital for the Infectious Diseases.
The thesis, according to which the rate of hemorrhagic complications in leg erysipelas is by 3.9 times higher than in facial erysipelas, was confirmed by laboratory findings. In particular, a significant decrease of protein C was noted in patients with leg erysipelas and concomitant chronic venous insufficiency. We have found increased D-dimer and decreased α2-macroglobulin levels, suggesting potent activation of proteolytic enzymes (plasmin, matrix metalloproteinases, neutrophil elastase), which can be one of the causes of bullae, erosion and ulceration formation in the erysipelas focus on the lower limb.
The signs of intravascular (latent) hemolysis - the decrease of haptoglobin concentration and the increase of indirect bilirubin and LDH blood level; the changes of rheological properties of erythrocytes - the increase of deformability (aggregation with lanthanum chloride) and the decrease of elasticity (aggregation with protamine sulfate) - have been identified as one of the main factors for DIC-like syndrome in erysipelas.
Erysipelas, being an acute infectious disease, is widely spread and does not depend from the level of industrial development and social security in different countries. Common and local predisposing factors form an important pathogenetic aspect of this condition’s development. Leg erysipelas is often associated with obesity, type 2 diabetes mellitus, chronic venous insufficiency (CVI) and feet and nail mycoses [4-7]. Facial erysipelas often develops in association with otomycosis and chronic diseases of the ORL- organs [8].
Despite modern methods of treatment, up to 10% cases of erysipelas are complicated by the development of skin necrosis at the sites of hemorrhages and vesicles formation, of venous incompetence (periphlebitis, phlebitis, thrombophlebitis). An increasing rate of hemorrhagic forms of erysipelas is being registered [9-15]. In this view, the study of the system of hemostasis and blood rheology during the developing infectious process in patients with lower-limb and facial erysipelas is of thrilling importance.
Primary facial erysipelas was diagnosed in 92% of cases and was more frequently seen in women (16 women, 8 men). Primary lower-limb erysipelas was registered in 50% of cases, repeated erysipelas in 31% and recurrent erysipelas in 19% of cases, unlike the facial erysipelas: primary (92%), repeated (4%) and recurrent (4%). The risk of erysipelas recurrence was statistically significantly higher when the inflammation focus was located on the leg, in comparison with the face (OR=5.55) (1,2,51), p=0.009.
Gender ratio in leg erysipelas was comparable (males-17, females - 19). Feet mycosis and onychomycosis were found to be the most frequent (88%) associated diseases. Obesity grade 2 to 4 was present in 11 patients, 5 patients had subcompensated type 2 diabetes mellitus.
Skin diseases (retroaural dermatitis, streptoderma, psoriasis) were the underlying pathology in 37.5% of patients with facial erysipelas, while 29% of these patients had chronic ORL pathology (otitis, tonsillitis, rhinitis). Four patients had type 2 diabetes mellitus.
The patients underwent in-hospital treatment in Erysipelas department of the 2nd Moscow Clinical Hospital for Infectious Diseases (Head Physician of the Hospital – Miasnikov V.A., M.D., Ph.D Head of Erysipelas department – Potekaeva S.A., M.D.). Thirty-two patients received antibacterial monotherapy: IM benzylpenicillin novocaine salt twice daily, 1,2 mln ME per day for 7-10 days, another two patients received IM Cephalosporin (Cephazolin) 1 g × 3 times daily for 5 days. Combined two-antibiotics therapy (IM benzylpenicillin novocaine salt twice daily, 1.2 ml ME per day for 7-10 days and Ciprolet twice daily per os, 1 g per day for 10 days) was used in 14 patients. Twelve patients got a three-antibiotics combination (benzylpenicillin novocaine salt 1.2 ml ME per day for 7 days+IV Ciprofloxacin 800 mg per day for 3 days with subsequent passage to 1 g per day per os for 10 days+IM Cefazolin 1 g three times per day for 5 days). Additionally, the patients received: antihistamine agents (Zodac, Diazolin); topical physiotherapy (UV irradiation therapy and low frequency current (LFC) for facial erysipelas; UV irradiation therapy, LFC and magnetic therapy for lower-limb erysipelas); the focus of erysipelas (on the legs) was regularly treated with tanning solution of potassium permanganate. The patients who participated in the study did not receive any additional drugs capable to influence their hemostasis’ state.
Mean duration of in-hospital stay was 11.9 + 4.1 days for the patients with leg erysipelas and 8.4 + 1.6 days for the patients with facial erysipelas. The hemostasis indices were studied in the initial stage of the disease (1st to 3rd days) - point 1, during the progression of the disease (days 4-6, 7-10) – points 2 and 3 and during the recovery period (days 11-15) – point 4 of the study. Every third patient with leg erysipelas underwent the follow-up study (in 5 months after the discharge), which allowed to separate the changes induced by erysipelas from the underlying concomitant diseases.
The control group comprised 32 normal subjects aged 24 to 50 years, with equal gender distribution.
The obtained results were proceeded with the use of Statistica 10.0 for Windows 7.0 software. The evaluation of statistically significant (reliable) differences between the groups was performed using: Mann-Whitney test for quantitative values, two independent groups; Kruskall-Wallis test for more than two independent groups; Wilcoxon test for quantitative values, related groups (pre-and post-therapy, in dynamics); chi-square test, and, if necessary, two-tailed Fisher’s exact test for qualitative values. The differences evaluated with the use of parametric and non-parametric methods were considered statistically significant with confidence level >95% (p<0.05). In case of statistically significant inter-group differences, the odds ratio (OR) and 95 confidence interval (CI) were calculated (OR[-95% CI, +95 CI]). The values in the Table are presented as Me (median) and SD - standard deviation [17].
Laboratory investigations of biochemical indices, the parameters of coagulogram, as well as the determination of blood serum protein fractions using electrophoresis were conducted together with the specialists from the express-laboratories of the 2nd Clinical Hospital for Infectious Diseases (Head of Laboratories T.N. Molotilova); the determination of protein C was performed together with the specialist in laboratory diagnosis Dr. T.N. Lagutina in the express-laboratories of Filatov Pediatric City Clinical Hospital 13 (Head of Laboratories T.N. Lagutina, M.D.). The study of aggregation properties of the platelets, the elasticity and the deformability of the erythrocytes was conducted together with senior researcher of the laboratories of hemostasis of the clinical department of Central Research Institute of Epidemiology, O.S. Astrina, the determination of von Willebrand factor-together with the research fellow N.I. Alioshina, (Head of clinical department of Central Research Institute of Epidemiology of Federal Service on Customers' Rights Protection and Human Well-being Surveillance – Professor A.V. Gorelov, M.D.).
Aggregation capacity of the platelets (using Born method) and the erythrocytes (using original technique suggested by Yu.A. Sheremetiev [18]) was determined using photometric technique with the help of SOLAR AP 2110 (Belarus) and LA 230 (scientific-production association BIOLA, RF) aggregometers.
Platelet aggregation was induced using 2 × 10-5 M ADP (Reanal, Hungary); erythrocyte aggregation using 1% protamine sulfate (PS) solution (Ellara, RF) and water solution of lanthanum chloride (LaCl3 Khimik, RF, at a dilution of 150 mg of the chemical agent in 100 ml Ð�2Ð�).
The indices of the external (prothrombin time, International Normalized Ratio, (INR), Quick prothrombin index) and the internal (activated partial thromboplastin time (aPTT) pathways of coagulation were determined using turbodimetric method with an automatic coagulometer ACL ELITE PRO (Instrumentation Laboratory, USA). The disfibrinogenemia degree was evaluated by the thrombin time (TT), functional fibrinogen activity (using Clauss method) and by the content of D-dimer.
The endothelial dysfunction was determined by the degree of athrombogenicity of the vascular wall endothelium (antithrombin III and protein C levels) as well as by the adhesive properties of the endothelium (using von Willebrand factor). Blood plasma levels of antithrombin-III (AT-III, AT) was determined using automatic coagulometer ACL ELITE PRO; of protein C (PC) using automatic coagulometer SYSMEX CA-500 (Siemens Healthcare, USA, chemical agents Siemens AG, Germany). Von Willebrand factor (fvW) was determined using the manual method (1982) and chemical agent Renam (RF).
Blood serum protein fractions were studied using electrophoretic method applied to agarous gel in HYDRASYS analyzer (Sebia, France). The level of the inflammatory reaction was estimated on the base of C-reactive protein (CRP) content using HITACHI 902 analyzer (Roche, Japan).The same analyzer was used for the determination of the content of transferrin, ceruloplasmin and haptoglobin, LDH and LDH1-2 fractions in blood serum. According to the design of the study, we have determined the substrates and the enzymes of biochemical passport [19,20], evaluated clinical blood and urine analyses.
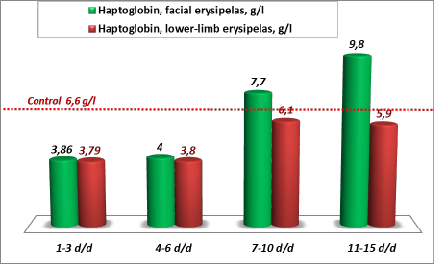
With the account of the increased blood levels of indirect bilirubin and LDH in patients with erysipelas, found in our earlier studies, as well as of the decreased level of haptoglobin found in this study (3.86 ± 26 g/l in facial erysipelas and 3.79 ± 0.3 g/l in lower-limb erysipelas, p<0.05 with the control), the picture of latent hemolysis in erysipelas becomes evident [16,38]. This conclusion is confirmed by further changes of haptoglobin level. The level of this protein increased twofold by ricochet: 253% of the initial values in facial erysipelas and 161% - in lower-limb erysipelas (Figure 3). Baseline values of protein С during the first 3 days of the disease (at admission) in the group of patients with lower-limb erysipelas (81.9 ± 4.9%) were significantly lower, than in patients with facial erysipelas (94.1 ± 6.0%), and reliably below the control values (100 ± 5%, Ñ�<0.05) (Table 1).
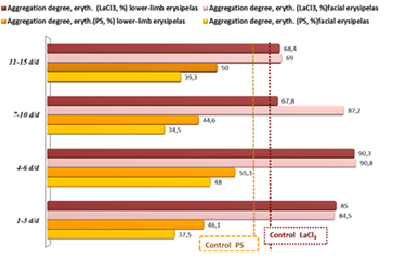
Hence, intravascular (latent) hemolysis is one of the leading pathogenetic mechanisms of DIC-like syndrome. Sime authors describe this syndrome in erysipelas as a clinically inapparent (local) DIC [4,11,12,14,40,43,47]. In our study, we have seen the transformation of DIC-like syndrome to the classical DIC in three (5%) of 60 studied patients [16,38]. We studied also rheological properties of erythrocytes, their elasticity (as judged by the aggregation with protamine sulfate) and deformability (by the aggregation with lanthanum chloride) in facial and lower-limb erysipelas. The degree of blood cells’ elasticity and deformability in normal subjects is almost equal: 62 ± 4.9% for PS and 66.4 ± 4.2% for LaCl3. With the decrement of the erysipelas focus, the level of protein C recovered gradually in both groups: 119.6 ± 3.1 at day 4-6 of the disease, 129 ± 6.4% at day 7-10, 153 ± 4.4% at day 11-15 in patients with facial erysipelas (Ñ�<0.05 between the study points 1/4, 2/4 and 3/4); and 103 ± 3.2%, 134.5 ± 4.7% , 139 ± 6.7% in patients with leg erysipelas (Ñ�<0.05 between the study points 1/4, 2/4).
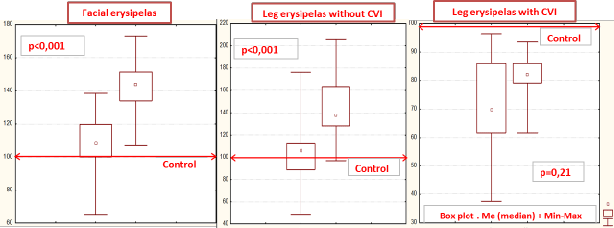
The level of protein C in patients with lower-limb erysipelas without CVI (n=28) was 99.8 ± 4.7% during the acute stage of the disease, and increased to 140 ± 4.5% during the recovery stage (Ñ�<0.001) (Figure 1). In lower-limb erysipelas with CVI, the low baseline level of protein C (69.8 ± 8.1%) did not significantly change during the therapy –79.15 ± 4.0%, Ñ�=0.21 and remained below the level seen during the recovery in patients with erysipelas without CVI (Figure 1). The level of protein C recovered to the normal value during the third week of the disease in patients with facial erysipelas (108.2 ± 5.1% at admission and 144 ± 4.6% at discharge; Ñ� for points 1/4<0.001); and during the fourth week in patients with leg erysipelas without CVI (99.8 ± 4.7% at admission and 140 ± 4.4% at discharge; Ñ� for points 1/4<0.001). In patients with leg erysipelas with CVI, the level of protein C remained refractory low during the follow-up: 69.8 ± 8.1% at admission, 79.15 ± 4.07% at discharge; Ñ� for points 1/4=0.21) (Table 1).
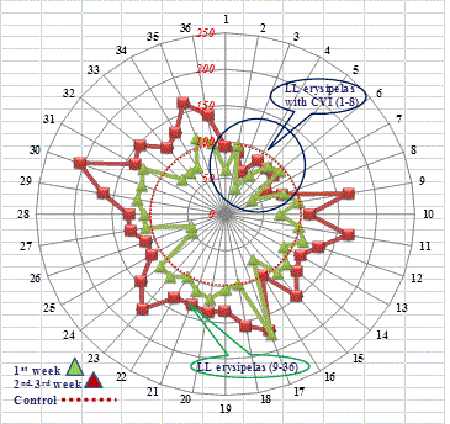
The changes in the level of protein C in the settings of endothelial dysfunction in certain patients (from 49.7% (point 1) to 112% (point 4) increase 125%; from 48.9% (point 1) to 110.7% (point 4) increase 126%; from 65.5% (point 1) to 119.7% (point 4) increase 83% deserves attention as an effective mechanism of native sanogenesis factors’ work during the recovery (Figure 2).
According to some authors, CVI leads to the prolongation of the healing time of erysipelas focus and of the recovery period [4,13-15,21]. We were able to prove, that in the presence of normal protein C levels (100 ± 5%), the chances for the favorable course of leg erysipelas are significantly higher (OR=2.89) (0,15,55) than in erysipelas with low protein C level and associated chronic venous insufficiency. In the absence of CVI, the recovery in our patients with lower-limb erysipelas was shorter.
Besides protein C, we have determined the level of another, not less important natural anticoagulant – the antithrombin III (AT-III). According to the literature, the thromboses (strokes, infarctions) develop when AT-III level is 80-90%. Antithrombin deficiency occurs in the presence of clinical manifestations of disseminated intravascular coagulation (DIC) and of the developing of multiorgan failure syndrome [13,25,28,40,43].
Antithrombin deficiency was more manifested in patients with lower-limb erysipelas, than with facial erysipelas (Table 1). The baseline values of AT-III in patients with leg erysipelas (81.6 ± 2.5%) were statistically lower than in facial erysipelas (91.8 ± 2.5%) (p<0.05 between the groups, the value in the control group being 97.3 ± 0.38%), and did not recover before the 15th day of the disease (Table 1). Antithrombin-III deficiency seen in patients with leg erysipelas can explain higher incidence of hemorrhagic forms of erysipelas (78%) in this group of patients in comparison with the patients with facial erysipelas (22%) (Table 1). Besides, AT-III, (as well as fibrinogen, CRP, orosomucoid) is an acute-phase protein. Our previous studies have shown that the level of acute-phase proteins in patients with lower-limb erysipelas was higher than in patients with facial erysipelas [6,16].
The concentration of α2-macroglobulin (α2–MG) decreased by 25%. The minimal level of α2–MG – an universal inhibitor of various proteases, including plasmin – was seen at the end of the 1st week of the disease: 3.78 ± 0.16% in facial erysipelas and 3.96 ± 0.16% in leg erysipelas. It is known that β-hemolytic streptococcus (β-HS) produces several pathogenicity factors (streptokinase, hyaluronidase, streptodornase), that destroy the protective level of heparansulfate, lining the vascular wall endothelium. This is accompanied by an increase of prothrombogenic properties of the vascular wall endothelium, the release of von Willebrand factor and the decrease of antithrombin III activity [9,10,12,16,27]. We have noted an increase of fvW within the interval from 220% to 187% during the first week of the disease in all patients with erysipelas, irrespective of the localization of the inflammation focus (face 187 ± 4.2%, lower limb 190 ± 2%, Ñ�<0.05 for the control value: 150.4 ± 3.9%). With the extinction of the erysipelas focus, there was a tendency for the decrease of high values of von Willebrand factor, however the studied index was not fully normalized (Table 1).
Thus, the obtained results of the study of endothelial markers in patients with erysipelas suggest that their endothelium-related hemostasis regulation was compromised. It concerned not only antithrombotic (decreased levels of protein C and antithrombin III), but also adhesive characteristics (high level of von Willebrand factor). The deficiency of natural anticoagulants was statistically more significant in leg erysipelas in comparison with facial erysipelas. The detected protein C deficiency without a tendency for recovery in the settings of conducted therapy, on the one hand, serves as a laboratory indicator of existing CVI in the presence of the lower-limb erysipelas, and, on the other hand, can be an indication for the prescription of vascular replacement therapy in patients with CVI [30-35]. It is known that the body uses natural anticoagulants for the isolation (delimitation) of infective inflammation area, and the decrease of anticoagulants’ concentration lowers the resisting barrier and opens the gate for the generalization of infective inflammation [29,30].
We have found the following shifts in the indices of the external (prothrombin time, prothrombin index, INR) and the internal (aPTT) coagulation pathways, in the degree of disfibrinogenemia (thrombin time, functional platelets’ activity and D-dimer concentration) in our patients with the lower-limb and facial erysipelas:
• The activation of coagulation cascade during the acute stage of the disease - the shortening of thrombin time to 12.2 ± 0.5 sec in facial erysipelas and to 10.9 ± 0.36 sec in leg erysipelas during the days 1-3 of the disease (p<0.05 with control index of 14.6 ± 0.26 sec), suggesting the presence of active processes of thrombin and fibrin formation in the blood flow;
• The activation of the external coagulation pathway – the lengthening of prothrombin time to 15.4 ± 0.5 sec in lower-limb erysipelas and to 12.2 ± 0.7 sec in facial erysipelas (normal subjects 10.9 ± 0.14 sec, p=0.033 with the control value), the decrease of prothrombin index and the increase of international normalized ratio (INR), (Table 2);
• The activation of the internal coagulation pathway – the lengthening of aPTT: 39.5 ± 1.4 sec (point 1) at admission and 37.4 ± 1.3 sec during the recovery period in leg erysipelas (Table 2). In facial erysipelas the baseline aPTT was shortened: 28.5 ± 1.3 sec (p<0.05 with the control value: 33.7 ± 0.66 sec) and lengthened to 45.1 ± 2.7 sec (p=0.025) by the end of the 1st week of the disease.
• The disfibrinogenemia – the shortening of thrombin time with the activation of the process of fibrin polymerization and the appearance of a great amount of D-dimers in the patient’s blood. During the 1st week of the disease, the fibrinogen level was higher (than during the 2nd week) in patients with erysipelas localization on the lower limb in comparison with the patients with facial erysipelas (Day 1-6 of the disease –8 ± 2.3 g/l leg vs 5.9 ± 1.8 g/l face, Ñ�=0.008, Day 7-15 of the disease 6.6 ± 2.4 g/l leg vs 4.3 ± 1.0 g/l face, Ñ�<0.0001), and by 2.7 times higher than the control values (2.96 ± 0.09 g/l, Ñ�<0.001).
Higher level of fibrinogen, an acute-phase protein (together with AT-III, C-reactive protein, presepsin, procalcitonin, TNF-alpha and interleukin- 6), can be considered as a more pronounced manifestation of inflammatory events [28,29,33,37]. Hence, the degree of disfibrinogenemia and the activation of the external and internal coagulation pathways were higher, when the erysipelatous focus was located on the lower limb. The peak of hemocoagulation changes was registered at days 4-6 of the disease (point 2 of the study), (Tables 2 and 3). While fibrinogen level at days 1-3 of lower-limb erysipelas (7.7 ± 0.38 g/l) was higher than in facial erysipelas (6.53 ± 0.49 g/l) by 18%, the concentration of plasma D-dimers in patients with lower-limb erysipelas (399 ± 46 ng/ml) was more than twofold in comparison with the patients with facial erysipelas (160.2 ± 41 mg/ml), and by 27 times higher than in normal subjects (14.5+3.18 ng/ml, Ñ�<0.001).
These differences in D-dimers blood concentration allowed us to conclude that the processes of intravascular coagulation in lower-limb erysipelas are more intense, as a necessary condition for the increase of D-dimer level consists in the presence of the clots of polymerized fibrin in the blood. The process of plasminogen transformation to plasmin, occurring on the principle “right here, right now”, takes place inside these clots [28,32,35,41]. The increase of D-dimer level and the decrease (expenditure) of alpha-2-macroglobulin are also are suggestive of a potent local activation of the system of proteolytic enzymes (plasmin, matrix metalloproteinases, neutrophil elastase), inducing the process of the erosion, ulcer and necrosis formation in the infectious inflammation focus [11,28-31]. Starting from the second week of the disease, the levels of acute-phase proteins (fibrinogen, CRP, α1-antitripsin (α1-AT) and orosomucoid) (=α1- acid glycoprotein) decreased with the improvement of patients’ condition (Table 3).
Disfibrinogenemia, activated external and internal coagulation pathways, as well as increased level of acute-phase proteins found in our study confirm a close relation between the systemic inflammatory response and the compromised hemostasis with their mutual potentiation [1-3,25,28,30,33,37,40]. The risk of the development of hemorrhagic (erythematous-hemorrhagic, bullous-hemorrhagic) erysipelas was significantly higher, when the focus of inflammation was located on the legs, than on the face (OR=9.88) (2,34,73,81). The risk of severe forms of erysipelas development on the lower limb (erythematous-bullous, erythematous-hemorrhagic, bullous-hemorrhagic) also was higher in comparison with the face (OR=4.9) (1,5,16).
In normal settings, the circulating platelets do not interfere with the internal surface of the vessel, covered by a thin layer of heparansulfate, that confers athrombogenic and anti-adhesive properties to vascular endothelium. The vascular wall injury results in the exposition of subendothelium components, mainly collagen, into the blood flow; in case of the participation of fvW (interaction with the platelet GP1b receptors) and of fibrinogen (interaction with the platelet GPIIb/Ша receptors), the processes of platelet adhesion and aggregation are significantly enhanced [27,28,41,42,46].
In our study, the ADP-induced platelet aggregation was minimal at days 1-3 of the disease: 41.7 ± 4% in facial erysipelas and 64.8 ± 3.9% in leg erysipelas (p<0.05), which is statistically lower in comparison with platelets’ functional activity in normal subjects (76 ± 3.1%, p<0.05). At days 11-15 of the disease, there was a tendency for the recovery of platelets’ functional activity (71.7 ± 3.4% in lower-limb erysipelas and 59 ± 3.2% in facial erysipelas). The number of platelets increased in both groups: from 224 ± 10 × 109/l (point 1) to 408 ± 21 × 109/l (point 3), (p=0.002) in leg erysipelas and from 234 ± 30 × 109/l to 317 ± 29 × 109/l (p=0.04) in facial erysipelas. Hence, the recovery of the number and the functional activity of the platelets in erysipelas occurred by the end of the second week of the disease.
In erysipelas, the cells’ elasticity decreased twofold (aggregation with PS), while the deformability (aggregation with LaCl3) increased by 37% (Table 4). We used aggregometer Biola to measure the size of erythrocytic aggregates and the height of aggregation curve simultaneously with two types of inductors (LaCl3 and PS). The aggregates were 3.6 times bigger, and the degree of aggregation by 7.8 times higher with LaCl3 than with PS. With the addition of LaCl3, the erythrocytes of erysipelas patients interacted faster (3-5 minutes), and formed the blocks, that quickly precipitated to the bottom of the test-tube forming large conglomerates apparent to the naked eye [16]. Other authors also have noted erysipelas-related changes of the erythrocyte membrane with cells’ form transformation from concave-discoid to spherical [4,14,18,47]. Our experiments confirm that with the addition of lanthanoid, the erythrocytes of erysipelas patients undergo a conformational rebuilding (the destruction of membrane’s cytoarchytectonics), which leads to fast adhesion between the cells and to the formation of cell conglomerates. Thus, the aggregation with LaCl3 helps to reproduce the picture of erysipelas-associated intravascular hemolysis in vitro. Protamine sulfate does not induce conformational rebuilding of the membrane. It displace, in a stepwise manner, various previously adsorbed substrates from the erythrocyte membrane, with their subsequent replacement by native proteins. This is accompanied by the repolarization of the erythrocyte membrane. With the loss of negative charge of the membrane that prevents cells adhesion to each other, the erythrocytes form the rouleaux. Visually, the aggregates on the PS are smaller and softer, and the aggregation reaction develops by several times slower (≥ 10 minutes), than on the lanthanoid [18,38].
The results of determination of the markers of hemostatic endothelial function in patients with erysipelas suggest the disturbances of endothelium-related regulation of hemostasis, as judged by antithrombotic (decreased levels of protein C and of antithrombin III), as well as by adhesive indices (high level of von Willebrand factor).
Increased level of D-dimer and decreased level of alpha-2-macroglobulin suggest potent activation of the system of proteolytic enzymes (plasmin, matrix metalloproteinases, neutrophil elastase), with is pathogenetically associated with the formation of bullae, erosions and ulcers in the erysipelas focus.
Low level of protein C, persisting during standard therapeutic procedures, is not only a laboratory indicator of CVI in leg erysipelas, but also serves as a marker of the development of DIC-like syndrome.
Documented protein C deficiency can serve as an indication for the solution of the question of antithrombotic therapy application. Maximally early start of the replacement therapy (with recombinant protein C) can contribute to the delimitation of the area of inflammatory focus and to the decrease of the severity of local proteolysis reactions in erysipelas.
We found the signs of intravascular (latent) hemolysis and of disturbed rheological properties of erythrocytes – increased deformability (aggregation with lanthanum chloride) and decreased elasticity (aggregation with protamine sulfate). The disturbed erythrocytes elasticity is an indication for the supplementation of standard erysipelas therapy with the agents contributing to the increase of red blood cells flexibility and to the improvement of rheological properties of the blood (e.g., Pentoxifylline).
The risk of hemorrhagic complications in lower-limb erysipelas is higher, than in facial erysipelas, OR = 9.88 [2,7,34].
References
- Chen D, Dorling A (2009) Critical roles for thrombin in acute and chronic inflammation. J ThrombHaemost 7 Suppl 1: 122-126.
- Kile BT (2009) The role of the intrinsic apoptosis pathway in platelet life and death. J ThrombHaemost 7 Suppl 1: 214-217.
- Castellino FJ, Ploplis VA (2009) The protein C pathway and pathologic processes. J ThrombHaemost 7 Suppl 1: 140-145.
- Erovichenkov AA (2003) Clinical and pathogenetic significance of hemostasis disturbances and their correction in patients with hemorrhagic erysipelas. Synopsis of Doctoral thesis. Moscow (in Russian).
- Potekaeva SA, Erovichenkov AA, Anokhina GI, Kolaeva NV, et al. (2010) Particularities of clinical course of erysipelas in the settings of metabolic syndrome Infectious diseases: Proc. 2nd Annual All-Russian Congress of Infectious diseases Moscow, pp. 252-253.
- Fokina EG, Rosly IM, Potekaeva SA (2014) Laboratory evaluation of erysipelatousimflammation. Epidemiologia i infekcionnyebolezni. Aktualnyevoprosy (Epidemiology and infectious diseases. Thrilling questions). 1: 28-32.
- Denis F, Martin C, Ploy MC (2001) [Erysipelas: microbiological and pathogenic data]. Ann DermatolVenereol 128: 317-325.
- Morozova SV, Timurzieva AB (2015) Professional view of the problem of external otitis. IDOCTOR. BionicaMedica Publishers, Moscow, Russia, pp. 8-11.
- Briko NI (2000) Streptococcal (group A) infection: view of situation, existing at the beginning of the 21st century. Vrach (Physician), Moscow, Russia, pp. 19-22.
- Marzhokhova AR (2015) Indices of intoxication syndrome in patients with erysipelas. Synopsis of PhD thesis./Nalchik pp. 173.
- Frolov AP (2003) Etiopathgenetic particularities of the development of necrotic form of erysipelas, its prediction and principles of complex therapy. Synopsis of PhD thesis. /Irkutsk, pp. 162.
- Mitrofanova My (2010) Disturbances of hemostasis and vascular endothelial function in patients with erysipelas. Synopsis of PhD thesis, Moscow, Russia.
- FazylovVKh (1996) Disturbances of hemostasis and immunity during the formation of erysipelas recurrence, their therapeutic correction. Synopsis of Doctoral thesis, St. Petersburg, Russia.
- Ratnikova LI, Dubovikova TA (2012) Evaluation of the state of vascular platelet hemostasis in patients with hemorrhagic forms of erysipelas Zhurnalinfectologii (Journal of infectology) 1: 53-57.
- Ziguidullina AI, FazylovVKh, ValeevaIKh (2003) State of lipids peroxidation in patients with hemorrhagic erysipelas, 6th Russian Congress of Infectionists,St. Petersburg, pp. 142.
- Fokina EG (2014) [Some features of primary facial erysipelas under the present conditions]. TerArkh 86: 70-77.
- RebrovaOYu (2002) Statistical analysis of medical data. Use of application programs STATISTICA, Media Sphera Publishers, Moscow, Russia, pp. 114.
- SheremetievYuA, Makin GI, SuslovFYu (1995) A method for the registration of the changes of surface erythrocyte charge (lanthanum chloride) Federal Service for Intellectual Property. Patent 2027188.
- Fokina EG, Rosly IM (2014) Individual biochemical passport. Vrach (Physician), Moscow, 6: 7-12.
- Papachristodoulou D, Snape A, Elliott WH, Elliott DC (2014) /Biochemistry and Molecular Biology. 5th edition «Oxford university press», Oxford, United Kingdom, pp. 592.
- Khasanov AG, Shaybakov DG (2013) The ways for increasing the effectiveness of antibacterial therapy in erysipelas.Mejdunarodnyzhurnalpricladnykh I fundamentalnykhissledovaniy (International Journal of Applied and Fundamental Investigations). Medical Sciences, pp. 43-48.
- Buyanov VM, Alekseev AA (1990) Lymphology of endotoxicosis, Meditsina Publishers, Moscow, Russia, pp. 272.
- Dahlbäck B (1997) Resistance to activated protein C as risk factor for thrombosis: molecular mechanisms, laboratory investigation, and clinical management. SeminHematol 34: 217-234.
- Egorova VV, Titova MI, Demidova VS (2013) Modern methodological aspects of laboratory diagnosis of protein C system and the importance of its study in surgery. Meditsinskyalfavit. Sovremennayalaboratoria (Medical alphabet. Modern laboratories) 3: 12-17.
- Bockeria LA, Klimovich LG, Potekhina AV (2004) Mechanisms of coagulation inhibitors involvement into the development of inflammatory reaction and perspective trends of anticoagulation therapy. Klinicheskayafisiologiakrovoobraschenia (Clinical physiology of the circulation). 1: 46-55.
- Erovichenkov AA, Potekaeva SA, Pak SG, BukholtzSYu (2008) Particularities of modern erysipelas clinic. Proc. of Euro-Asiatic Congress of Infectious Diseases. Vitebsk 1: 138-139.
- PervushinYuV, RogovaSSh, Kovalevich NI (2009) Laboratory methods of studying the hemostasis system and the diagnosis of hemocogaulation disturbances. Manual of State Educational Institution «Stavropol State Medical Academy, Stavropol,Moscow, pp. 60.
- Samsonova NN, Klimovich LG, Rogalskaya EA (2014) Pathogenesis of postoperative coagulopathy. . Klinicheskayafisiologiakrovoobraschenia (Clinical physiology of the circulation). Publishing house of Bakoulev Center for Cardiovascular Surgery , Moscow, pp. 17 -24.
- Esmon CT, Taylor FB Jr, Snow TR (1991) Inflammation and coagulation: linked processes potentially regulated through a common pathway mediated by protein C. ThrombHaemost 66: 160-165.
- Esmon CT (2001) Role of coagulation inhibitors in inflammation – Thromb 86: 51-56.
- Coughlin SR (2001) Protease-activated receptors in vascular biology ThrombHaemost 86: 298-307.
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, et al. (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344: 699-709.
- Study of the effectiveness and the safety of Coagil-VII-Eptacog alpha (activated) in patients with bleeding disorders operated under extracorporeal circulation N.N. Samsonova, M.M. Rybka, G.V. Lobacheva, L.G. Klimovich EF, Kozar TS, Schegolkova EA (2012) RogalskayaTromboz, guemostas i reologia (Thrombosis, hemostasis and rheology) 3: 52-56.
- Klimovich LG, Kozar EF, Kraynichenko TV (2007) Anti-inflammatory and anticoagulation effects of recombinant activated protein C in complex intensive therapy of sepsis in neonates and infants after heart surgery, 3rd All-Russian Conference “Clinical hemostasiology and hemorheology in cardiovascular surgery: proceedings, pp.101.
- Bockeria LA, Lobacheva GV, Kharkin AV, Ochakovskaya E Yu, Samsonova, LG (2006) First experience with the use of recombinant activated protein C in complex intensive therapy of sepsis in neonates and infants after cardiac surgery. KlimovichDetskiebolezniserdza i sosudov (Pediatric cardiovascular diseases) 35: pp. 60-64.
- Sridharan P, Chamberlain RS (2013) The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills? Surg Infect (Larchmt) 14: 489-511.
- Velkov VV Presepsin (2014) Early and highly effective sepsis marker . Meditsinskyalfavit. Sovremennayalaboratoria N4 (Medical alphabet. Modern laboratories N4). 22: 42-48.
- Fokina EG, Rosly IM (2014) Laboratory evaluation of erysipelas. Epidemiologia i infekcionnyebolezni. Aktualnyevoprosy (Epidemiology and infectious diseases. Thrilling questions). Bionika publishers, Moscow, Russia, pp. 28-32.
- Fokina EG (2014) Some particularities of primary facial erysipelas in present-day settings.Terapevticheskyarkhiv (Therapeutic archives). Media Sphera publishers, Moscow, Russia, pp. 70-77.
- Bokarev IN, Kozlova TV, Popova LV (2007) Laboratory diagnosis of hemocoagulation system. Tromby, krovotochivost i boleznisosudov (Thrombi, bleeding disorders and vascular diseases) 53-56.
- Vavilova TV (2005) Hemostasiology in clinical practice (textbook for physicians), St. Petersburg, Publishing house of St. Petersburg Pavlov State Medical University, pp. 92.
- Schiffman FJ (2007) Pathophysiology of blood, BINOM publishers, Moscow, Russia, pp.446.
- Maleev VV, Poliakova AM, Kravchenko AV (2005) Hemostasis disturbances in infectious diseases. DeNovo publishers, Moscow, pp. 160.
- Mechanisms of adaptation in diphtheria and erysipelas. Round Table./ Fokina E.G. Epidemiologia i infekcionnyebolezni. Aktualnyevoprosy (Epidemiology and infectious diseases. Thrilling questions). Bionika publishers, Moscow, pp. 53 -57.
- Rosly IM, Shuliak Yu A (2004) Practical biochemistry. Borgues publishers, Moscow, Russia, pp. 166.
- Bouriachkovskaya LI (2007) Heterogenicity of human and animal platelets Relation between morphological particularities and functional state. Synopsis of Doctoral thesis in Biology.Moscow,pp. 47.
- GV (2002) Clinical and pathogenetical significance of morphofunctional erythrocyte disturbances in erysipelas. Synopsis of PhD thesis in medicine, pp. 23.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11265
- [From(publication date):
December-2015 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 10328
- PDF downloads : 937
