Research Article Open Access
Hemoglobin Loaded Alginate Particles
Alberto Dessy*,#, Anna Maria Piras# and Federica Chiellini
Laboratory of Bioactive Polymeric Materials for Biomedical and Environmental Applications (BIOlab), via Vecchia Livornese 1291, 56122, San Piero a Grado (Pi), UdR INSTM, Department of Chemistry and Industrial Chemistry, University of Pisa, Pisa, Italy
- Corresponding Author:
- Alberto Dessy
Laboratory of Bioactive Polymeric Materials for
Biomedical and Environmental Applications (BIOlab)
via Vecchia Livornese 1291, 56122, San Piero a Grado (Pi)
UdR INSTM, Department of Chemistry and Industrial Chemistry
University of Pisa, Pisa, Italy
E-mail: albertodessy@virgilio.it
- Anna Maria Piras
Laboratory of Bioactive Polymeric Materials for
Biomedical and Environmental Applications (BIOlab)
via Vecchia Livornese 1291, 56122, San Piero a Grado (Pi)
UdR INSTM, Department of Chemistry and Industrial Chemistry
University of Pisa, Pisa, Italy
E-mail: apiras@ns.dcci.unipi.it
Received date June 01, 2013; Accepted date July 07, 2013; Published date July 15, 2013
Citation: Dessy A, Piras AM, Chiellini F (2013) Hemoglobin Loaded Alginate Particles. J Biomim Biomater Tissue Eng 18:102. doi: 10.4172/1662-100X.1000102
Copyright: © 2013 Dessy A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
Over the last few years medical and pharmaceutical industries have shown an increasing interest in alginate, an anionic polysaccharide widely distributed in the cell walls of brown algae. The present work aims at loading Human Hemoglobin (Hb) into alginate particles; the modification of some formulation parameters was carefully investigated by analysing changes on particles size and protein physicochemical properties. Particles were prepared by dropping alginate into an aqueous solution containing Hb and CaCl2, which permits the formation of particles through ionic crosslinking. Hb loaded alginate beads were obtained possessing an average diameter of about 2 mm and a protein loading of about 5%. Physicochemical characterizations showed that the protein maintained its functional ability of reversibly binding oxygen and its quaternary structure once loaded into alginate beads. In a refinement of the first formulation trials, Hb loaded alginate microparticles with diameter around 150 μm were obtained with a protein loading of about 50%.
Keywords
Natural polymers; Polysaccharides; Ionotropic gelation; Proteins encapsulation
Introduction
Alginates are linear, unbranched, biocompatible polysaccharides directly extracted from different seaweed such as Laminaria hyperborea, Ascophyllum nodosum and Macrocystis pyrifera. Alginates are composed of (1-4)-linked β-R-mannuronic acid (M units) and α-Lguluronic acid (G units) monomers which vary in amount on the basis of the extraction source. Because of their biocompatibility, abundance in source, and low prices, they have been widely used in the food industry as thickeners and emulsifying agents. Alginates are commonly used also in pharmaceutical applications such as floating drug delivery systems, in situ forming ophthalmic drug delivery systems, mefenamic acid loaded alginate beads and insulin loaded oral drug delivery system [1,2]. Drug loaded alginate particles are mainly prepared by dropping a sodium alginate solution containing the desired drug into a crosslinking solution, where divalent ions such as Ca2+ Sr2+ or Ba2+ are dissolved [3].
The gelation and crosslinking of the polymers are achieved by exchanging the counter ions (generally sodium ions) of the guluronic moieties with the divalent cations, giving rise to the so called egg-box structure. The divalent cations bind the α-L-guluronic acid blocks in a highly cooperative manner; the size of the cooperative unit is generally bigger than 20 monomers. Each alginate chain can dimerize to form junctions with many other chains; as a result gel networks are formed rather than insoluble precipitates. The use of alginate based drug delivery systems presents many advantages mainly related to the encapsulation process which takes place under mild conditions, i.e. room temperature and without the use of organic solvents. Moreover alginate based particles present a high gel porosity which allows macromolecules diffusion [4].
Although few studies reporting the loading of Human Hemoglobin (Hb) into alginate particles are present in the literature [5,6], little is known regarding the maintenance of Hb physicochemical properties after its loading into alginate particles, and how particles size affects this parameter. In this perspective the present work is aimed at the preparation and characterization of alginate based polymeric particles loaded with Hb; the evaluation of changes in the protein biochemical properties after particles size reduction is also part of the present research activity. Beads possessing an average diameter of 2 mm were easily obtained by means of ionic gelation; the loaded protein was characterized by a functional and a structural point of view, suggesting that Hb preserve its activity of reversibly binding oxygen. One of the main findings arising from the present study is related to the feasibility of reducing alginate particles dimensions to a micrometric scale. Although a physicochemical interaction between alginate and Hb was observed, the protein functional properties of reversibly binding oxygen were maintained also when loaded into smaller particles.
Experimental
Materials
Sodium Alginate (250 cps at 25°C, 2% solution), Sodium Chloride, Agarose, Alginate Lyase, Tris base, Glycine, Bromophenol Blue, Glycerol and Drabkin’s reagent were purchased from Sigma Aldrich. Calcium Chloride was purchased from Carlo Erba. Human Serum Albumin (HSA) and Human Hemoglobin (Hb) were kindly supplied by Kedrion S.p.A., Castelvecchio Pascoli, Lucca, Italy.
Phosphate Buffer Saline (PBS 0.1M) was prepared by dissolving 2.0 g of KCl, 2.0 g of KH2PO4·H2O, 80 g of NaCl, and 15.6 g of Na2HPO4·12H2O in 1 liter of distilled water. The pH was adjusted to 7.4 with 10 N NaOH and the resulting solution was sterilized in an autoclave (121°C for 20 min) before use and storage. Working solutions were obtained by diluting PBS 0.1M with distilled water at 1:10 v/v (PBS 0.01 M).
50 mM Tris buffer solution was prepared by dissolving 7.09 g of Trizma buffer in 1 liter of distilled water. The pH was adjusted to 8.0 and the resulting solution was autoclaved (121°C for 20 min) before use and storage.
Physiological solution was prepared by dissolving 9.0 g of NaCl in 1 liter of distilled water. The solution was sterilized in an autoclave (121°C for 20 min) before use and storage.
Electrophoresis sample buffer was prepared by adding 8 mL of glycerol, 4 mL of Tris buffer solution (1.5 M, pH 9.2) and 0.01 g of Bromophenol blue to 28 mL of deionized water under magnetic stirring.
Tris Glycine buffer were prepared by adding 8 g of Tris Base and 36 g of Glycine to 500 mL of water under magnetic stirring.
The commercial products were used without any preliminary purification if not otherwise stated.
Methods
Hb Loaded Alginate Particles
Hb loaded alginate particles (beads and microparticles) were obtained by means of ionotropic gelation. Alginate was dissolved in physiological solution and mixed with Hb in Tris Buffer pH 8. The resulting solution was added dropwise, using a syringe equipped with a 22G needle, to a water solution containing calcium chloride, under magnetic stirring. Formulations details relevant to three different procedures indicated as A, B and C are reported in Table 1.
Alginate particles characterization
Dimensional analyses were carried out using laser diffraction. Alginate particles suspension were added into the analysis cell until 30– 50% obscuration of the PIDS detector was reached. Deionised water was used as background and the diameter distribution was processed using Fraunhofer optical model. Three runs were performed on each sample.
Alginate particles degradation
In order to investigate the maintenance of Hb functional activity after its loading into alginate particles, samples were degraded using alginate lyase as briefly described as follows: particles were washed three times with PBS 1X pH 7, in order to remove the excess calcium ions. After sedimentation (beads) and centrifugation at 8000 g for 30 minutes at 37°C (microparticles), the alginate particles were suspended under magnetic stirring in a 10 mL PBS 1X pH 7 solution containing 0.08% (w/l) of alginate lyase. The solution was kept at 37°C under magnetic stirring until complete degradation was achieved (30 minutes).
Encapsulation efficacy and Hb loading into alginate particles
| Formulation Name | Alginate(mg)/Physiological solution(mL) | Hb(mg)/Tris Buffer(mL) | CaCl2(mg)/Water(mL) |
|---|---|---|---|
| BsHb | 40/2 | 8/0.16 | 150/10 |
| MPsHb_A | 10/10 | 2/0.04 | 60/30 |
| MPsHb_B | 10/10 | 2/0.04 | 60/30 |
| MPsHb_C | 10/10 | 20/0.04 | 60/30 |
Table 1: Details of Hb-Loaded Alginate Particles Composition.
Hb encapsulation in alginate particles was evaluated by a colorimetric assay based on Drabkin’s reagent. Experiments were carried out on particles collected after the lyase dissolution. Once dissolved, three particles samples were collected and 0.4 mL of each of them were added to 2.0 mL of Drabkin’s solution. Samples were then incubated for 1 h at room temperature (20 ± 2°C), in the dark, and the UV spectrum between 450-700 nm was measured (UNICAM UV 500 ThermoSpectronic). The same procedure was applied to a blank sample prepared with Tris buffer pH 8. Hb concentration was determined using a calibration curve obtained by plotting the absorbance at 540 nm of standard Hb solutions (250–2500 μg/mL) vs. their concentration in μg/mL (R2: 0.99). All standards and sample spectra were normalised on their absorbance at 650 nm (reference wavelength). The Drabkin solution was prepared as indicated by the producer. One vial of the reagent and 0.5 mL of Brij 35 solution were added to 1 L of water. After vigorous stirring, the solution was stored in the dark at room temperature. In parallel three samples of the dissolved alginate particles were lyophilised, weighted and used as a reference.
The Encapsulation Efficacy (EE) was calculated as a percentage of recovered Hb with respect to the total protein employed in the preparation process.
The Protein Loading (Ldg) was calculated as percentage of recovered Hb with respect to particles weight.
Evaluation of hemoglobin capability of reversibly binding oxygen
Hb capability of reversibly binding oxygen was detected by analysing changes in Hb UV absorption spectra (200-700 nm) in oxygenated and deoxygenated solutions. Oxygenated solutions were generated by means of a 10 minutes atmospheric oxygen exposure, while deoxygenated solutions were obtained by means of a 10 minutes nitrogen flux exposure.
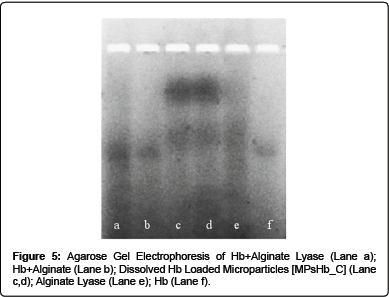
Agarose gel electrophoresis of Hb loaded alginate particles
Dissolved Hb loaded alginate particles were submitted to electrophoretic analysis in 1.5 % agarose gel. 7 × 7 cm agarose gels (0.75 mm thick) were prepared by adding 0.75 g of agarose to 50 mL of Tris Glycine electrophoresis buffer; in order to dissolve agarose, the solution was warmed using a magnetic hot plate. After the agarose solution was cooled to 50-60°C, it was put into a gel caster. The so formed agarose gel was placed into the electrophoretic chamber and covered with the Glycine Electrophoresis buffer. Samples were prepared by mixing 500 μl of dissolved particles, possessing a protein concentration of 0.4 mg, with 500 μl of electrophoretic sample buffer. The diluted samples were loaded into agarose gel wells and the electrophoretic run was carried out for 30 minutes at 150 V.
Instrumentation
UV-Vis absorption spectra measurements were performed using a Jasco V–530 spectrophotometer. Dimensional analyses were carried out by using a Coulter LS230 Laser Diffraction Particle Size Analyzer, equipped with small volume module plus. Samples were added into the cell until 30–50% obscuration of PIDS detector. Particles purification processes and cell recovering were performed in ALC® PK121R centrifuged, equipped with AM-10 and AM-21 rotors. Protein Electrophoretic profiles were detected by placing agarose gels into the Bio Rad Gel Doc XR, using a Bio Rad PowerPac Universal Power Supply.
Results and Discussion
Hb loaded alginate beads
Hb loaded alginate beads (BsHb) were prepared by means of ionic gelation. BsHb with an average diameter of about 2 mm were obtained as shown in Figure 1.
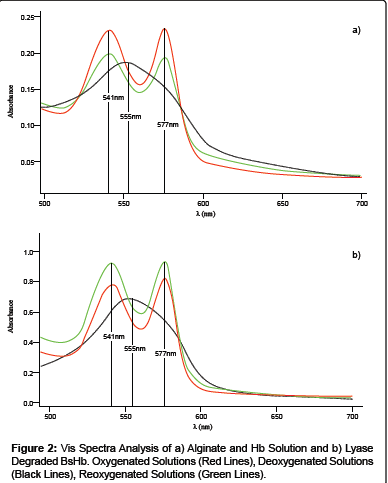
In order to check whether the polymer used in the preparation of Hb loaded particles could interfere with the protein ability of reversibly binding oxygen, UV-Vis spectra analysis were performed on oxygenated, deoxygenated and reoxygenated solutions containing alginate and Hb.
As reported in Figure 2, the protein showed the oxy-form UV region characteristic peaks in the oxygenated and reoxygenated solutions (541 and 577 nm) and the deoxy-form UV region characteristic peak in the deoxygenated solutions (555 nm). The observed results indicated that the polymer does not interfere with the protein ability of reversibly binding oxygen.
To check whether Hb maintained its own functional properties after the loading into alginate based beads, UV-Vis Absorbance spectra analysis were performed on degraded BsHb. Particles degradation was carried out by using alginate lyase. Alginate lyases are enzymes isolated from a wide range of organisms, including algae, marine invertebrates and marine and terrestrial microorganisms [7] that are able to cleave alginate by the β-elimination mechanism [8].
UV-Vis spectra analyses were performed on oxygenated, deoxygenated and reoxygenated solutions containing the dissolved BsHb. The analyses revealed that Hb maintain its own functional activity after the loading into the beads, as revealed by oxy-form UV region characteristic peaks in the oxygenated and reoxygenated solutions and the deoxy-form UV region characteristic peak in the deoxygenated solutions (Figure 2). Moreover the presence of alginate lyase and alginate metabolites didn’t interfere with Hb characterization.
The protein content determination of BsHb was carried out by means of Drabkin’s assay performed on dissolved particles; results showed a protein Encapsulation Efficacy (EE) of about 30 ± 2% and a protein Loading (Ldg) of about 5 ± 0.3%.
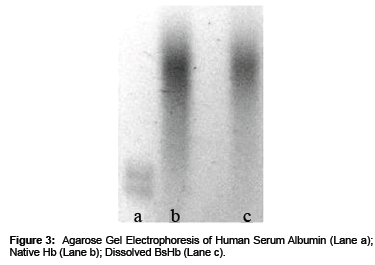
The maintenance of the protein quaternary structure after its loading into BsHb was detected by monitoring the Hb profile on Agarose gel under non-denaturing alkaline conditions [9-11].
As shown in Figure 3 the electrophoretic profile of Hb was comparable to the native protein electrophoretic profile, suggesting the maintenance of Hb quaternary structure after its loading into the Beads.
Hb loaded alginate microparticles
In order investigate the feasibility of reducing alginate particles dimension, formulation studies were carried out. Microparticles were obtained by ionic gelation lowering alginate concentration to 0.1 % (w/v) and Calcium chloride to 0.2 % (w/v) in agreement with literature data [12] (Formulation A). The prepared Hb loaded Microparticles (MPsHb_A) were characterized in terms of dimension, highlighting an average diameter of about 110 μm ± 20 μm; protein EE and Ldg, analysed by means of Drabkin’s assay, was estimated to be around 5.38 ± 0.8% and 1.4 ± 0.2% respectively. To increase Hb content into alginate microparticles the pH of the alginate solution was adjusted to 6 before dropping the polymer (Formulation B). As widely reported in literature Hb isoelectric point is 6.81 whereas alginate pKa value is around 3.6 [13,14]. At pH 6 the two components have opposite net charge, thus resulting in an increase of the electrostatic interaction between the protein and the polyanionic alginate; this interaction might lead to a higher Hb encapsulation into alginate particles [15]. Particles originating from Formulation B (MPsHb_B) were analysed for dimension and protein loading. No alteration regarding the average diameter was observed, while a significant increase in protein content was detected; 47 ± 4% of EE and 11 ± 2% of protein Ldg were obtained.
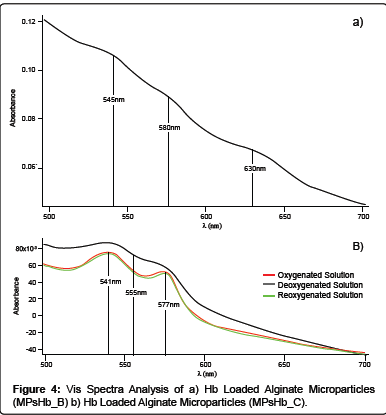
In order to check the maintenance of Hb functional activity once loaded inside MPsHb_B, UV-Vis spectra analysis were performed on the solutions obtained after alginate lyase activity. The typical absorbance peaks of Ferryl-Hb (545, 580 nm) and MetHb (630 nm) were present suggesting that an oxidation process occurring during microparticles preparation, might lead to the oxidation of Oxy- Hb to MetHb and Ferryl-Hb (Figure 4). It is well known that Hb autoxidation phenomena can be correlated to its concentration. At low concentrations Hb tetramers are easily separated in dimers [16], in particular this phenomenon is maximised when the Hb concentration is lower than 10-5 M [17]. As a consequence of the dimer conversion, a 17-fold increase in the rate of Hb autoxidation can be observed as well as an enhancement of hemichrome formation [18-20]. It is possible to speculate that the oxidative phenomena noticed in MPsHb_B formulation can be attributed to Hb dimers formation since a highly diluted Hb solution is needed to reduce alginate particles dimensions.
With the aim of minimizing the oxidation of the protein, Hb concentration into the formulation system was augmented from 3.4 × 10-6 M up to 3.4 × 10-5 M (Formulation C). MPsHb_C microparticles were characterized in terms of dimensions, highlighting the feasibility of obtaining Hb loaded particles possessing an average diameter of about 156 μm ± 94 μm. The observed increase in the average particle diameter of MPsHb_C with respect to the MPsHb_B microparticles formulation can be attributed to the higher protein content. In fact, although the EE of MPsHb_C was estimated to be 38 ± 3%, the protein Ldg was 50 ± 4%, as revealed by Drabkin’s assay. Moreover, as reported in Figure 4, UV-Vis spectra analysis showed the presence of the oxy-form region with characteristic peaks in the dissolved MPsHb_C microparticles oxygenated solution, suggesting that the oxidative phenomena observed in the MPsHb-B formulation seemed to be avoided.
On the basis of the observed results, it is possible to hypothesize that the dimerization of the protein, responsible for the Hb oxidation during the particles formulation process, is reduced by increasing the protein concentration in the formulation system. Further investigations aimed at analysing the maintenance of the protein ability of reversibly binding oxygen were also carried out.
As reported in Figure 4, only a partial conversion of Hb from the oxy-state to the deoxy-state was obtained. Since no oxidative processes seem to be responsible for the alteration of protein functionality, it can thus be suggested that a physicochemical interaction between alginate, calcium and a portion of loaded Hb can occur, slowing down the R-T transition of the protein.
To confirm this hypothesis, Hb electrophoretic profile on Agarose gel under non-denaturing alkaline conditions was analysed. As shown in Figure 5, single bands were detectable for native Hb and for Hb in the presence of both alginate lyase and alginate; on the other side 2 distinct bands were evident in dissolved Hb loaded Microparticles lanes.
These results are in agreement with our hypothesis, since dissolved Hb loaded Microparticles lower band can be attributed to free Hb portion, while the presence of an upper band can be caused by the interaction between the polymer, calcium ions and Hb which retards the protein migration into the gel.
Conclusions
The employment of alginate for the production of Hb loaded particles showed interesting results. The performed formulation studies aimed at reducing particles dimension, clearly demonstrated that the proposed system posses highly tunable properties. Moreover, as widely reported by the literature [21] a key issue in the development of Hb loaded particles concerns the need of avoiding oxidative phenomena which may lead to alteration on the protein functional properties; as a consequence the obtainment of particles carrying a non-oxidised form of the protein is an important result achieved in the present work, which may open promising perspective for the employment Hb loaded alginate particles for therapeutic applications.
Interestingly, a tight interaction between alginate and Hb was observed that affects the release of oxygen by the protein after its loading into the polymeric particles based on alginate. Since a low and a high Hb oxygen affinity is usually required for oxygen contribution to hypoxic and ischemic tissues respectively [22], polymeric particles loaded with functionally altered Hb can possibly be applied for therapeutic approaches aimed at achieving the remediation of particular pathological conditions.
Declaration of Interest
The authors report no declarations of interest.
References
- Sachan NK, Pushkar S, Jha A, Bhattcharya A (2009) Sodium alginate: the wonder polymer for controlled drug delivery. J Pharm Res 8: 1191-1199.
- Dalmoro A, Barba AA, Lamberti G, Grassi M, D'Amore M (2012) Pharmaceutical applications of biocompatible polymer blends containing sodium alginate. Adv Polym Tech 31: 219-230.
- Chiellini F, Piras AM, Errico C, Chiellini E (2008) Micro/nanostructured polymeric systems for biomedical and pharmaceutical applications. Nanomedicine (Lond) 3: 367-393.
- Reis CP, Neufeld RJ, Vilela S, Ribeiro AJ, Veiga F (2006) Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J Microencapsul 23: 245-257.
- Silva CM, Ribeiro AJ, Figueiredo M, Ferreira D, Veiga F (2006) Microencapsulation ofHemoglobin in Chitosan-coated Alginate Microspheres Prepared by Emulsification/Internal Gelation. AAPS J 7: E903-E913.
- Swain S, Behera A, Beg S, Patra CN, Dinda SC, et al. (2012) Modified alginate beads for mucoadhesive drug delivery system: an updated review of patents. Recent Pat Drug Deliv Formul 6: 259-277.
- Wong TY, Preston LA, Schiller NL (2000) ALGINATE LYASE: review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu Rev Microbiol 54: 289-340.
- Sutherland IW (2006) FEMS Microb Rev 16: 332.
- Kuznetsov SA, Vaisberg EA, Shanina NA, Magretova NN, Chernyak VY (1988) Gelfand VI. The quaternary structure of bovine brain kinesin. EMBO J 7: 353-356.
- Reddy PL, Bowie LJ, Callistein S (1997) Binding of nitric oxide to thiols and hemes in hemoglobin H: implications for alpha-thalassemia and hypertension. Clin Chem 43: 1442-1447.
- Gonzalez-Vogel A, Eyzaguirre J, Oleas G, Callegari E, Navarrete M (2011) Proteomic analysis in non-denaturing condition of the secretome reveals the presence of multienzyme complexes in Penicillium purpurogenum. Appl Microbiol Biotechnol 89: 145-155.
- Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P (1993) Development of a new drug carrier made from alginate. J Pharm Sci 82: 912-917.
- Gao HL, Li CY, Ma FX, Wang K, Xu JJ, et al. (2012) A nanochannel array based device for determination of the isoelectric point of confined proteins. Phys Chem Chem Phys 14: 9460-9467.
- Paul W, Sharma CP (2012) Proc Biochem 47: 882.
- Coppi G, Iannuccelli V, Leo E, Bernabei MT, Cameroni R (2001) Chitosan-alginate microparticles as a protein carrier. Drug Dev Ind Pharm 27: 393-400.
- Sugawara Y, Kadono E, Suzuki A, Yukuta Y, Shibasaki Y, et al. (2003) Hemichrome formation observed in human haemoglobin A under various buffer conditions. Acta Physiol Scand 179: 49-59.
- Arisaka F, Nagai Y, Nagai M (2011) Dimer-tetramer association equilibria of human adult hemoglobin and its mutants as observed by analytical ultracentrifugation. Methods 54: 175-180.
- Maqsood S, Benjakul S (2011) Food Chem 124: 875.
- Culbertson DS, Olson JS (2010) Role of heme in the unfolding and assembly of myoglobin. Biochemistry 49: 6052-6063.
- Vergara A, Franzese M, Merlino A, Bonomi G, Verde C, et al. (2009) Correlation between hemichrome stability and the root effect in tetrameric hemoglobins. Biophys J 97: 866-874.
- Piras AM, Dessy A, Chiellini F, Chiellini E, Farina C, et al. (2008) Polymeric nanoparticles for hemoglobin-based oxygen carriers. Biochim Biophys Acta 1784: 1454-1461.
- Sakai H, Cabrales P, Tsai AG, Tsuchida E, Intaglietta M (2005) Oxygen release from low and normal P50 Hb vesicles in transiently occluded arterioles of the hamster window model. Am J Physiol Heart Circ Physiol 288: H2897-903.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 21848
- [From(publication date):
July-2013 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 17106
- PDF downloads : 4742