Research Article Open Access
Hemocompatibility Evaluation of Polyurethane Film with Surface-Grafted Sugar- Based Amphipathic Compounds
Zhirong Xin1*, Binbin Du1, Yanming Wang1, Shengxu Qian1, Weijia Li1, Yuan Gao1, Miao Sun1, Shifang Luan2* and Jinghua Yin2
1School of Chemistry and Chemical Engineering, Yantai University, Yantai 264005, China
2State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, People’s Republic of China
- *Corresponding Author:
- Zhirong Xin
School of Chemistry and Chemical Engineering
Yantai University, Yantai 264005, China
Tel: 865356902232
Email: xinzhirong2012@126.com
Shifang Luan
State Key Laboratory of Polymer Physics and Chemistry
Changchun Institute of Applied Chemistry, Chinese Academy of Sciences
Changchun 130022, People’s Republic of China
Tel: 861068597521
E-mail: sfluan@ciac.ac.cn
Received Date: March 20, 2017; Accepted Date: April 01, 2017; Published Date: April 05, 2017
Citation: Xin Z, Du B, Wang Y, Qian S, Li W, et al. (2017) Hemocompatibility Evaluation of Polyurethane Film with Surface-Grafted Sugar-Based Amphipathic Compounds. J Anal Bioanal Tech 8:357. doi: 10.4172/2155-9872.1000357
Copyright: © 2017 Xin Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Sugar-based amphipathic compounds (BA-CnAG) were successfully prepared. Polyurethane (PU) was grafted with glycidyl methacrylate (GMA) by the means of UV irradiation, and further modified with the BA-CnAG based on the ring opening of the epoxy groups. The surface graft polymerization was confirmed by ATR-FTIR and XPS. Water contact angle, protein adsorption, and platelet adhesion measurements were used to evaluate the hydrophilicity and hemocompatibility of the films. The results demonstrated that amphiphilic surfactant-containing polymer surfaces presented protein-resistant behavior and anti-platelet adhesion after functionalization with BA-CnAG. Besides, the hemocompatibility of the modified surface deteriorated as the length of hydrophobic chain of BA-CnAG increased.
Keywords
Polyurethane; Sugar-based amphipathic compounds; Photo-grafting polymerization; Protein adsorption; Platelet adhesion
Introduction
Polyurethane (PU) has been reported in a variety of processing biomedical devices such as artificial hearts [1,2], cardiovascular biomaterials [3-5], and scaffolds [6,7] for over four decades. This synthetic polymer contains soft and hard segments which contributes to different degree of micro-phase separation, consequently affects its physical and mechanical properties. Despite the use of PU in certain blood-contacting applications, the contributing effects of the numerous chemical ingredients of polyurethane in thrombogenesis is nonignorable [8-10]. When in contact with blood or serum for long periods of time, the surface of PU films will result in significant adsorption of proteins, and induce platelet adhesion by activating the coagulation pathway, eventually leading to the formation of microscopic thrombi and microemboli. Lelah et al. [11] has investigated the surface properties for a number of different polyurethanes and believed that the reason was of significance in determining blood response.
Nowadays, Hemocompatible surfaces have been of great interest in the development of biomedical materials. Many studies, including physical deposition, “grafting to” or “grafting-from” strategies, have focused on the modification of different materials in order to improve hemocompatibility [12]. However, as reported, most of the surface modification techniques mentioned above usually results in deteriorating of the bulk properties of the substrates and/or a short period remaining for the modification effects. Compared with other methods, photografting polymerization of functional monomers induced by UV irradiation showed some special advantages, such as low cost of equipment, fast rate and mild reaction conditions without affecting the bulk performance of materials [13,14]. A large number of biocompatible moieties, including sugars [15-18], heparin [19,20], phospholipid polymers [21-23], and hyaluronic acid [24] have been used with photografting polymerization to modify the surface properties to obtain the satisfied biocompatibility substrate.
Recently, a series of amphiphilic polymeric materials was fabricated to explore the effect of nonfouling performance of surfaces [25-28]. Chen et al. [29] studied the interactions of amphiphilic polymers films with proteins and found that despite the high affinity of the hydrophilic or hydrophobic functional groups by themselves toward proteins, the films showed great reduced nonspecific protein adsorption. Colak et al. [26] designed a series of novel zwitterion-based, amphiphilic coatings. The hydrophobicity of the system was tuned by the incorporation of side chains to the unique zwitterionic functionality at a repeat unit level and the result showed that the hydrophobic/lipophilic interactions can be tuned to obtain an effective nonfouling material. However, the mechanism behind this effectiveness is controversial, the general consensus is that the amphiphilic molecules with two extreme chemistries can entangle with each other and form a nonleaching surface, weakening the interaction of the organism with the surface.
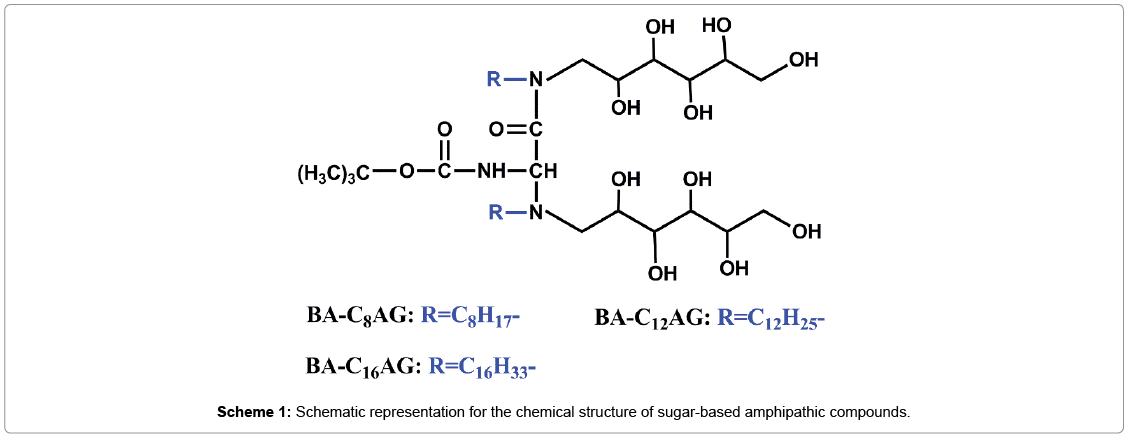
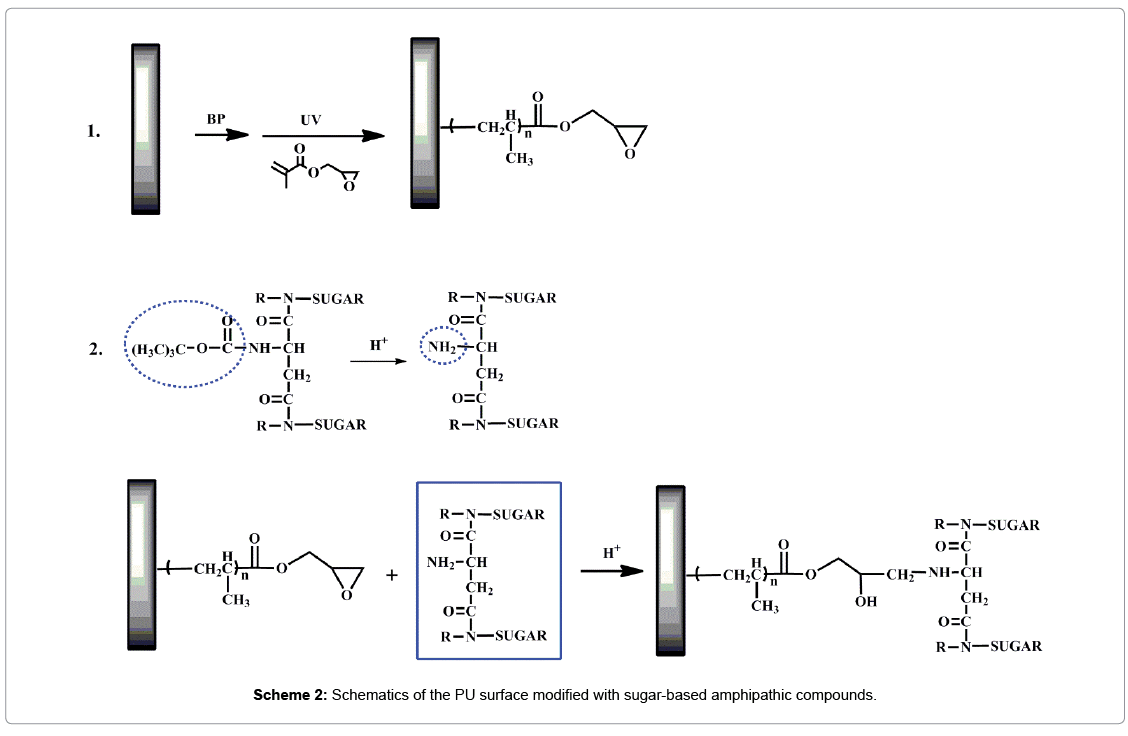
In this work, a series of sugar-based amphipathic compounds (BAC nAG, Scheme 1) with glucose as hydrophilic head-groups and alkyl groups as hydrophobic tails were synthesized. Then the as-prepared compounds were covalently immobilized on the pGMA modified PU based on the ring opening of the epoxy groups (Scheme 2). The surfaces were characterized by attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), X-ray photoelectron spectroscopy (XPS), and contact angle measurements. Furthermore, surface hemocompatibility of the modified PU was examined by Bovine serum albumin (BSA) adsorption and the platelet adhesion assay.
Experimental
Materials
L-aspartic acid (Asp), di-tert-butyl dicarbonate ((BOC)2O), N,N′- dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), and glycidyl methacrylate (GMA) were obtained from Shanghai Aladdin Chemicals (China). Benzophenone (BP) was supplied by Peking Ruichen Chemicals (China). Bovine serum albumin (BSA), sodium dodecyl sulfate (SDS) and phosphate buffered solution (PBS, 0.01 mol/L, pH=7.4) were provided by Dingguo Bio-technology (China). Micro BCATM protein assay reagent kits were purchased from Boster Biological Technology (AR1110, China). The platelet-rich plasma (PRP) was obtained from the fresh rabbit blood by centrifugation at 1000 rpm for 15 min. The other solvents and reagents were AR grade chemicals and used without further purification.
Preparation and characterization of sugar-based amphipathic compounds
Synthesis of Boc-Asp: To a solution of Asp (10 mmol) in acetone/ ultrapure water (10:1, 44 mL), was added triethylamine (20 mmol), and the resulting solution was stirred in an ice-water bath. (BOC)2O (11 mmol) was added dropwise, stirred for 3 h. The solution was concentrated under reduced pressure, diluted with H2O (20 mL), and extracted with ethylacetate (3 × 30 mL). The aqueous layer was acidified using HCL (1 mol/L) in an ice-water bath and extracted with ethylacetate (3 × 30 mL). The combined organic extracts were washed with brine (2 × 20 mL), dried with Na2SO4. After evaporation of the solvent, the solid residue was washed several times with hexane, and dried in a desiccator. The structure of the product was confirmed by proton nuclear magnetic resonance spectroscopies (1H NMR, 400 MHz Bruker Avance DPX-300 spectrometer).
1H-NMR (CD3OD): (δï¼┬?ppm) 1.34ï¼┬?s, 9H, 3CH3, 2.83ï¼┬?s, 2H, CH2), 4.43ï¼┬?s,1H,CH).
Synthesis of sugar-based amphipathic compounds: The sugarbased amphipathic compounds (N-Alkyl gluconamide, denoted as BA-CnAG, n=8, 12, 16) were synthesized as follows, the structural characteristics of BA-CnAG are shown in Scheme 1:
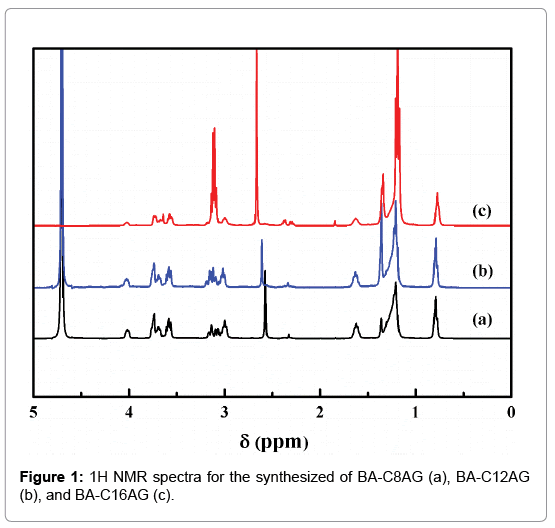
The solution of Boc-Asp (7.5 mmol), DCC (15 mol), and NHS (15 mol) was stirred in an ice-water bath for 2 hours, and then react to another 12 hours at room temperature. The mixture was filtered to remove insoluble by-product. After the filtrate was evaporated, the residue was washed with ethanol, and the carboxyl active ester was obtained after dried. The active ester (0.01 mol) and N-alkyl-1-amino- 1-deoxy-D-glucitol surfactants (0.02 mol, denoted as CnAG, n=8, 12, 16, were synthesized in our laboratory in early according to a method described in detail elsewhere [30]), were added to a three-necked bottle with acetone, and the pH of the mixture was adjusted to 8 with triethylamine. Then the reaction was carried out at room temperature for 48 h. After evaporation of the solvent, the residue was washed with hexane and acetonitrile, and dried in a desiccator. The structures of the final products were confirmed by proton nuclear magnetic resonance spectroscopies (Figure 1).
BA-C8AG, 1H-NMR (D2O): (δ,ppm) 0.80 (s, 6H, CH3), 1.20-1.36 (m, 29H, C(CH3)3, CH3-(CH2)5-), 1.62 (m, 4H, CH3-(CH2)5-CH2), 2.32 (s, 1H, NH-CH-CH2-), 2.57 (m, 4H, CH3-(CH2)6-CH2), 4.01 (m, 2H, NH-CH-CH2), 2.96-3.77 (m, 6H, CH-OH and -CH2-OH, sugar).
BA-C12AG, 1H-NMR (D2O): (δ,ppm) 0.79 (s, 6H, CH3), 1.20-1.37 (m, 45H, C(CH3)3, CH3-(CH2)9-), 1.63 (m, 4H, CH3-(CH2)9-CH2), 2.34 (s, 1H, NH-CH-CH2-), 2.60 (m, 4H, CH3-(CH2)10-CH2), 4.03 (m, 2H, NH-CH-CH2), 3.00-3.76 (m, 6H, CH-OH and -CH2-OH, sugar).
BA-C16AG, 1H-NMR (D2O): (δ,ppm) 0.77 (s, 6H, CH3), 1.18-1.34 (m, 61H, C(CH3)3, CH3-(CH2)13-), 1.63 (m, 4H, CH3-(CH2)13-CH2), 2.34 (s, 1H, NH-CH-CH2-), 2.67 (m, 4H, CH3-(CH2)14-CH2), 3.99 (m, 2H, NH-CH-CH2), 3.10-3.72 (m, 6H, CH-OH and -CH2-OH, sugar).
Surface modification of PU films
Preparation of the pGMA-modified films: The solution-casted PU films were washed with deionized water and ethanol several times, and dried for use. The dried films were dipped into a BP solution of ethanol (1.0 wt%) for 30 minutes, and dried at room temperature. Then the PU films were placed on the glass plate with a drop of ethanol solution containing GMA monomer (10 vol%) deposited on each PU film, and a quartz plate was placed on the top. After that the PU films were irradiated under a high-pressure mercury lamp (400 W, main wavelength 380 nm) for 8 min. Finally, the films (denoted as PU-g- GMA) were washed with water and ethanol several times to remove residual GMA or pGMA, and dried in a vacuum oven.
Preparation of the BA-CnAG-modified films: To a solution of BA-CnA (300 mg) in ethanol solution (30 mL), HCL (1 mol/L) was added to removal tert-butyl of BA-CnA with stirring for 30 min. With the amino groups exposed, the PU-g-GMA films were immersed with the reaction of the epoxy groups and amino groups for 12 h at 60°C under reflux. Finally, the modified films (denoted as PU-g-GMA-BAC nAG) were rinsed with ethanol and water, and dried under vacuum at room temperature.
Characterization
ATR-FTIR measurement was performed on a Fourier transform infrared spectrometer (FTIR, BRUKER Vertex 70) with a resolution of 4 cm-1 in absorbance mode.
XPS analysis was carried out on a VG Scientific ESCA MK II Thermo Avantage V 3.20 analyzer with an Al/K (hν=1486.6 eV) anode mono-X-ray source. The releasing angle of the photoelectron for each atom was fixed at 90°. The survey spectra were collected over a range of 0-1200 eV and high-resolution spectra of C1s were also collected.
The wettability of the films was determined by using contact angle measurements performed with a contact angle measuring system (DSA, KRÜSS GmbH, Germany). Before measuring, the films were dried overnight in a vacuum oven at room temperature. Measurements were carried out using the sessile drop method with 2.0 μL deionized water drops. Reported results were the averages of measurements on five samples.
Protein adsorption
The films were placed into a 24-well tissue culture plate and immersion in PBS for at least 2 h to equilibrate the surfaces. Then, the PBS was removed and a protein solution of BSA (1.0 mg/mL) was poured into the well and incubated for 2 h at 37°C. After that, the samples were washed with PBS and finally, the proteins adsorbed on the surface were removed by immersing the samples in a 1.0% (w/v) SDS solution for 20 min. According to a bicinchoninic acid (BCA) protein assay kit method, the amounts of proteins desorbed were determined by a microplate reader (TECAN SUNRISE, Swiss).
Platelet adhesion
After equilibration with PBS overnight, the films with 20 μL PRP dropped on were incubation for 2 h at 37°C. Then the films were rinsed four times with PBS to remove weakly adsorbed platelets and treated by glutaraldehyde (2.5 wt%) for more than 2 h at 4°C. After fixation, the samples were dehydrated with a series of ethanol-water solutions (30, 50, 70, 90, 100% (v/v)) for 30 min per step. Finally, the films were coated with gold and the adhered platelets were observed with a field emitted scanning electron microscopy (SEM, XL 30 ESEM FEG, FEI Company, USA). The average number of adherent platelets was counted by SEM images in three different domains.
Results and Discussion
Surface chemistry
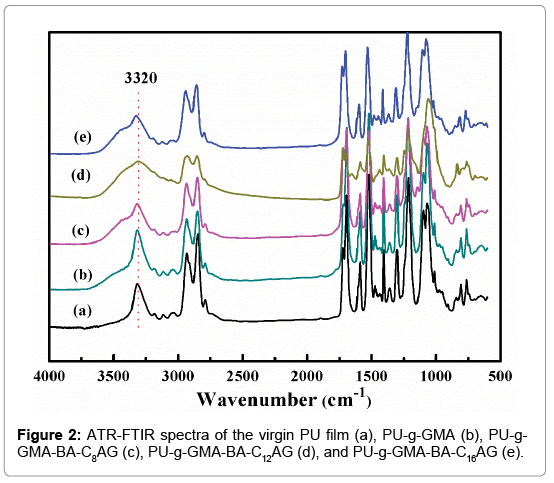
The ATR-FTIR spectra of PU-g-GMA (b), PU-g-GMA-BA-C8AG (c), PU-g-GMA-BA-C12AG (d), PU-g-GMA-BA-C16AG (e) surfaces, as well as the unmodified PU (a) surface are shown in Figure 2. The absorbance at 3320 cm-1 (O-H) was increased in Figure 2c, 2d and 2e), providing supporting evidence of the surface grafting of BA-CnAG onto the pGMA-modified PU films. However, the absorbance of grafted films was not significantly different from that of the pure PU film, and this was attributed to the fact that peaks of newly grafted groups of GMA and BA-CnAG were overlapped with that in the PU itself [31,32].
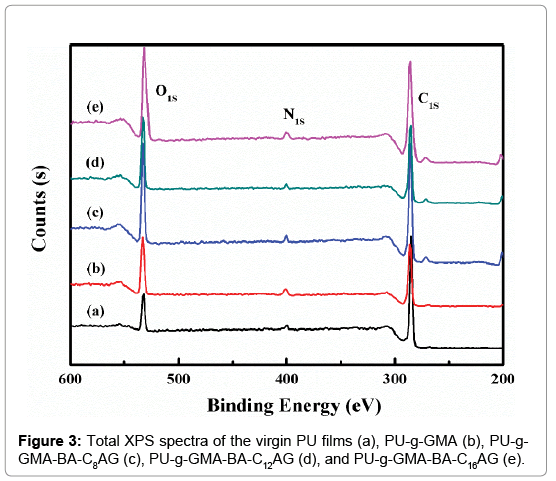
The surface composition of the outermost layer of the virgin and modified PU films was determined by XPS (Figure 3). The large peak at about 284 eV attributing to C1s, the small peak at about 400 eV corresponding to N1s, and the absorbance at 532 eV was attributed to O1s. As expected, the oxygen content of the grafted surface increased due to the grafting of ester groups, cyclooxy groups in GMA and hydroxyl groups in BA-CnAG. According to the above XPS data, the elemental compositions and the atomic percentage of the surfaces were calculated in Table 1. The O/C ratio increased from 0.18 to 0.30 after grafted with GMA. With further immobilization of BA-CnAG, the O/C ratio decreased from 0.35 to 0.31 with the increasing of n, and this ascribed to the increasing of alkyl chain length of BA-CnAG molecules. These verified the successful surface modification of PU via the procedures as shown in Scheme 2.
| Samples | Atomic compositions (%) | Atomic ratio (%) | ||
|---|---|---|---|---|
| C1s | N1s | O1s | O/C | |
| Virgin PU | 83.1 | 1.8 | 15.1 | 18.2 |
| PU-g-GMA | 75.0 | 2.2 | 22.6 | 30.1 |
| PU-g-GMA-BA-C8AG | 71.9 | 2.9 | 25.2 | 35.0 |
| PU-g-GMA-BA-C12AG | 73.2 | 2.3 | 24.5 | 33.5 |
| PU-g-GMA-BA-C16AG | 74.5 | 2.6 | 22.9 | 30.7 |
Table 1: Atomic compositions and atomic ratio from XPS.
Wettability and surface energy of the PU films
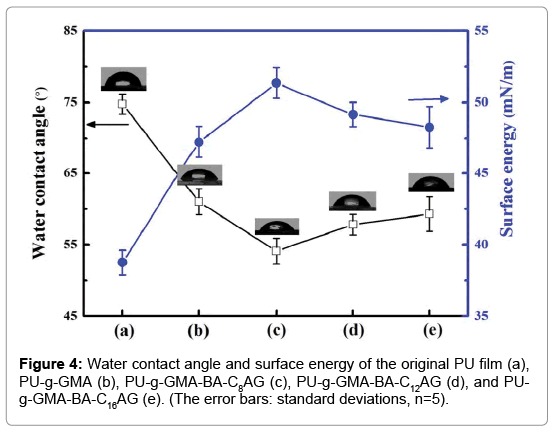
The surface of PU film was almost smooth which became rough by grafting GMA, due to the reason that increasing the amount of grafted polymer lead to a rougher surface. The contact angle measurement results of PU film and grafted PU films were shown in Figure 4. The average contact angle of the virgin PU was 75°. After grafting of GMA, the hydrophilicity was enhanced and a decrease of contact angle down to approximately 61°, attributed to the cyclooxy groups in the GMA molecule. With the further immobilization of BA-CnAG, the films were even more hydrophilic and the average contact angle was reduced to 54°, 57° and 59°, indicated that the hydroxyl groups contributed significantly to the hydrophilicity of the copolymers. In addition, comparing of the parameters in Figure 4c, 4d and 4e, the surface wettability had a dependence on hydrophobic groups of the BA-CnAG. When the length of alkyl chain was shortest, the contact angle was lowest, presented a highly hydrophilic surface. Correspondingly, for the modified films, the surface energy was enhanced from 38.76 mJ/m2 to approximately 47.22, 51.36, 49.15 and 48.28 mJ/m2.
Protein adsorption
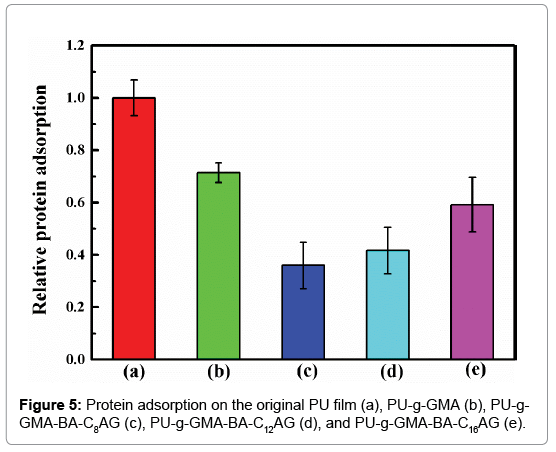
The initial adsorption of plasma proteins takes place when an artificial surface comes into contact with blood. Therefore, the main purpose for biomaterial development is to build ultra-low fouling or even non-fouling surfaces. In this study, bovine serum albumin (BSA) was selected as a model protein for total protein adsorption investigations. As shown in Figure 5, the PU-g-GMA did not provide an effective resistance to protein adsorption compared with the original film. Among the samples investigated, the PU film modified by BA-C8AG possessed the most favorable surface, and the adsorption of BSA on its hydrophilic surface decreased by at least 64%. In contrast, the relative protein adsorption of PU-g-GMA-BA-C12AG and PU-g- GMA- BA-C16AG decreased by at least 58% and 41% compared with the original film. Among the amphiphilic BA-CnAG modified samples, we hypothesized that the alkyl chain length of the sugar-based compounds would influence the extent of protein adsorption. It showed that when an appropriate balance between hydrophobicity and hydrophilicity was reached, amphiphilic polymer surface with antifouling property can be expected.
Platelet adhesion
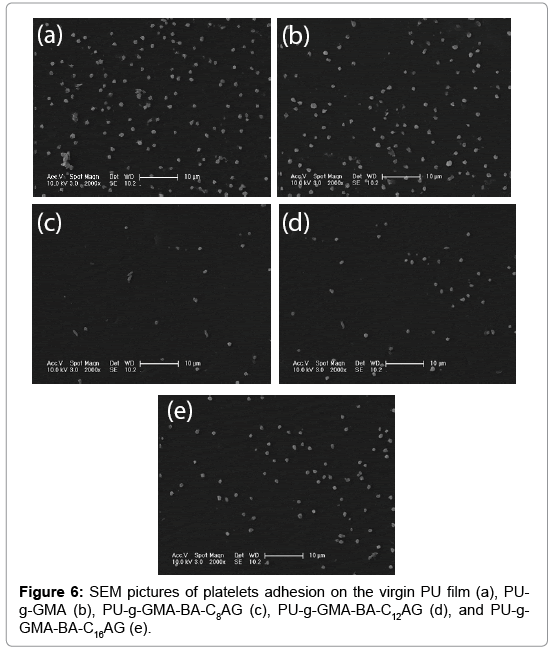
The interactions between the blood and samples were assayed by platelet adhesion experiment. Platelet aggregation is symbol of platelet activation and is considered to be a major mechanism of thrombosis [33,34]. Meanwhile, platelet adhesion is one of the intuitive methods to measure the blood compatibility of biomaterials. As shown in Figure 6, the unmodified PU surface exhibited significant adhesion of platelets and the average platelet density was 63.1 × 103 cells/mm2. For pGMAgrafted film (Figure 6b), the situation of platelet adhesion was somewhat improved and the average platelet density was 52.9 × 103 cells/mm2. On PU-g-GMA-BA-CnAG substrates (Figure 6c, 6d and 6e), platelet adhesion was almost entirely inhibited (9.2 × 103, 17.7 × 103, 35.0 × 103 cells/mm2), and quantitatively, the adhered platelet densities were reduced by 85%, 71.9% and 45%. This improvement was presumably derived from water hydration and the configurational entropy repulsion of the grafted polymers, preventing the protein from approaching the surfaces, and hence, lead to an excellent hemocompatibility of the modified films. Comparing of the average platelet density in Figure 6c, 6d and 6e), the film own the worst hemocompatibility with the BA-C16AG immobilized on pGMA-PU surface, indicating that hydrophobicity functionalities in amphiphilic structure dominating the surface may be responsible for their fierce platelet adhesion. These results well coincide with the protein adsorption assays.
Conclusions
Sugar-based amphiphilic compounds have been successfully immobilized on PU via a pGMA spacer. The chemical structure of the BA-CnAG and the surface chemistry of the relevant modified films were investigated by 1H NMR, ATR-FTIR and XPS, respectively. Moreover, a decrease of the contact angle also demonstrated thegrafting of this hydrophilic monomer. After modification with BACnAG, the amphiphilic surfactant-containing polymer surfaces presented relatively high excellent protein-resistant and anti-platelet adhesion performance. The delicate balance between hydrophilicity and hydrophobicity accounted for the satisfied hemocompatibility of BA-CnAG-modified substrates.
Acknowledgements
The authors acknowledge the financial support of the Shandong Natural Science Foundation of China (Grant No. ZR2015EM036), and the financial support of the National Science Foundation of China (Grant No. 21404088).
References
- Bélanger MC, Marois Y, Roy R, Mehri Y, Wagner E, et al. (2000) Selection of a polyurethane membrane for the manufacture of ventricles for a totally implantable artificial heart: blood compatibility and biocompatibility studies. Artificial organs24:879-888.
- Alves P, Cardoso R, Correia T, Antunes B, Correia I, et al. (2014) Surface modification of polyurethane films by plasma and ultraviolet light to improve haemocompatibility for artificial heart valves. Colloids and Surfaces B: Biointerfaces113:25-32.
- Meng J, Kong H, Xu H, Song L, Wang C, et al. (2005) Improving the blood compatibility of polyurethane using carbon nanotubes as fillers and its implications to cardiovascular surgery. Journal of Biomedical Materials Research Part A74:208-214.
- Lam MT, Wu JC (2012) Biomaterial applications in cardiovascular tissue repair and regeneration.
- Kucinska-Lipka J, Gubanska I, Janik H, Sienkiewicz M (2015) Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Materials Science and Engineering: C46:166-176.
- Verdonk P, Beaufils P, Bellemans J, Djian P, Heinrichs E-L, et al. (2012) Successful treatment of painful irreparable partial meniscal defects with a polyurethane scaffold two-year safety and clinical outcomes. The American journal of sports medicine40:844-853.
- Li L, Zhao M, Li J, Zuo Y, Zou Q, et al. (2015) Preparation and cell infiltration of lotus-type porous nano-hydroxyapatite/polyurethane scaffold for bone tissue regeneration. Materials Letters149:25-28.
- Stokes KB (1988) Polyether polyurethanes: biostable or not? Journal of biomaterials applications3:228-259.
- Burke A, Hasirci N (2004) Polyurethanes in biomedical applications. Biomaterials, Springer,pp: 83-101.
- Francolini I, Crisante F, Martinelli A, D’Ilario L, Piozzi A (2012) Synthesis of biomimetic segmented polyurethanes as antifouling biomaterials. Acta biomaterialia8:549-558.
- Lelah MD, Grasel TG, Pierce JA, Cooper SL (1986) Ex vivo interactions and surface property relationships of polyetherurethanes. Journal of biomedical materials research20:433-468.
- Meyers SR, Grinstaff MW (2012) Biocompatible and bioactive surface modifications for prolonged in vivo efficacy. Chemical reviews112:1615-1632.
- Deng J, Wang L, Liu L, Yang W (2009) Developments and new applications of UV-induced surface graft polymerizations. Progress in Polymer Science34:156-193.
- Benson RS (2002) Use of radiation in biomaterials science. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms191:752-757.
- Xin Z, Hou J, Ding J, Yang Z, Yan S, et al. (2013) Surface functionalization of polyethylene via covalent immobilization of O-stearoyl-chitosan. Applied Surface Science279:424-431.
- Yang Q, Strathmann M, Rumpf A, Schaule G, Ulbricht M (2010) Grafted glycopolymer-based receptor mimics on polymer support for selective adhesion of bacteria. ACS applied materials & interfaces2:3555-3562.
- Yang Q, Xu ZK, Dai ZW, Wang JL, Ulbricht M (2005) Surface modification of polypropylene microporous membranes with a novel glycopolymer. Chemistry of materials17:3050-3058.
- Ren Z, Chen G, Wei Z, Sang L, Qi M (2013) Hemocompatibility evaluation of polyurethane film with surfaceâ┬?┬Égrafted poly (ethylene glycol) and carboxymethylâ┬?┬Échitosan. Journal of Applied Polymer Science127:308-315.
- Gao Q, Chen Y, Wei Y, Wang X, Luo Y (2013) Heparin-grafted poly (tetrafluoroethylene-co-hexafluoropropylene) film with highly effective blood compatibility via an esterification reaction. Surface and Coatings Technology228:S126-S30.
- Guowei Z, Yashao C, Tao D, Xiaoli W (2007) Surface modification of polyethylene by heparin for improvement of antithrombogenicity. Plasma Science and Technology9:202.
- Hoven VP, Chombanpaew K, Iwasaki Y, Tasakorn P (2009) Improving blood compatibility of natural rubber by UVâ┬?┬Éinduced graft polymerization of hydrophilic monomers. Journal of applied polymer science112:208-217.
- Xiong D, Deng Y, Wang N, Yang Y (2014) Influence of surface PMPC brushes on tribological and biocompatibility properties of UHMWPE. Applied Surface Science298:56-61.
- Song L, Zhao J, Jin J, Ma J, Liu J, et al. (2014) Fabricating antigen recognition and anti-bioadhesion polymeric surface via a photografting polymerization strategy. Materials Science and Engineering: C36:57-64.
- Li X, Luan S, Shi H, Yang H, Song L, et al. (2013) Improved biocompatibility of poly (styrene-b-(ethylene-co-butylene)-b-styrene) elastomer by a surface graft polymerization of hyaluronic acid. Colloids and surfaces B, Biointerfaces102:210-217.
- Feng S, Huang Y, Wang Q, Qing F-L (2011) Nonbiofouling surface based on amphiphilic alkanethiol self-assembled monolayers. Surface and Interface Analysis43:770-776.
- Colak S, Tew GN (2012) Amphiphilic polybetaines: the effect of side-chain hydrophobicity on protein adsorption. Biomacromolecules13:1233-1239.
- Goli KK, Rojas OJ, Genzer J (2012) Formation and antifouling properties of amphiphilic coatings on polypropylene fibers. Biomacromolecules13:3769-3779.
- van Zoelen W, Buss HG, Ellebracht NC, Lynd NA, Fischer DA, et al. (2014) Sequence of Hydrophobic and Hydrophilic Residues in Amphiphilic Polymer Coatings Affects Surface Structure and Marine Antifouling/Fouling Release Properties. ACS Macro Letters3:364-368.
- Chen Y, Thayumanavan S (2009) Amphiphilicity in Homopolymer Surfaces Reduces Nonspecific Protein Adsorption†. Langmuir25:13795-13799.
- Laska U, Wilk KA, Maliszewska I, Syper L (2006) Novel glucose-derived Gemini surfactants with a 1,1'-ethylenebisurea spacer: preparation, thermotropic behavior, and biological properties. Journal of Surfactants and Detergents9:115-124.
- Choi H-S, Kim Y-S, Zhang Y, Tang S, Myung S-W, et al. (2004) Plasma-induced graft co-polymerization of acrylic acid onto the polyurethane surface. Surface and Coatings Technology182:55-64.
- Yuan J, Chen L, Jiang X, Shen J, Lin S (2004) Chemical graft polymerization of sulfobetaine monomer on polyurethane surface for reduction in platelet adhesion. Colloids and surfaces B, Biointerfaces39:87-94.
- Vogler EA, Siedlecki CA (2009) Contact activation of blood-plasma coagulation. Biomaterials30:1857-1869.
- Williams DF (2008) On the mechanisms of biocompatibility. Biomaterials29:2941-2953.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 4007
- [From(publication date):
April-2017 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 3112
- PDF downloads : 895